Probiotics and Phytoantioxidants Target Coronary Endothelial Dysfunction in Irregular Sleep- and Obesity-Associated Cardiometabolic Syndrome
Abstract
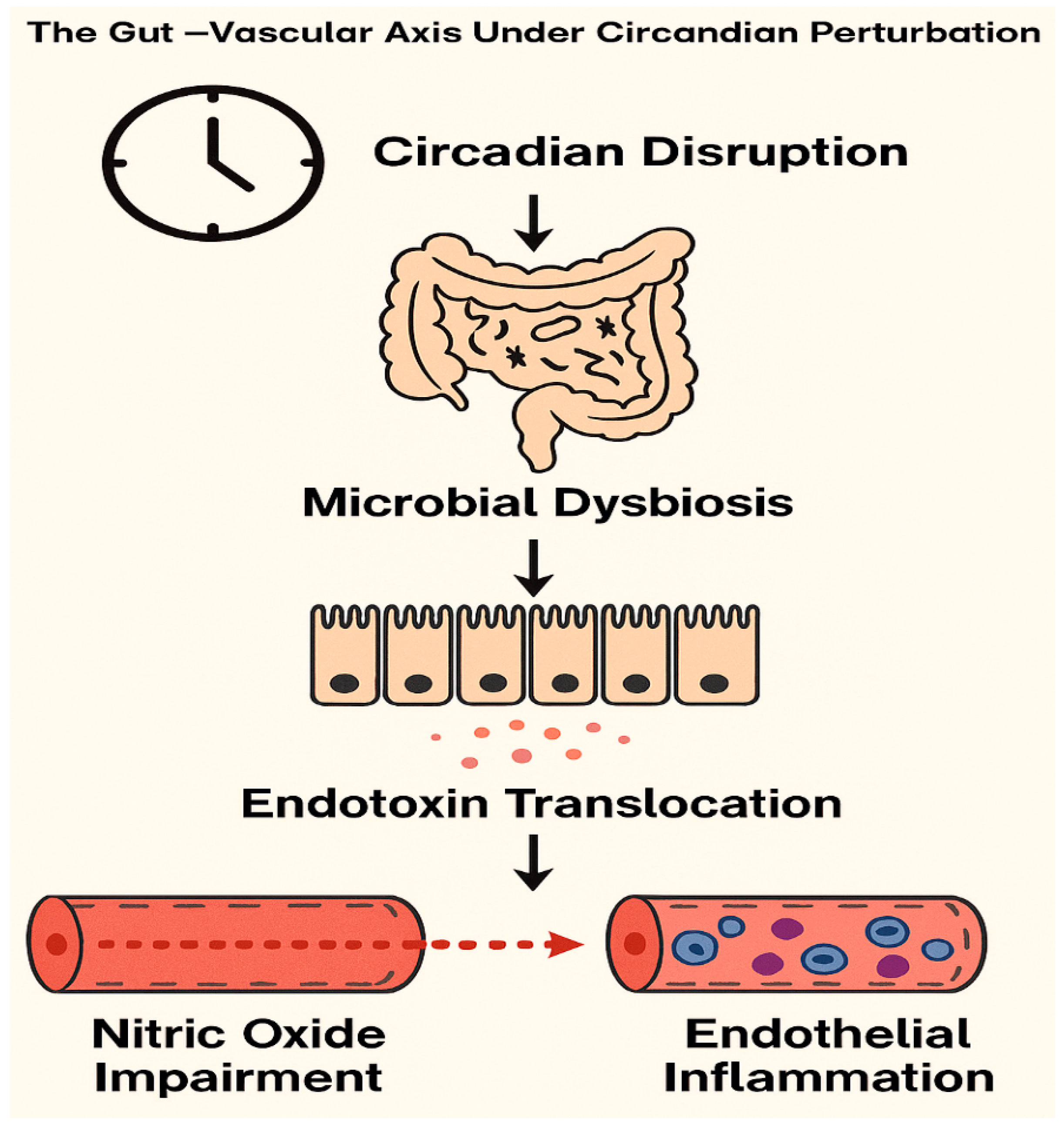
1. Introduction
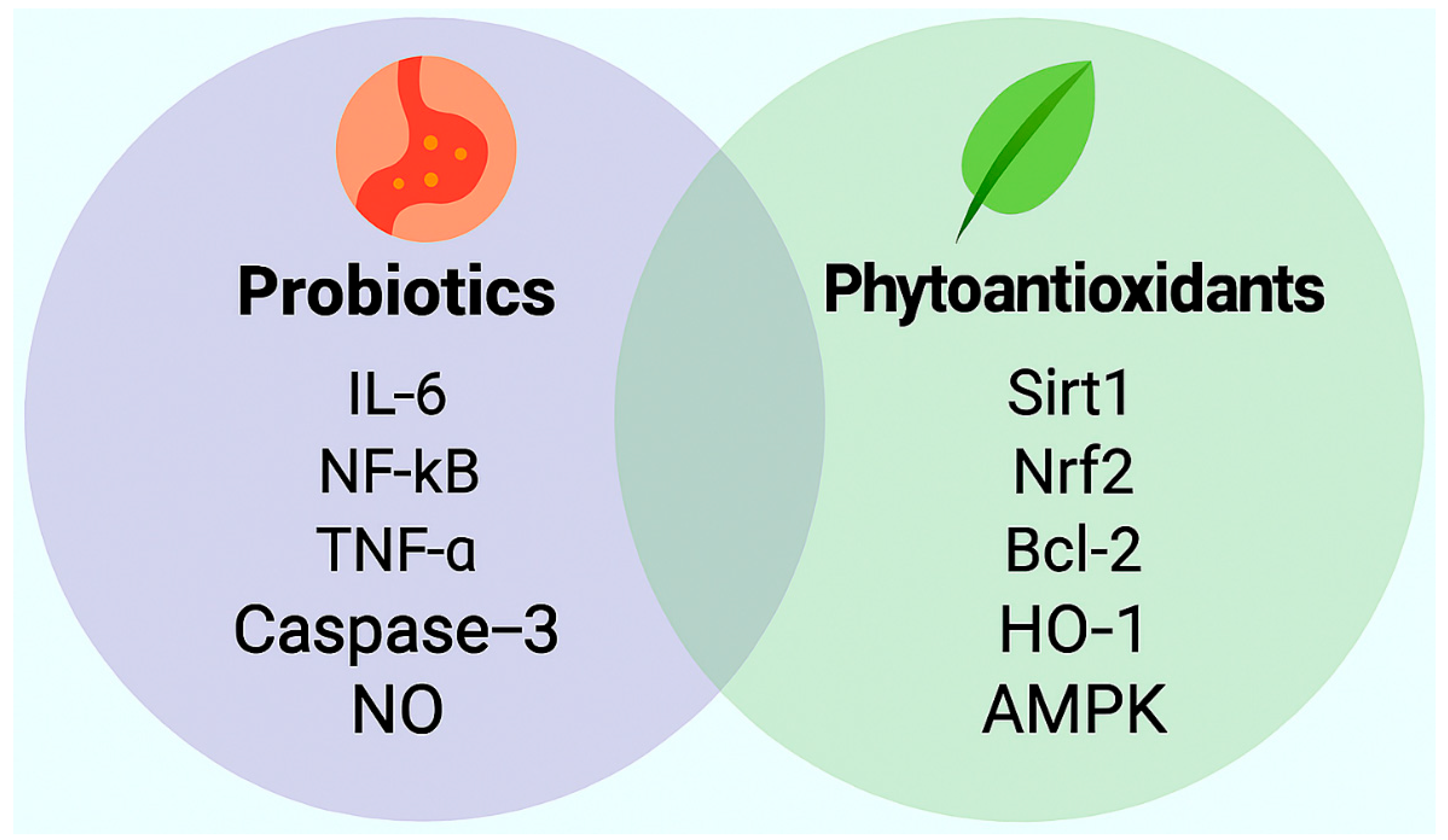
2. Probiotics: Mechanistic, Preclinical, and Clinical Evidence
- Lactobacillus plantarum 299v (109 CFU/day for 8 weeks) improved flow-mediated dilation and reduced IL-6 and TNF-α levels in adults with metabolic syndrome [30].
- Bifidobacterium longum BB536 (109 CFU/day for 12 weeks) lowered systolic blood pressure and oxidized LDL in hypertensive adults, with concurrent improvement in endothelial-dependent vasodilation [31].
- Lactobacillus casei Shirota (109 CFU/day for 8 weeks) significantly attenuated renal inflammation and fibrosis, en-hanced regulatory T-cell activity, and suppressed NF-κB signaling, indicating im-munoregulatory and nephroprotective effects [32].
| Bacterial Species | Intervention Details | Mechanistic Effects | Vascular Outcomes | Reference |
|---|---|---|---|---|
| Lactobacillus acidophilus ATCC 4356 | 1 × 109 CFU/mL, daily for 4 weeks (preclinical) | Activates SIRT1, Nrf2, and HO-1; upregulates antioxidant enzymes | Mitigates myocardial injury; enhances endothelial resilience | [28] |
| Lactobacillus plantarum 299v | 109 CFU/day for 8 weeks (clinical) | Reduces IL-6 and TNF-α; enhances SCFA biosynthesis | Improves flow-mediated dilation in metabolic syndrome | [30] |
| Bifidobacterium longum BB536 | 109 CFU/day for 12 weeks (clinical) | Lowers oxidized LDL; modulates NO signaling | Reduces systolic blood pressure; improves endothelial vasodilation | [31] |
| Lactobacillus casei Shirota | 1010 CFU/day for 12 weeks (clinical) | Enhances NO bioavailability; reduces VCAM-1 expression | Improves endothelial function in overweight individuals | [32] |
| Lactobacillus spp. (observational) | Elevated abundance in STEMI patients | Associated with lower IL-1β, TNF-α, and malondialdehyde | Attenuates systemic inflammation and oxidative stress | [28] |
| Lactobacillus rhamnosus GG | 109 CFU/day for 6–8 weeks (clinical and preclinical) | Suppresses TLR4 signaling; enhances tight junction integrity | Reduces CRP and improves endothelial-dependent vasodilation | [24,25,26] |
| Bifidobacterium breve B-3 | 109 CFU/day for 12 weeks (clinical) | Increases SCFA production; reduces TMAO and inflammatory cytokines | Improves arterial stiffness and metabolic markers | [27,29] |
3. Phytoantioxidants
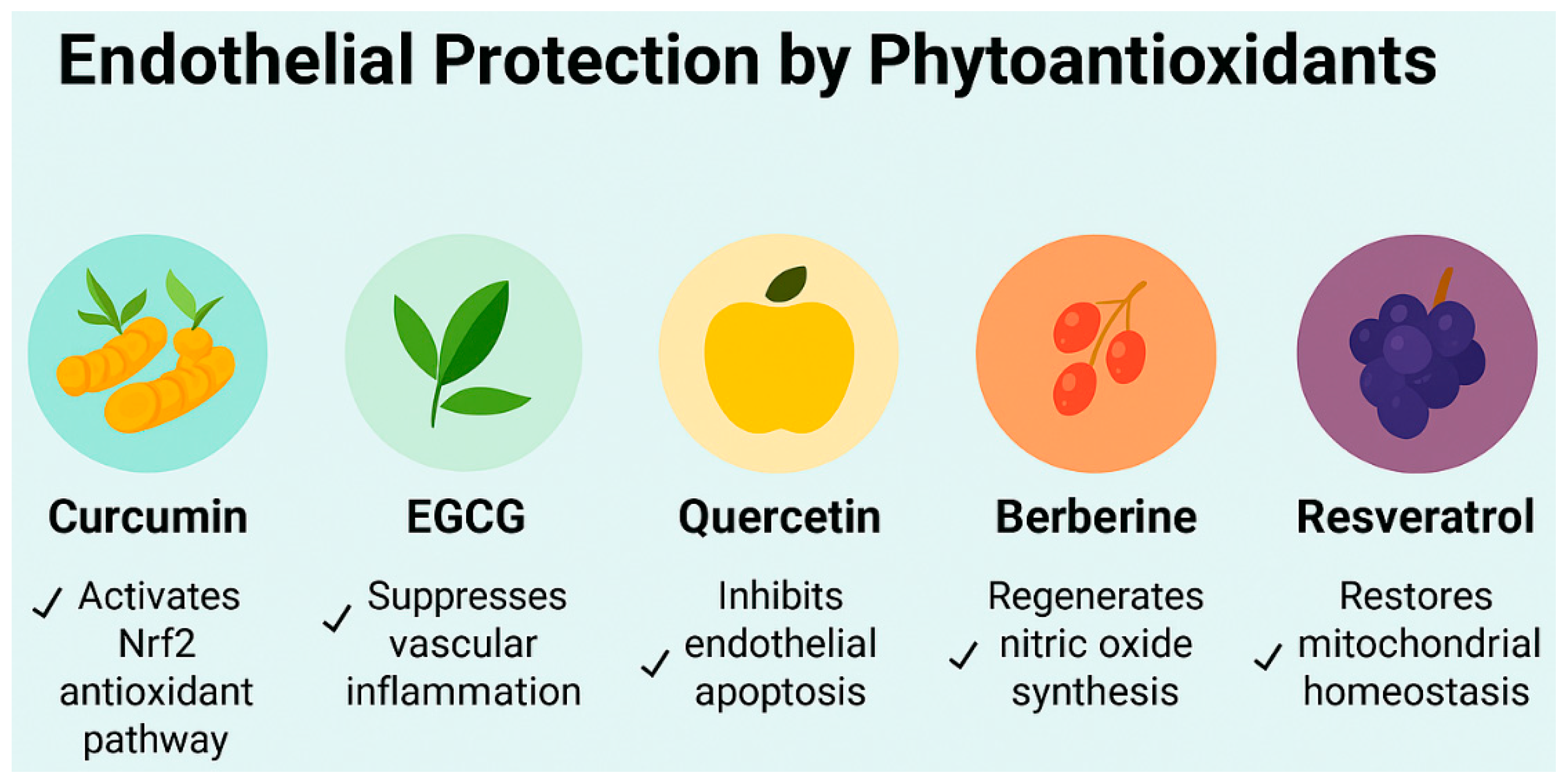
3.1. Curcumin: Mechanistic, Preclinical, and Clinical Evidence
3.2. Epigallocatechin Gallate: Mechanistic, Preclinical, and Clinical Evidence
3.3. Quercetin: Mechanistic, Preclinical, and Clinical Evidence
3.4. Berberine: Mechanistic, Preclinical, and Clinical Evidence
3.5. Resveratrol: Mechanistic, Preclinical, and Clinical Evidence
| Class | Mechanisms | Dosage & Duration | Reference | |
|---|---|---|---|---|
| Curcumin | Non-flavonoid polyphenol | NF-κB inhibition, AMPK activation, gut barrier modulation | 500–1000 mg/day, 8-12 weeks | [33,34,35,36,37,38,39,40,41,42] |
| EGCG | Flavonoid | Antioxidant, anti-inflammatory, microbiota–gut–brain axis modulation | 300–800 mg/day, 8–12 weeks | [43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
| Quercetin | Flavonoid | Nrf2 activation, endothelial protection, metabolic regulation | 300–800 mg/day, 8–12 weeks | [57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Berberine | Alkaloid | FXR/TGR5 modulation, insulin sensitization, bile acid signaling | 1000 mg twice daily, 12 weeks | [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] |
| Resveratrol | Non-flavonoid polyphenol | SIRT1 activation, mitochondrial biogenesis, anti-inflammatory and vascular effects | 150–500 mg/day, 8–12 weeks | [16,25,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] |
4. Translational Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sejbuk, M.; Siebieszuk, A.; Witkowska, A.M. The role of gut microbiome in sleep quality and health: Dietary strategies for microbiota support. Nutrients 2024, 16, 2259. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut microbiota and cardiovascular disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Circadian rhythms: A regulator of gastrointestinal health and dysfunction. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Chassaing, B.; Langella, P. Exploring the interaction and impact of probiotic and commensal bacteria on vitamins, minerals and short chain fatty acids metabolism. Microb. Cell Fact. 2024, 23, 172. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A.; Atkin, S.L.; Johnston, T.P.; Jamialahmadi, T.; et al. Curcumin and endothelial function: A systematic review. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Alam, M.; Gulzar, M.; Akhtar, M.S.; Rashid, S.; Zulfareen; Tanuja; Shamsi, A.; Hassan, M.I. Epigallocatechin-3-gallate therapeutic potential in human diseases: Molecular mechanisms and clinical studies. Mol. Biomed. 2024, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Wang, Y.; Tu, S. Potential implications of quercetin in autoimmune diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Phytother. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a promising nutraceutical: Implications in gut microbiota modulation, inflammatory disorders, and colorectal cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.W.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor δ. Pharmacol. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef]
- Sung, M.M.; Dyck, J.R. Therapeutic potential of resveratrol in heart failure. Ann. N. Y. Acad. Sci. 2015, 1348, 123–131. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Ruiz-Ros, J.A.; María, T.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory biomarkers and improves cardiovascular health. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, Z.; Zhang, D.X.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host–gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Nutr. Rev. 2014, 72, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Zhao, X.; Shang, C.; Xiang, M.; Li, L.; Cui, X. Microbiota-derived short-chain fatty acids: Implications for cardiovascular and metabolic disease. Front. Cardiovasc. Med. 2022, 9, 900381. [Google Scholar] [CrossRef]
- Kavyani, B.; Ahmadi, S.; Nabizadeh, E.; Abdi, M. Anti-oxidative activity of probiotics; focused on cardiovascular disease, cancer, aging, and obesity. Microb. Pathog. 2024, 196, 107001. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Wang, Q.; Zhou, H. Gut-derived endotoxemia and TMAO in coronary endothelial dysfunction: Role of microbial metabolites. J. Clin. Med. 2022, 11, 2987. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, L.; Zhang, X.; Chen, Y.; Wang, H.; Liu, Q.; Lu, P.; Wang, J.; Liu, Y.; Gao, J.; et al. Lactobacillus ameliorates myocardial ischemia–reperfusion injury by attenuating apoptosis, inflammation, oxidative stress, and ferroptosis. BMC Med. 2025, 23, 377. [Google Scholar] [CrossRef]
- Lopes, M.R.; Direito, R.; Guiguer, E.L.; Strozze Catharin, V.C.; Zutin, T.L.M.; Rubira, C.J.; Sloan, K.P.; Sloan, L.A.; Yanaguizawa Junior, J.L.; Laurindo, L.F.; et al. Bridging the gut microbiota and the brain, kidney, and cardiovascular health: The role of probiotics. Probiotics Antimicrob. Proteins 2025, 17, e10680. [Google Scholar] [CrossRef]
- Andersson, K.; Nilsson, A.; Johansson, M.; Bergström, J. Lactobacillus plantarum 299v improves endothelial function in metabolic syndrome: A cohort study. Microorganisms 2023, 11, 456. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Chan, C.W.; Chen, Y.T.; Lin, B.F. Renal protective and immunoregulatory effects of Lactobacillus casei strain Shirota in nephropathy-prone mice. Front. Nutr. 2024, 11, 1438327. [Google Scholar] [CrossRef]
- Alam, M.S.; Anwar, M.J.; Maity, M.K. Curcumin modulates eNOS and NF-κB signaling in vascular inflammation. Pharmaceuticals 2024, 17, 1674. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Phytother. Res. 2014, 28, 514–518. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.C.; Ursoniu, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Joshi, P.; Joshi, S.; Semwal, D.K.; Rawat, S.; Bhatt, V.; Nautiyal, V.; Verma, K.; Dwivedi, J.; Sharma, S.; Semwal, R.B.; et al. Role of curcumin in ameliorating hypertension and associated conditions: A mechanistic insight. Mol. Cell. Biochem. 2022, 488, 45–62. [Google Scholar] [CrossRef]
- Feng, J. Role of curcumin in altering gut microbiota for anti-obesity and anti-hyperlipidemic effects. Front. Microbiol. 2025, 16, 1625098. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The modulatory effects of curcumin on the gut microbiota: A potential strategy for disease treatment and health promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Julanon, N.; Chattranukulchai Shantavasinkul, P.; Polruang, N.; Numthavaj, P.; Thakkinstian, A. Umbrella Review of Systematic Reviews and Meta-Analyses of Randomized Controlled Trials Investigating the Effect of Curcumin Supplementation on Lipid Profiles. Nutr. Rev. 2025, 83, 1520–1536. [Google Scholar] [CrossRef]
- Ferreira, M.; Tan, J.; Alavi, S.; Chen, Y.; Kumar, R.; Singh, A.; Patel, V.; Gupta, N.; Wang, L.; Zhang, Y.; et al. Curcumin–phospholipid complex improves lipid profile but not endothelial biomarkers in adults with metabolic syndrome: A longitudinal cohort analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1121–1130. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R. Enhancing the bioavailability and bioactivity of curcumin for disease prevention and treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Chen, Y.; Shen, H.; Chen, L.; Ding, L.; Tang, Q.; Yang, Z.; Chen, W.; Shen, Z. Precision cardiac targeting: Empowering curcumin therapy through smart exosome-mediated drug delivery. Regen. Biomater. 2024, 11, rbad108. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative delivery systems for curcumin: Exploring nanosized and conventional formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ellis, K.; Scipione, C.A.; Fish, J.E.; Howe, K.L. Epigallocatechin gallate (EGCG) modulates senescent endothelial cell–monocyte communication in age-related vascular inflammation. Front. Cardiovasc. Med. 2025, 11, 1506360. [Google Scholar] [CrossRef]
- Das, M.; Yagnik, U.; Raninga, I. EGCG improves endothelial biomarkers in metabolic syndrome. Front. Endocrinol. 2025, 16, 1655875. [Google Scholar] [CrossRef]
- Rezaei, M.; Akhavan, N.; Fathi, F.; Alavi, S.M.; Fadaii, M.J.; Askarpour, M. Effect of green tea supplementation on blood pressure in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Blood Press. 2025, 34, e2517122. [Google Scholar] [CrossRef]
- Yan, R.; Cao, Y. EGCG improves short-chain fatty acid levels and endothelial function in adults with poor sleep quality. Biomedicines 2025, 13, 206. [Google Scholar] [CrossRef]
- Margareto, J. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Sun, Y. EGCG suppresses TLR4 signaling and enhances Nrf2 antioxidant response. Mol. Nutr. Food Res. 2025, 69, e2300123. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Wu, S.; Dong, R.; Xie, Y.; Chen, W.; Liu, W.; Weng, Y. CO-loaded hemoglobin/EGCG nanoparticles functional coatings for inflammation modulation of vascular implants. Regen. Biomater. 2025, 12, rbae148. [Google Scholar] [CrossRef]
- Patel, P.; Garala, K.; Singh, S.; Prajapati, B.G.; Chittasupho, C. Lipid-based nanoparticles in delivering bioactive compounds for improving therapeutic efficacy. Pharmaceuticals 2025, 17, 329. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Alharbi, H.O.; Alshebremi, M.; Babiker, A.Y.; Rahmani, A.H. Quercetin as a modulator of endothelial nitric oxide and oxidative stress pathways. Biomolecules 2025, 15, 151. [Google Scholar] [CrossRef]
- Mi, W.; Hu, Z.; Xu, L.; Bian, X.; Lian, W.; Yin, S.; Zhao, S.; Gao, W.; Guo, C.; Shi, T. Quercetin positively affects gene expression profiles and metabolic pathways of antibiotic-treated mouse gut microbiota. Front. Microbiol. 2022, 13, 983358. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Huang, Y.; Zhang, X.; Yang, T.; Zhan, K.; Zhao, G. Quercetin alleviates lipopolyssacharide-induced oxidative stress and inflammatory responses via regulation of the TLR4–NF-κB signaling pathway in bovine rumen epithelial cells. Toxins 2023, 15, 512. [Google Scholar] [CrossRef]
- Seong, H.J.; Baek, Y.; Lee, S.; Jin, H.J. Gut microbiome and metabolic pathways linked to sleep quality. Front. Microbiol. 2024, 15, 1418773. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Y.; Zhang, Y.; Xie, J.; Guo, Q.; Yang, H.; Yang, Y.; Chen, J.; Su, L. Quercetin ameliorates obesity and inflammation via microbial metabolite indole-3-propionic acid in high-fat diet-induced obese mice. Front. Nutr. 2025, 12, 1574792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lv, H.; Ainiwan, M.; Yesitayi, G.; Abudesimu, A.; Siti, D.; Aizitiaili, A.; Ma, X. Untargeted metabolomics identifies indole-3-propionic acid to relieve Ang II-induced endothelial dysfunction in aortic dissection. Mol. Cell. Biochem. 2024, 479, 1767–1786. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, W.; Zhang, P.; Qu, Y.; Zhong, H.; Zhou, L.; Zhou, W.; Yang, W.; Xu, H.; Zhao, X.; et al. The gut microbiota–bile acid–TGR5 axis orchestrates platelet activation and atherothrombosis. Nat. Cardiovasc. Res. 2025, 4, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Kerby, R.L.; Aquino-Martinez, R.; Evered, A.H.; Cross, T.-W.L.; Everhart, J.; Ulland, T.K.; Kay, C.D.; Bolling, B.W.; Bäckhed, F.; et al. Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis. NPJ Biofilms Microbiomes 2025, 11, 12. [Google Scholar] [CrossRef]
- Kim, W.G.; Kim, H.I.; Kwon, E.K.; Han, M.J.; Kim, D.H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct. 2018, 9, 4255–4265. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin reprograms immunometabolism of macrophages via the SIRT1/PGC-1α signaling pathway to ameliorate lipopolysaccharide-induced oxidative damage. Int. J. Mol. Sci. 2023, 24, 5542. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Su, L.; Zeng, Y.; Li, G.; Chen, J.; Chen, X. Quercetin improves high-fat diet-induced obesity by modulating gut microbiota and metabolites in C57BL/6J mice. Phytother Res. 2022, 36, 4558. [Google Scholar] [CrossRef]
- Shi, M.; Sun, L.; Wei, J.; Shen, Y.; Wang, J.; Zhang, P.; Yang, X.; Ding, Y.; Yin, W.; Lu, X.; et al. Quercetin alleviates endothelial dysfunction in preeclampsia by inhibiting ferroptosis and inflammation through EGFR binding. Commun. Biol. 2025, 8, 547. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Menni, C.; Berry, S.E.; Valdes, A.M.; Spector, T.D.; Segata, N. Cardiometabolic health, diet and the gut microbiome: A meta-omics perspective. Nat. Med. 2023, 29, 1174–1182. [Google Scholar] [CrossRef]
- Zieniuk, B.; Pawełkowicz, M. Berberine as a Bioactive Alkaloid: Multi-Omics Perspectives on Its Role in Obesity Management. Metabolites 2025, 15, 467. [Google Scholar] [CrossRef]
- Chang, J.; Sun, C.; Wang, M.; Li, W.; Jia, Y.; Zhang, J.; Qiu, F. Berberine inhibits phagocytosis through the TLR4-PI3K-CDC42 pathway. Acta Mater. Medica 2025, 4, 280. [Google Scholar] [CrossRef]
- Dong, C.; Yu, J.; Yang, Y.; Zhang, F.; Su, W.; Fan, Q.; Wu, C.; Wu, S. Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed. Pharmacother. 2021, 139, 111595. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ji, L.; Wang, Y.; Zhang, Y.; Wang, H.; Wang, J.; Zhu, Q.; Xie, M.; Ou, W.; Liu, J.; et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption. Cell Metab. 2024, 36, 1998–2014.e15. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Hu, J.; Deng, Y.; Zou, J.; Ding, W.; Peng, Q.; Duan, R.; Sun, J.; Zhu, J. Berberine mediates the production of butyrate to ameliorate cerebral ischemia via the gut microbiota in mice. Nutrients 2023, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, B.; Dong, X.; Sun, J.; Miao, Z.; Pan, L. Gut microbiota-derived butyrate prevents aortic dissection via GPR41. Acta Pharmacol. Sin. 2025, 46, 1123–1135. [Google Scholar] [CrossRef]
- Ma, S.R.; Tong, Q.; Lin, Y.; Pan, L.B.; Fu, J.; Peng, R.; Zhang, X.-F.; Zhao, Z.X.; Li, Y.; Yu, J.B.; et al. Berberine treats atherosclerosis via a vitamin-like effect down-regulating choline–TMA–TMAO production pathway in gut microbiota. Signal Transduct. Target Ther. 2022, 7, 207. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.-J.; Park, W. Berberine modulates hyper-inflammation in mouse macrophages stimulated with polyinosinic-polycytidylic acid via calcium–CHOP/STAT pathway. Sci. Rep. 2021, 11, 11234. [Google Scholar] [CrossRef]
- Zhao, G.-L.; Yu, L.-M.; Gao, W.-L.; Duan, W.-X.; Jiang, B.; Liu, X.-D.; Zhang, B.; Liu, Z.-H.; Zhai, M.-E.; Jin, Z.-X.; et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 2016, 37, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, R.; Xu, S.; Zhou, X.-Y.; Cai, K.; Chen, Y.-L.; Zhou, Z.-Y.; Sun, X.; Shi, Y.; Wang, F.; et al. NOTCH1 mitochondria localization during heart development promotes mitochondrial metabolism and the endothelial-to-mesenchymal transition in mice. Nat. Commun. 2024, 15, 7074. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, C.; Jiang, Z.; Yang, Y.; Zhang, Y.; Yang, M.; Zhang, X.; Du, Y.; Zhang, J.; Wang, L.; et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, B.; Meng, X.; Yao, S.; Jin, L.; Yang, J.; Wang, J.; Zhang, H.; Zhang, Z.; Cai, D.; et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci. Rep. 2016, 6, 20848. [Google Scholar] [CrossRef]
- He, R.; Liu, B.; Geng, B.; Li, N.; Geng, Q. The role of HDAC3 and its inhibitors in regulation of oxidative stress and chronic diseases. Cell Death Discov. 2023, 9, 1399. [Google Scholar] [CrossRef]
- Cole, L.K.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Berberine elevates cardiolipin in heart of offspring from mouse dams with high-fat diet-induced gestational diabetes mellitus. Sci. Rep. 2021, 11, 95353. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci Rep. 2021, 11, 87759. [Google Scholar] [CrossRef]
- Zhao, J.V.; Yeung, W.F.; Chan, Y.H.; Vackova, D.; Leung, J.Y.Y.; Ip, D.K.M.; Zhao, J.; Ho, W.K.; Tse, H.F.; Schooling, C.M. Effect of berberine on cardiovascular disease risk factors: A mechanistic randomized controlled trial. Nutrients 2021, 13, 2550. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, H.; Nie, R.; Zhang, X.; Zhang, H.; Nian, X.; Liu, Y.; Chen, J.; Wang, Q.; Li, M. Berberine as a multi-target therapeutic agent for obesity: From pharmacological mechanisms to clinical evidence. Eur. J. Med. Res. 2025, 30, 477. [Google Scholar] [CrossRef]
- Godos, J.; Romano, G.L.; Gozzo, L.; Laudani, S.; Paladino, N.; Dominguez Azpíroz, I.; Martínez López, N.M.; Giampieri, F.; Quiles, J.L.; Battino, M.; et al. Resveratrol and vascular health: Evidence from clinical studies and mechanisms of actions related to its metabolites produced by gut microbiota. Front. Pharmacol. 2024, 15, 1368949. [Google Scholar] [CrossRef]
- Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and gut microbiota synergy: Preventive and therapeutic effects. Int. J. Mol. Sci. 2024, 24, 17573. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Resveratrol and the interaction between gut microbiota and arterial remodelling. Nutrients 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Förstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind placebo-controlled one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Wang, F.; Zhang, J. Resveratrol improves mitochondrial biogenesis by activating SIRT1/PGC-1α signaling in SAP. Sci. Rep. 2024, 14, 26216. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Kasprzak, P.; Kondracka, A.; Oniszczuk, T.; Rusinek, A.; Oniszczuk, A. Does resveratrol improve metabolic dysfunction-associated steatotic liver disease (MASLD)? Int. J. Mol. Sci. 2024, 25, 3746. [Google Scholar] [CrossRef]
- Fernandez-Quintela, A.; Macarulla, M.T.; Gómez-Zorita, S.; González, M.; Milton-Laskibar, I.; Portillo, M.P. Relationship between changes in microbiota induced by resveratrol and its anti-diabetic effect on type 2 diabetes. Front. Nutr. 2023, 9, 1084702. [Google Scholar] [CrossRef]
- Wu, W.; Meng, T.; Jin, F.; Li, J.; Huang, J.; Guo, Z.; Yu, M.; Zhou, Y. Effects of resveratrol on postmenopausal women: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1588284. [Google Scholar] [CrossRef]
- Damay, V.A.; Ivan, I. Resveratrol as an anti-inflammatory agent in coronary artery disease: A systematic review, meta-analysis and meta-regression. Chin. J. Integr. Med. 2024, 30, 927–937. [Google Scholar] [CrossRef]
- Bullón-Vela, V.; Abete, I.; Zulet, M.A.; Xu, Y.; Martínez-González, M.A.; Sayón-Orea, C.; Ruiz-Canela, M.; Toledo, E.; Martín Sánchez, V.; Estruch, R.; et al. Urinary resveratrol metabolites output: Differential associations with cardiometabolic markers and liver enzymes in house-dwelling subjects featuring metabolic syndrome. Molecules 2020, 25, 4340. [Google Scholar] [CrossRef]
- Molani-Gol, R.; Rafraf, M. Effects of resveratrol on anthropometric indices and inflammatory markers: An umbrella meta-analysis. Eur. J. Nutr. 2024, 63, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gong, A.; Zhang, B.; Cheng, H.; Huang, L.; Wu, X.; Zhang, D.; Dai, W.; Li, S.; Xu, H. The chronobiological and neuroprotective mechanisms of resveratrol in improving sleep. Mediators Inflamm. 2025, 2025, 4954030. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O.L. High-dose resveratrol supplementation (1500 mg/day for 4 weeks) in obese men: A randomized, placebo-controlled trial of insulin sensitivity, blood pressure, and endothelial function. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jia, Y.; Ren, F. Multidimensional biological activities of resveratrol and its prospects and challenges in the health field. Front. Nutr. 2024, 11, 1408651. [Google Scholar] [CrossRef]
- Borshchev, Y.Y.; Burovenko, I.Y.; Karaseva, A.B.; Minasian, S.M.; Protsak, E.S.; Borshchev, V.Y.; Semenova, N.Y.; Borshcheva, O.V.; Suvorov, A.N.; Galagudza, M.M. Probiotic therapy with Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis results in infarct size limitation in rats with obesity and chemically induced colitis. Microorganisms 2022, 10, 2293. [Google Scholar] [CrossRef]
- Borshchev, Y.Y.; Protsak, E.S.; Burovenko, I.Y.; Semenova, N.Y.; Zubkov, I.G.; Galagudza, M.M. Effect of probiotic therapy on hemodynamic response associated with systemic inflammatory reaction and antibiotic-induced dysbiosis in chronic experiments in rats. Bull. Exp. Biol. Med. 2022, 172, 676–680. [Google Scholar] [CrossRef]
- Hu, J.; Mesnage, R.; Tuohy, K.; Heiss, C.; Rodriguez-Mateos, A. (Poly)phenol-related gut metabotypes and human health: An update. Food Funct. 2024, 15, 2814–2835. [Google Scholar] [CrossRef]
- Bah, Y.R.; Baba, K.; Mustafa, D.N.A.B.; Watanabe, S.; Takeda, A.K.; Yamashita, T.; Kasahara, K. Bacteroides and Prevotella enriched gut microbial clusters associate with metabolic risks. Gut Pathog. 2025, 17, 55. [Google Scholar] [CrossRef]
- Ji, L.; Ma, J.; Ma, Y.; Cheng, Z.; Gan, S.; Yuan, G.; Liu, D.; Li, S.; Liu, Y.; Xue, X.; et al. Berberine ursodeoxycholate for the treatment of type 2 diabetes: A randomized clinical trial. JAMA Netw. Open 2025, 8, e2462185. [Google Scholar] [CrossRef]
- Li, X.; Zheng, P.; Cao, W.; Cao, Y.; She, X.; Yang, H.; Ma, K.; Wu, F.; Gao, X.; Fu, Y.; et al. Lactobacillus rhamnosus GG ameliorates noise induced cognitive deficits and systemic inflammation in rats by modulating the gut brain axis. Front. Cell Infect. Microbiol. 2023, 13, 1067367. [Google Scholar] [CrossRef]
- Srivastava, R.; Gupta, M.K. Gut bacteria derived metabolites and their implications in mental health and neurological diseases. World J. Microbiol. Biotechnol. 2025, 41, 423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-N.; Chu, Y. Probiotics and Phytoantioxidants Target Coronary Endothelial Dysfunction in Irregular Sleep- and Obesity-Associated Cardiometabolic Syndrome. Life 2025, 15, 1740. https://doi.org/10.3390/life15111740
Tseng C-N, Chu Y. Probiotics and Phytoantioxidants Target Coronary Endothelial Dysfunction in Irregular Sleep- and Obesity-Associated Cardiometabolic Syndrome. Life. 2025; 15(11):1740. https://doi.org/10.3390/life15111740
Chicago/Turabian StyleTseng, Chi-Nan, and Yen Chu. 2025. "Probiotics and Phytoantioxidants Target Coronary Endothelial Dysfunction in Irregular Sleep- and Obesity-Associated Cardiometabolic Syndrome" Life 15, no. 11: 1740. https://doi.org/10.3390/life15111740
APA StyleTseng, C.-N., & Chu, Y. (2025). Probiotics and Phytoantioxidants Target Coronary Endothelial Dysfunction in Irregular Sleep- and Obesity-Associated Cardiometabolic Syndrome. Life, 15(11), 1740. https://doi.org/10.3390/life15111740






