Targeting GM-CSF in Rheumatoid Arthritis: Advances in Cytokine-Directed Immunotherapy and Clinical Implications
Abstract
1. Introduction
2. Fundamental Aspects of RA
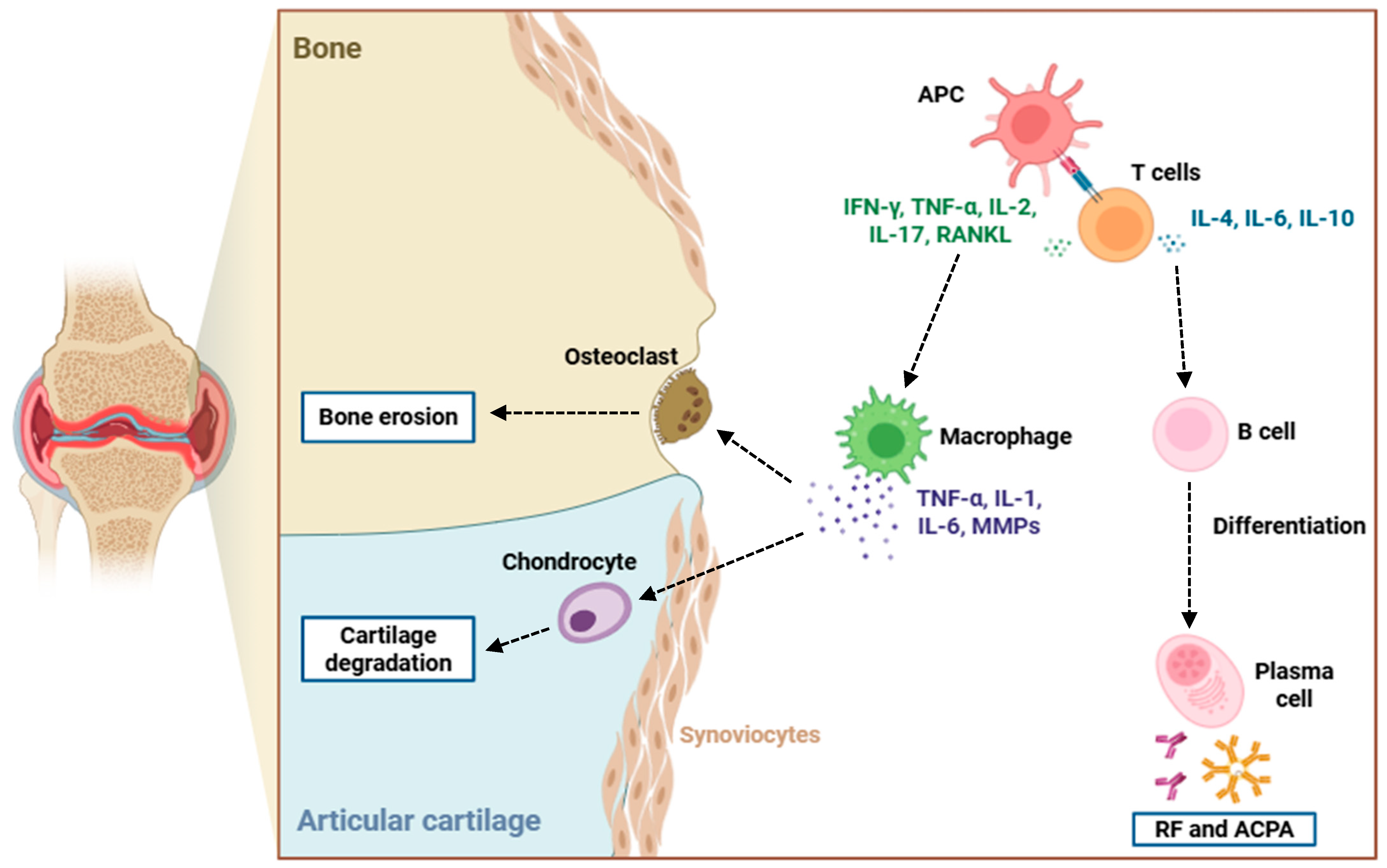
3. Role of GM-CSF in RA
3.1. Molecular Signaling Mechanisms of GM-CSF
3.2. Impact of GM-CSF on Immune Effector Cell Differentiation and Activation

4. Therapeutic Targeting of GM-CSF
4.1. Overview of Monoclonal Antibody Strategies: GM-CSF Neutralization vs. Receptor Blockade
4.2. Neutralization of GM-CSF Pathway in RA: Preclinical Efficacy of Monoclonal Antibodies
4.3. Monoclonal Antibodies Against GM-CSF/GM-CSFR in RA: Clinical Trial Insights
5. Next-Generation Strategies for GM-CSF-Targeted Therapies in RA
5.1. Long-Term Safety and Immunovigilance
5.2. Precision Medicine and Predictive Biomarkers
5.3. Combination and Sequencing Strategies
5.4. Extra-Articular Implications and Comorbidity Management
5.5. Biomarker-Driven Patient Selection
5.6. Pediatric and Global Health Applications
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACPA | Anti-citrullinated protein antibody |
| ACR | American College of Rheumatology |
| ACR20 | American College of Rheumatology 20% improvement criteria |
| ACR50 | American College of Rheumatology 50% improvement criteria |
| ACR70 | American College of Rheumatology 70% improvement criteria |
| Akt | Protein kinase B |
| anti-CCP | Anti-cyclic citrullinated peptide antibody |
| AP-1 | Activator protein 1 |
| APC | Antigen-presenting cell |
| bDMARD | Biologic disease-modifying antirheumatic drug |
| BCL2 | B-cell lymphoma 2 |
| BCL-XL | B-cell lymphoma-extra large |
| C3a | Complement component 3a |
| C5a | Complement component 5a |
| CCL17 | Chemokine (C-C motif) ligand 17 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCL22 | Chemokine (C-C motif) ligand 22 |
| CCL3 | Chemokine (C-C motif) ligand 3 |
| CCL5 | C-C motif chemokine ligand 5 |
| CCR2 | C-C chemokine receptor type 2 |
| CD4 | Cluster of differentiation 4 |
| CD40 | Cluster of differentiation 40 |
| CD80 | Cluster of differentiation 80 |
| CD86 | Cluster of differentiation 86 |
| CDAI | Clinical disease activity index |
| c-Fos | Cellular FOS proto-oncogene |
| CHD | Cytokine receptor homology domain |
| CIA | Collagen-induced arthritis |
| CRP | C-reactive protein |
| CTLA4 | Cytotoxic T-lymphocyte antigen 4 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCL8 | Chemokine (C-X-C motif) ligand 8 |
| CX3CR1 | CX3C chemokine receptor 1 |
| DAS28 | Disease activity score in 28 joints |
| DAS28-CRP | Disease activity score in 28 joints with C-reactive protein |
| DCE-MRI | Dynamic contrast-enhanced MRI |
| DMARD | Disease-modifying antirheumatic drug |
| Elk-1 | ETS-like protein 1 |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ESR | Erythrocyte sedimentation rate |
| EULAR | European League Against Rheumatism |
| FLS | Fibroblast-like synoviocyte |
| GLS | Glutaminase |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GM-CSFR | Granulocyte-macrophage colony-stimulating factor receptor |
| GM-CSFRα | Granulocyte-macrophage colony-stimulating factor receptor alpha subunit |
| GM-CSFRβc | Granulocyte-macrophage colony-stimulating factor receptor β common subunit |
| GM-DM | GM-CSF-differentiated macrophage |
| HAQ-DI | Health assessment questionnaire disability index |
| HK | Hexokinase 2 |
| HLA-DRB1 | Human leukocyte antigen-DR beta 1 |
| IFN-γ | Interferon gamma |
| IgG1 | Immunoglobulin G1 |
| IKK | IκB Kinase |
| IL-12 | Interleukin 12 |
| IL-17 | Interleukin 17 |
| IL-17A | Interleukin 17A |
| IL-17F | Interleukin 17F |
| IL-1β | Interleukin 1 beta |
| IL-22 | Interleukin 22 |
| IL-23 | Interleukin 23 |
| IL-3 | Interleukin 3 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| iNOS | Inducible nitric oxide synthase |
| IRF4 | Interferon regulatory factor 4 |
| IRF5 | Interferon regulatory factor 5 |
| IκBα | Inhibitor of kappa B alpha |
| i.v. | Intravenous injection |
| JAK2 | Janus kinase |
| JAK2 | Janus kinase 2 |
| JIA | Juvenile idiopathic arthritis |
| Ly-6Chigh | Lymphocyte antigen 6C high-expressing monocytes |
| M1 | Classically activated macrophage phenotype |
| mAb | Monoclonal antibody |
| MAPK | Mitogen-activated protein kinase |
| MAPK/ERK | Mitogen-activated protein kinase/extracellular signal-regulated kinase |
| MCL1 | Myeloid cell leukemia 1 |
| M-CSF | Macrophage colony-stimulating factor |
| MEK1/2 | Mitogen-activated protein kinase kinase 1/2 |
| MHC | Major histocompatibility complex |
| MHC-II | Major histocompatibility complex type II |
| MMP | Matrix metalloproteinase |
| MMP-1 | Matrix metalloproteinase 1 |
| MMP-13 | Matrix metalloproteinase 13 |
| MMP-3 | Matrix metalloproteinase 3 |
| MMP-9 | Matrix metalloproteinase 9 |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger ribonucleic acid |
| mTOR | Mechanistic target of rapamycin |
| mTORC2 | Mechanistic target of rapamycin complex 2 |
| NET | Neutrophil extracellular trap |
| NFATc1 | Nuclear factor of activated T-cells, cytoplasmic 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| OMERACT | Outcome measures in rheumatology clinical trials |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PET | Positron emission tomography |
| PFK1 | Phosphofructokinase 1 |
| PI3K | Phosphoinositide 3-kinase |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PK | Pharmacokinetics |
| PTPN22 | Protein tyrosine phosphatase non-receptor type 22 |
| RA | Rheumatoid arthritis |
| RA-ILD | Rheumatoid arthritis-associated interstitial lung disease |
| Raf-1 | Rapidly accelerated fibrosarcoma 1 |
| RAMRIS | Rheumatoid arthritis magnetic resonance imaging score |
| RAMRIQ | Rheumatoid arthritis magnetic resonance imaging quantitative |
| RANK | Receptor activator of nuclear factor κB |
| RANKL | Receptor activator of nuclear factor κB ligand |
| Ras | Rat sarcoma |
| RF | Rheumatoid factor |
| ROS | Reactive oxygen species |
| s.c. | Subcutaneous injection |
| SH2 | Src homology 2 |
| sJIA | Systemic juvenile idiopathic arthritis |
| SOCS3 | Suppressor of cytokine signaling 3 |
| STAT | Signal transducer and activator of transcription |
| STAT4 | Signal transducer and activator of transcription 4 |
| STAT5 | Signal transducer and activator of transcription 5 |
| Th | T helper lymphocyte |
| Th1 | T helper 1 cell |
| Th17 | T helper 17 cell |
| TNF-α | Tumor necrosis factor alpha |
| Treg | Regulatory T cell |
References
- Gao, Y.; Zhang, Y.; Liu, X. Rheumatoid arthritis: Pathogenesis and therapeutic advances. MedComm 2024, 5, e509. [Google Scholar] [CrossRef]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef]
- Edilova, M.I.; Akram, A.; Abdul-Sater, A.A. Innate immunity drives pathogenesis of rheumatoid arthritis. Biomed. J. 2021, 44, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H. Adaptive immunity in the joint of rheumatoid arthritis. Immunol. Med. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Brzustewicz, E.; Bryl, E. The role of cytokines in the pathogenesis of rheumatoid arthritis—Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 2015, 76, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Fujimoto, S.; Niiro, H. Pathogenic Role of Cytokines in Rheumatoid Arthritis. J. Clin. Med. 2025, 14, 6409. [Google Scholar] [CrossRef]
- Fuentelsaz-Romero, S.; Cuervo, A.; Estrada-Capetillo, L.; Celis, R.; García-Campos, R.; Ramírez, J.; Sastre, S.; Samaniego, R.; Puig-Kröger, A.; Cañete, J.D. GM-CSF Expression and Macrophage Polarization in Joints of Undifferentiated Arthritis Patients Evolving to Rheumatoid Arthritis or Psoriatic Arthritis. Front. Immunol. 2021, 11, 613975. [Google Scholar] [CrossRef]
- Su, J.; Hu, W.; Ding, Y.; Zhang, P.; Li, T.; Liu, S.; Xing, L. Serum GM-CSF level is a predictor of treatment response to tocilizumab in rheumatoid arthritis patients: A prospective observational cohort study. Arthritis Res. Ther. 2024, 26, 130. [Google Scholar] [CrossRef]
- Shiomi, A.; Usui, T.; Mimori, T. GM-CSF as a therapeutic target in autoimmune diseases. Inflamm. Regen. 2016, 36, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.C.; Achuthan, A.A.; Hamilton, J.A. GM-CSF: A Promising Target in Inflammation and Autoimmunity. Immunotargets Ther. 2020, 9, 225–240. [Google Scholar] [CrossRef]
- Woodcock, J.M.; McClure, B.J.; Stomski, F.C.; Elliott, M.J.; Bagley, C.J.; Lopez, A.F. The human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor exists as a preformed receptor complex that can be activated by GM-CSF, interleukin-3, or interleukin-5. Blood 1997, 90, 3005–3017. [Google Scholar] [CrossRef]
- Hansen, G.; Hercus, T.R.; McClure, B.J.; Stomski, F.C.; Dottore, M.; Powell, J.; Ramshaw, H.; Woodcock, J.M.; Xu, Y.; Guthridge, M.; et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 2008, 134, 496–507. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Nagenborg, J.; Jin, H.; Ruder, A.V.; Temmerman, L.; Mees, B.; Schalkwijk, C.; Müller-Klieser, D.; Berg, T.; Goossens, P.; Donners, M.M.P.C.; et al. GM-CSF-activated STAT5A regulates macrophage functions and inflammation in atherosclerosis. Front. Immunol. 2023, 14, 1165306. [Google Scholar] [CrossRef]
- Larbi, A.; Douziech, N.; Fortin, C.; Linteau, A.; Dupuis, G.; Fulop, T., Jr. The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun. Ageing 2005, 2, 6. [Google Scholar] [CrossRef]
- Han, N.-R.; Park, H.-J.; Moon, P.-D. Resveratrol Downregulates Granulocyte-Macrophage Colony-Stimulating Factor-Induced Oncostatin M Production through Blocking of PI3K/Akt/NF-κB Signal Cascade in Neutrophil-like Differentiated HL-60 Cells. Curr. Issues Mol. Biol. 2022, 44, 541–549. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, M.; Zhao, Y.; Xu, H.; Feng, F.; Bai, Z.; Zhang, K.; Fu, L.; Wang, F.; Cheng, Y.; et al. GM-CSF potentiates macrophages to retain an inflammatory feature from their circulating monocyte precursors in rheumatoid arthritis. J. Transl. Med. 2025, 23, 883. [Google Scholar] [CrossRef]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF in inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef]
- Domańska-Poboża, J.; Wisłowska, M. Evolving strategies in the treatment of rheumatoid arthritis: A historical perspective. Reumatologia 2025, 63, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; McDermott, G.C.; Juge, P.A.; Chang, S.H.; Vanni, K.M.; Qian, G.; Bade, K.J.; Mueller, K.T.; Kowalski, E.N.; Saavedra, A.A.; et al. Disease-modifying antirheumatic drugs and risk of incident interstitial lung disease among patients with rheumatoid arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2024, 69, 152561. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Crotti, C.; Agape, E.; Becciolini, A.; Biggioggero, M.; Favalli, E.G. Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects. Drugs 2019, 79, 1741–1755. [Google Scholar] [CrossRef]
- Bykerk, V.P. The efficacy and safety of targeting GM-CSF in arthritis. Lancet Rheumatol. 2020, 2, e648–e650. [Google Scholar] [CrossRef]
- Taylor, P.C.; Weinblatt, M.E.; McInnes, I.B.; Atsumi, T.; Strand, V.; Takeuchi, T.; Bracher, M.; Brooks, D.; Davies, J.; Goode, C.; et al. Anti-GM-CSF otilimab versus sarilumab or placebo in patients with rheumatoid arthritis and inadequate response to targeted therapies: A phase III randomised trial (contRAst 3). Ann. Rheum. Dis. 2023, 82, 1527–1537. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Vencovsky, J.; Guo, X.; White, W.I.; Ryan, P.C.; Godwood, A.; Albulescu, M.; et al. A Randomized Phase IIb Study of Mavrilimumab and Golimumab in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 49–59. [Google Scholar] [CrossRef]
- Buckley, C.D.; Simón-Campos, J.A.; Zhdan, V.; Becker, B.; Davy, K.; Fisheleva, E.; Gupta, A.; Hawkes, C.; Inman, D.; Layton, M.; et al. Efficacy, patient-reported outcomes, and safety of the anti-granulocyte macrophage colony-stimulating factor antibody otilimab (GSK3196165) in patients with rheumatoid arthritis: A randomised, phase 2b, dose-ranging study. Lancet Rheumatol. 2020, 2, e677–e688. [Google Scholar] [CrossRef]
- Genovese, M.C.; Buckley, C.D.; Saurigny, D.; Schett, G.; Davy, K.; Gupta, A.; Smith, J.E.; Patel, J.; Tak, P.P. Targeting GM-CSF in rheumatological conditions: Risk of PAP—Authors’ reply. Lancet Rheumatol. 2021, 3, e473–e474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; Liu, S.; Wang, Q.; Wang, Y.; Hou, S.; Wang, J.; Zhang, Y. Global, regional, and national epidemiology of rheumatoid arthritis among people aged 20–54 years from 1990 to 2021. Sci. Rep. 2025, 15, 10736. [Google Scholar] [CrossRef] [PubMed]
- Maranini, B.; Bortoluzzi, A.; Silvagni, E.; Govoni, M. Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 499. [Google Scholar] [CrossRef]
- Scott, D.L.; Steer, S. The course of established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007, 21, 943–967. [Google Scholar] [CrossRef]
- Rawla, P. Cardiac and vascular complications in rheumatoid arthritis. Reumatologia 2019, 57, 27–36. [Google Scholar] [CrossRef]
- Wu, D.; Luo, Y.; Li, T.; Zhao, X.; Lv, T.; Fang, G.; Ou, P.; Li, H.; Luo, X.; Huang, A.; et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front. Immunol. 2022, 13, 1051082. [Google Scholar] [CrossRef]
- Dedmon, L.E. The genetics of rheumatoid arthritis. Rheumatology 2020, 59, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, T.; Olesińska, M.; Paradowska-Gorycka, A. Current Understanding of an Emerging Role of HLA-DRB1 Gene in Rheumatoid Arthritis–From Research to Clinical Practice. Cells 2020, 9, 1127. [Google Scholar] [CrossRef]
- Mustelin, T.; Bottini, N.; Stanford, S.M. The Contribution of PTPN22 to Rheumatic Disease. Arthritis Rheumatol. 2019, 71, 486–495. [Google Scholar] [CrossRef]
- Bravo-Villagra, K.M.; Muñoz-Valle, J.F.; Baños-Hernández, C.J.; Cerpa-Cruz, S.; Navarro-Zarza, J.E.; Parra-Rojas, I.; Aguilar-Velázquez, J.A.; García-Arellano, S.; López-Quintero, A. STAT4 Gene Variant rs7574865 Is Associated with Rheumatoid Arthritis Activity and Anti-CCP Levels in the Western but Not in the Southern Population of Mexico. Genes 2024, 15, 241. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, S.; Yuan, X.; Shu, Z.; Li, S.; Sun, X.; Xiao, J.; Liu, H. Association between CTLA-4 gene polymorphism and risk of rheumatoid arthritis: A meta-analysis. Aging 2021, 13, 19397–19414. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, L.; Olsson, T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Oduro, P.K.; Sun, T.; Chen, H.; Yi, Y.; Zeng, W.; Wang, Q.; Leng, L.; Yang, L.; et al. The Relationship Between Porphyromonas Gingivalis and Rheumatoid Arthritis: A Meta-Analysis. Front. Cell Infect. Microbiol. 2022, 12, 956417. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Zhang, R.D.; Musonye, H.A.; Zhao, H.Y.; He, Y.S.; Zhao, C.N.; He, T.; Tian, T.; Gao, Z.X.; Fang, Y.; et al. Hormonal and reproductive factors in relation to the risk of rheumatoid arthritis in women: A prospective cohort study with 223 526 participants. RMD Open 2024, 10, e003338. [Google Scholar] [CrossRef]
- Shuai, Z.; Zheng, S.; Wang, K.; Wang, J.; Leung, P.S.C.; Xu, B. Reestablish immune tolerance in rheumatoid arthritis. Front. Immunol. 2022, 13, 1012868. [Google Scholar] [CrossRef]
- Carlé, C.; Degboe, Y.; Ruyssen-Witrand, A.; Arleevskaya, M.I.; Clavel, C.; Renaudineau, Y. Characteristics of the (Auto)Reactive T Cells in Rheumatoid Arthritis According to the Immune Epitope Database. Int. J. Mol. Sci. 2023, 24, 4296. [Google Scholar] [CrossRef]
- van Loosdregt, J.; Rossetti, M.; Spreafico, R.; Moshref, M.; Olmer, M.; Williams, G.W.; Kumar, P.; Copeland, D.; Pischel, K.; Lotz, M.; et al. Increased autophagy in CD4+ T cells of rheumatoid arthritis patients results in T-cell hyperactivation and apoptosis resistance. Eur. J. Immunol. 2016, 46, 2862–2870. [Google Scholar] [CrossRef]
- Luo, P.; Wang, P.; Xu, J.; Hou, W.; Xu, P.; Xu, K.; Liu, L. Immunomodulatory role of T helper cells in rheumatoid arthritis: A comprehensive research review. Bone Jt. Res. 2022, 11, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Kotschenreuther, K.; Deng, S.; Kofler, D.M. Regulatory T cells in rheumatoid arthritis: Functions, development, regulation, and therapeutic potential. Cell. Mol. Life Sci. 2022, 79, 533. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Tsaltskan, V.; Firestein, G.S. Targeting fibroblast-like synoviocytes in rheumatoid arthritis. Curr. Opin. Pharmacol. 2022, 67, 102304. [Google Scholar] [CrossRef]
- Brescia, A.C.; Simonds, M.M.; Sullivan, K.E.; Rose, C.D. Secretion of pro-inflammatory cytokines and chemokines and loss of regulatory signals by fibroblast-like synoviocytes in juvenile idiopathic arthritis. Proteom. Clin. Appl. 2017, 11, 1600088. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2022, 63, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Banda, N.K.; Hyatt, S.; Antonioli, A.H.; White, J.T.; Glogowska, M.; Takahashi, K.; Merkel, T.J.; Stahl, G.L.; Mueller-Ortiz, S.; Wetsel, R.; et al. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J. Immunol. 2012, 188, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Youinou, P.; Mackenzie, L.; Katsikis, P.; Merdrignac, G.; Isenberg, D.A.; Tuaillon, N.; Lamour, A.; Le Goff, P.; Jouquan, J.; Drogou, A.; et al. The relationship between CD5-expressing B lymphocytes and serologic abnormalities in rheumatoid arthritis patients and their relatives. Arthritis Rheum. 1990, 33, 339–348. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Grimstad, K.; Thorarinsdottir, K.; Forslind, K.; Glinatsi, D.; Leu Agelii, M.; Aranburu, A.; Sundell, T.; Jonsson, C.A.; Camponeschi, A.; et al. Correlation of Professional Antigen-Presenting Tbet+CD11c+ B Cells With Bone Destruction in Untreated Rheumatoid Arthritis. Arthritis Rheumatol. 2024, 76, 1263–1277. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Wu, R. Neutrophils in Rheumatoid Arthritis Synovium: Implications on Disease Activity and Inflammation State. J. Inflamm. Res. 2025, 18, 4741–4753. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, K.; Jiang, P.; Zhao, J.; Shan, Y.; Shi, Y.; Zhao, F.; Chang, C.; Li, Y.; Zhou, M.; et al. Macrophage polarization in rheumatoid arthritis: Signaling pathways, metabolic reprogramming, and crosstalk with synovial fibroblasts. Front. Immunol. 2024, 15, 1394108. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Chu, Y.; Xie, J.; Ding, W.; Wang, F. Tissue factor expression in rheumatoid synovium: A potential role in pannus invasion of rheumatoid arthritis. Acta Histochem. 2013, 115, 692–697. [Google Scholar] [CrossRef]
- Tanaka, S. RANKL is a therapeutic target of bone destruction in rheumatoid arthritis. F1000Research 2019, 8, F1000, Faculty Rev-533. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK–RANKL–OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef]
- Rolph, D.; Das, H. Transcriptional Regulation of Osteoclastogenesis: The Emerging Role of KLF2. Front. Immunol. 2020, 11, 937. [Google Scholar] [CrossRef]
- DI Matteo, A.; Emery, P. Rheumatoid arthritis: A review of the key clinical features and ongoing challenges of the disease. Panminerva Med. 2024, 66, 427–442. [Google Scholar] [CrossRef]
- Zimba, O.; Baimukhamedov, C.; Kocyigit, B.F. Late-onset rheumatoid arthritis: Clinical features, diagnostic challenges, and treatment approaches. Rheumatol. Int. 2025, 45, 152. [Google Scholar] [CrossRef]
- Pavlov-Dolijanovic, S.; Bogojevic, M.; Nozica-Radulovic, T.; Radunovic, G.; Mujovic, N. Elderly-Onset Rheumatoid Arthritis: Characteristics and Treatment Options. Medicina 2023, 59, 1878. [Google Scholar] [CrossRef]
- Chmielewski, G.; Majewski, M.S.; Kuna, J.; Mikiewicz, M.; Krajewska-Włodarczyk, M. Fatigue in Inflammatory Joint Diseases. Int. J. Mol. Sci. 2023, 24, 12040. [Google Scholar] [CrossRef]
- Debreova, M.; Culenova, M.; Smolinska, V.; Nicodemou, A.; Csobonyeiova, M.; Danisovic, L. Rheumatoid arthritis: From synovium biology to cell-based therapy. Cytotherapy 2022, 24, 365–375. [Google Scholar] [CrossRef]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D.; Tanasescu, R. Extra-articular Manifestations in Rheumatoid Arthritis. Maedica 2010, 5, 286–291. [Google Scholar]
- Sanghavi, N.; Ingrassia, J.P.; Korem, S.; Ash, J.; Pan, S.; Wasserman, A. Cardiovascular Manifestations in Rheumatoid Arthritis. Cardiol. Rev. 2024, 32, 146–152. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef]
- Aggarwal, R.; Liao, K.; Nair, R.; Ringold, S.; Costenbader, K.H. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009, 61, 1472–1483. [Google Scholar] [CrossRef]
- Kgoebane, K.; Ally, M.M.T.M.; Duim-Beytell, M.C.; Suleman, F.E. The role of imaging in rheumatoid arthritis. SA J. Radiol. 2018, 22, 1316. [Google Scholar] [CrossRef]
- Hercus, T.R.; Thomas, D.; Guthridge, M.A.; Ekert, P.G.; King-Scott, J.; Parker, M.W.; Lopez, A.F. The granulocyte-macrophage colony-stimulating factor receptor: Linking its structure to cell signaling and its role in disease. Blood 2009, 114, 1289–1298. [Google Scholar] [CrossRef]
- Weng, S.; Zhang, D.-E. The GM-CSF Receptor Alpha Chain (CSF2RA) Functions As a Novel Ligand-Independent Tumor Suppressor in t(8;21) AML. Blood 2015, 126, 3589. [Google Scholar] [CrossRef]
- Mirza, S.; Walker, A.; Chen, J.; Murphy, J.M.; Young, I.G. The Ig-like domain of human GM-CSF receptor alpha plays a critical role in cytokine binding and receptor activation. Biochem. J. 2010, 426, 307–317. [Google Scholar] [CrossRef]
- Pundavela, J.; Hall, A.; Dinglasan, S.A.; Choi, K.; Rizvi, T.A.; Trapnell, B.C.; Wu, J.; Ratner, N. Granulocyte-Macrophage Colony Stimulating Factor Receptor Contributes to Plexiform Neurofibroma Initiation. Cancers 2025, 17, 905. [Google Scholar] [CrossRef]
- Carr, P.D.; Gustin, S.E.; Church, A.P.; Murphy, J.M.; Ford, S.C.; Mann, D.A.; Woltring, D.M.; Walker, I.; Ollis, D.L.; Young, I.G. Structure of the complete extracellular domain of the common beta subunit of the human GM-CSF, IL-3, and IL-5 receptors reveals a novel dimer configuration. Cell 2001, 104, 291–300. [Google Scholar] [CrossRef]
- Zsiros, V.; Katz, S.; Doczi, N.; Kiss, A.L. Endocytosis of GM-CSF receptor β is essential for signal transduction regulating mesothelial-macrophage transition. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1450–1462. [Google Scholar] [CrossRef]
- Zhao, Y.; Wagner, F.; Frank, S.J.; Kraft, A.S. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 1995, 270, 13814–13818. [Google Scholar] [CrossRef]
- Liu, R.Y.; Fan, C.; Garcia, R.; Jove, R.; Zuckerman, K.S. Constitutive activation of the JAK2/STAT5 signal transduction pathway correlates with growth factor independence of megakaryocytic leukemic cell lines. Blood 1999, 93, 2369–2379. [Google Scholar] [CrossRef]
- Kimura, A.; Rieger, M.A.; Simone, J.M.; Chen, W.; Wickre, M.C.; Zhu, B.M.; Hoppe, P.S.; O’Shea, J.J.; Schroeder, T.; Hennighausen, L. The transcription factors STAT5A/B regulate GM-CSF-mediated granulopoiesis. Blood 2009, 114, 4721–4728. [Google Scholar] [CrossRef]
- Rumore-Maton, B.; Elf, J.; Belkin, N.; Stutevoss, B.; Seydel, F.; Garrigan, E.; Litherland, S.A. M-CSF and GM-CSF regulation of STAT5 activation and DNA binding in myeloid cell differentiation is disrupted in nonobese diabetic mice. Clin. Dev. Immunol. 2008, 2008, 769795. [Google Scholar] [CrossRef]
- Vázquez Marrero, V.R.; Dresler, M.; Haggadone, M.D.; Lu, A.; Shin, S. GM-CSF engages multiple signaling pathways to enhance pro-inflammatory cytokine responses in human monocytes during Legionella infection. Infect. Immun. 2025, 93, e0056524. [Google Scholar] [CrossRef]
- López-Navarro, B.; Simón-Fuentes, M.; Ríos, I.; Schiaffino, M.T.; Sanchez, A.; Torres-Torresano, M.; Nieto-Valle, A.; Castrejón, I.; Puig-Kröger, A. Macrophage re-programming by JAK inhibitors relies on MAFB. Cell Mol. Life Sci. 2024, 81, 152. [Google Scholar] [CrossRef]
- Boyer, S.; Lee, H.J.; Steele, N.; Zhang, L.; Sajjakulnukit, P.; Andren, A.; Ward, M.H.; Singh, R.; Basrur, V.; Zhang, Y.; et al. Multiomic characterization of pancreatic cancer-associated macrophage polarization reveals deregulated metabolic programs driven by the GM-CSF-PI3K pathway. Elife 2022, 11, e73796. [Google Scholar] [CrossRef]
- Jücker, M.; Feldman, R.A. Identification of a new adapter protein that may link the common beta subunit of the receptor for granulocyte/macrophage colony-stimulating factor, interleukin (IL)-3, and IL-5 to phosphatidylinositol 3-kinase. J. Bol. Chem. 1995, 270, 27817–27822. [Google Scholar] [CrossRef]
- Mafi, S.; Mansoori, B.; Taeb, S.; Sadeghi, H.; Abbasi, R.; Cho, W.C.; Rostamzadeh, D. mTOR-Mediated Regulation of Immune Responses in Cancer and Tumor Microenvironment. Front. Immunol. 2022, 12, 774103. [Google Scholar] [CrossRef]
- de Carvalho Oliveira, V.; Tatsiy, O.; McDonald, P.P. Phosphoinositol 3-kinase-driven NET formation involves different isoforms and signaling partners depending on the stimulus. Front. Immunol. 2023, 14, 1042686. [Google Scholar] [CrossRef]
- Kolonics, A.; Apáti, A.; Jánossy, J.; Brózik, A.; Gáti, R.; Schaefer, A.; Magócsi, M. Activation of Raf/ERK1/2 MAP kinase pathway is involved in GM-CSF-induced proliferation and survival but not in erythropoietin-induced differentiation of TF-1 cells. Cell Signal. 2001, 13, 743–754. [Google Scholar] [CrossRef]
- Schallenberg, M.; Charalambous, P.; Thanos, S. GM-CSF regulates the ERK1/2 pathways and protects injured retinal ganglion cells from induced death. Exp. Eye Res. 2009, 89, 665–677. [Google Scholar] [CrossRef]
- Parajuli, B.; Sonobe, Y.; Kawanokuchi, J.; Doi, Y.; Noda, M.; Takeuchi, H.; Mizuno, T.; Suzumura, A. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J. Neuroinflamm. 2012, 9, 268. [Google Scholar] [CrossRef]
- Hu, N.; Qiu, Y.; Dong, F. Role of Erk1/2 signaling in the regulation of neutrophil versus monocyte development in response to G-CSF and M-CSF. J. Biol. Chem. 2015, 290, 24561–24573. [Google Scholar] [CrossRef]
- Rodriguez, R.M.; Suarez-Alvarez, B.; Lavín, J.L.; Ascensión, A.M.; Gonzalez, M.; Lozano, J.J.; Raneros, A.B.; Bulnes, P.D.; Vidal-Castiñeira, J.R.; Huidobro, C.; et al. Signal Integration and Transcriptional Regulation of the Inflammatory Response Mediated by the GM-/M-CSF Signaling Axis in Human Monocytes. Cell Rep. 2019, 29, 860–872.e5. [Google Scholar] [CrossRef]
- Ebner, K.; Bandion, A.; Binder, B.R.; de Martin, R.; Schmid, J.A. GMCSF activates NF-kappaB via direct interaction of the GMCSF receptor with IkappaB kinase beta. Blood 2003, 102, 192–199. [Google Scholar] [CrossRef]
- Guerrero, P.; Bono, C.; Sobén, M.; Guiu, A.; Cheng, Q.J.; Gil, M.L.; Yáñez, A. GM-CSF receptor expression determines opposing innate memory phenotypes at different stages of myelopoiesis. Blood 2024, 143, 2763–2777. [Google Scholar] [CrossRef]
- Cook, A.D.; Louis, C.; Robinson, M.J.; Saleh, R.; Sleeman, M.A.; Hamilton, J.A. Granulocyte macrophage colony-stimulating factor receptor α expression and its targeting in antigen-induced arthritis and inflammation. Arthritis Res. Ther. 2016, 18, 287. [Google Scholar] [CrossRef]
- Yoshitomi, H. Regulation of Immune Responses and Chronic Inflammation by Fibroblast-Like Synoviocytes. Front. Immunol. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Tu, J.; Hong, W.; Zhang, P.; Wang, X.; Körner, H.; Wei, W. Ontology and Function of Fibroblast-Like and Macrophage-Like Synoviocytes: How Do They Talk to Each Other and Can They Be Targeted for Rheumatoid Arthritis Therapy? Front. Immunol. 2018, 9, 1467. [Google Scholar] [CrossRef]
- Xiang, C.; Li, H.; Tang, W. Targeting CSF-1R represents an effective strategy in modulating inflammatory diseases. Pharmacol. Res. 2023, 187, 106566. [Google Scholar] [CrossRef]
- Zhan, Y.; Lew, A.M.; Chopin, M. The Pleiotropic Effects of the GM-CSF Rheostat on Myeloid Cell Differentiation and Function: More Than a Numbers Game. Front. Immunol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Lotfi, N.; Zhang, G.X.; Esmaeil, N.; Rostami, A. Evaluation of the effect of GM-CSF blocking on the phenotype and function of human monocytes. Sci. Rep. 2020, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 2013, 39, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Hirota, K.; Sakaguchi, S. Synovial Tissue Inflammation Mediated by Autoimmune T Cells. Front. Immunol. 2019, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Fuhler, G.M.; Cadwallader, K.A.; Knol, G.J.; Chilvers, E.R.; Drayer, A.L.; Vellenga, E. Disturbed granulocyte macrophage-colony stimulating factor priming of phosphatidylinositol 3,4,5-trisphosphate accumulation and Rac activation in fMLP-stimulated neutrophils from patients with myelodysplasia. J. Leukoc. Biol. 2004, 76, 254–262. [Google Scholar] [CrossRef]
- Zhang, Y.; McCluskey, K.; Fujii, K.; Wahl, L.M. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J. Immunol. 1998, 161, 3071–3076. [Google Scholar] [CrossRef]
- Schreck, R.; Baeuerle, P.A. NF-kappa B as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol. Cell. Biol. 1990, 10, 1281–1286. [Google Scholar] [CrossRef]
- Weiss, M.; Blazek, K.; Byrne, A.J.; Perocheau, D.P.; Udalova, I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediat. Inflamm. 2013, 2013, 245804. [Google Scholar] [CrossRef]
- Helft, J.; Böttcher, J.P.; Chakravarty, P.; Zelenay, S.; Huotari, J.; Schraml, B.U.; Goubau, D.; Reis e Sousa, C. Alive but Confused: Heterogeneity of CD11c(+) MHC Class II(+) Cells in GM-CSF Mouse Bone Marrow Cultures. Immunity 2016, 44, 3–4. [Google Scholar] [CrossRef]
- Mashima, H.; Zhang, R.; Kobayashi, T.; Hagiya, Y.; Tsukamoto, H.; Liu, T.; Iwama, T.; Yamamoto, M.; Lin, C.; Nakatsuka, R.; et al. Generation of GM-CSF-producing antigen-presenting cells that induce a cytotoxic T cell-mediated antitumor response. Oncoimmunology 2020, 9, 1814620. [Google Scholar] [CrossRef]
- Korniotis, S.; Saichi, M.; Trichot, C.; Hoffmann, C.; Amblard, E.; Viguier, A.; Grondin, S.; Noel, F.; Mattoo, H.; Soumelis, V. GM-CSF-activated human dendritic cells promote type 1 T follicular helper cell polarization in a CD40-dependent manner. J. Cell Sci. 2022, 135, jcs260298. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, B.C.; Corthay, A.; Øynebråten, I. High production of IL-12 by human dendritic cells stimulated with combinations of pattern-recognition receptor agonists. npj Vaccines 2024, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hashimoto, M.; Ito, Y.; Matsuura, M.; Ito, H.; Tanaka, M.; Watanabe, H.; Kondoh, G.; Tanaka, A.; Yasuda, K.; et al. Autoimmune Th17 Cells Induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis. Immunity 2018, 48, 1220–1232.e5. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, K.; Roesler, J.; Emmendoerffer, A.; Elsner, J.; Welte, K. Functional features of neutrophils induced by G-CSF and GM-CSF treatment: Differential effects and clinical implications. Leukemia 1997, 11, 466–478. [Google Scholar] [CrossRef]
- Chao, J.R.; Wang, J.M.; Lee, S.F.; Peng, H.W.; Lin, Y.H.; Chou, C.H.; Li, J.C.; Huang, H.M.; Chou, C.K.; Kuo, M.L.; et al. mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol. Cell. Biol. 1998, 18, 4883–4898. [Google Scholar] [CrossRef]
- He, K.; Liu, X.; Hoffman, R.D.; Shi, R.Z.; Lv, G.Y.; Gao, J.L. G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors. FEBS Open Bio 2022, 12, 1268–1285. [Google Scholar] [CrossRef]
- Chen, J.; Cao, Y.; Xiao, J.; Hong, Y.; Zhu, Y. The emerging role of neutrophil extracellular traps in the progression of rheumatoid arthritis. Front. Immunol. 2024, 15, 1438272. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Thiruppathi, M.; Elshabrawy, H.A.; Alharshawi, K.; Kumar, P.; Prabhakar, B.S. GM-CSF: An immune modulatory cytokine that can suppress autoimmunity. Cytokine 2015, 75, 261–271. [Google Scholar] [CrossRef]
- Singh, P.; González-Ramos, S.; Mojena, M.; Rosales-Mendoza, C.E.; Emami, H.; Swanson, J.; Morss, A.; Fayad, Z.A.; Rudd, J.H.; Gelfand, J.; et al. GM-CSF Enhances Macrophage Glycolytic Activity In Vitro and Improves Detection of Inflammation In Vivo. J. Nucl. Med. 2016, 57, 1428–1435. [Google Scholar] [CrossRef]
- Sun, H.W.; Wu, W.C.; Chen, H.T.; Xu, Y.T.; Yang, Y.Y.; Chen, J.; Yu, X.J.; Wang, Z.; Shuang, Z.Y.; Zheng, L. Glutamine Deprivation Promotes the Generation and Mobilization of MDSCs by Enhancing Expression of G-CSF and GM-CSF. Front. Immunol. 2021, 11, 616367. [Google Scholar] [CrossRef]
- Guo, X.; Wang, S.; Godwood, A.; Close, D.; Ryan, P.C.; Roskos, L.K.; White, W.I. Pharmacodynamic biomarkers and differential effects of TNF- and GM-CSF-targeting biologics in rheumatoid arthritis. Int. J. Rheum. Dis. 2019, 22, 646–653. [Google Scholar] [CrossRef]
- Senolt, L. Emerging therapies in rheumatoid arthritis: Focus on monoclonal antibodies. F1000Research 2019, 8, F1000, Faculty Rev-1549. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; Taylor, P.C.; McInnes, I.B.; Atsumi, T.; Strand, V.; Takeuchi, T.; Bracher, M.; Brooks, D.; Curtis, P.; Gupta, A.; et al. Long-term safety and efficacy of anti-GM-CSF otilimab in patients with rheumatoid arthritis: Long-term extension of three phase 3 randomised trials (contRAst X). BMJ Open 2025, 15, e088869. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, T.W.; Batalov, A.; Stoilov, R.; Lloyd, E.; Wagner, T.; Saurigny, D.; Souberbielle, B.; Esfandiari, E. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Corbera-Bellalta, M.; Alba-Rovira, R.; Muralidharan, S.; Espígol-Frigolé, G.; Ríos-Garcés, R.; Marco-Hernández, J.; Denuc, A.; Kamberovic, F.; Pérez-Galán, P.; Joseph, A.; et al. Blocking GM-CSF receptor α with mavrilimumab reduces infiltrating cells, pro-inflammatory markers and neoangiogenesis in ex vivo cultured arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2022, 81, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Feist, E.; Sleeman, M.A.; Wang, B.; White, B.; Magrini, F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: A randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann. Rheum. Dis. 2011, 70, 1542–1549. [Google Scholar] [CrossRef]
- Davda, J.P.; Hansen, R.J. Properties of a general PK/PD model of antibody-ligand interactions for therapeutic antibodies that bind to soluble endogenous targets. MAbs 2010, 2, 576–588. [Google Scholar] [CrossRef]
- Cook, A.D.; Braine, E.L.; Campbell, I.K.; Rich, M.J.; Hamilton, J.A. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): Requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001, 3, 293–298. [Google Scholar] [CrossRef]
- Cook, A.D.; Turner, A.L.; Braine, E.L.; Pobjoy, J.; Lenzo, J.C.; Hamilton, J.A. Regulation of systemic and local myeloid cell subpopulations by bone marrow cell-derived granulocyte-macrophage colony-stimulating factor in experimental inflammatory arthritis. Arthritis Rheum. 2011, 63, 2340–2351. [Google Scholar] [CrossRef]
- Greven, D.E.; Cohen, E.S.; Gerlag, D.M.; Campbell, J.; Woods, J.; Davis, N.; van Nieuwenhuijze, A.; Lewis, A.; Heasmen, S.; McCourt, M.; et al. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 1924–1930. [Google Scholar] [CrossRef]
- Minter, R.R.; Cohen, E.S.; Wang, B.; Liang, M.; Vainshtein, I.; Rees, G.; Eghobamien, L.; Harrison, P.; Sims, D.A.; Matthews, C.; et al. Protein engineering and preclinical development of a GM-CSF receptor antibody for the treatment of rheumatoid arthritis. Br. J. Pharmacol. 2013, 168, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; van der Heijde, D.; Strand, V.; Atsumi, T.; McInnes, I.B.; Takeuchi, T.; Taylor, P.C.; Bracher, M.; Brooks, D.; Davies, J.; et al. Anti-GM-CSF otilimab versus tofacitinib or placebo in patients with active rheumatoid arthritis and an inadequate response to conventional or biologic DMARDs: Two phase 3 randomised trials (contRAst 1 and contRAst 2). Ann. Rheum. Dis. 2023, 82, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Berkowitz, M.; Conaghan, P.G.; Peterfy, C.; Davy, K.; Fisheleva, E.; Gupta, A.; Inman, D.; Janiczek, R.; Layton, M.; et al. MRI of the joint and evaluation of the granulocyte-macrophage colony-stimulating factor-CCL17 axis in patients with rheumatoid arthritis receiving otilimab: A phase 2a randomised mechanistic study. Lancet Rheumatol. 2020, 2, e666–e676. [Google Scholar] [CrossRef]
- Taylor, P.C.; Saurigny, D.; Vencovsky, J.; Takeuchi, T.; Nakamura, T.; Matsievskaia, G.; Hunt, B.; Wagner, T.; Souberbielle, B.; NEXUS Study Group. Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: A randomized, controlled trial. Arthritis Res. Ther. 2019, 21, 101. [Google Scholar]
- Kivitz, A.; Hazan, L.; Hoffman, K.; Wallin, B. FRI0209 MORAb-022, an Anti-Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) Monoclonal Antibody (MAB): RESULTS of the First Study in Patients with Mild-to-Moderate Rheumatoid Arthritis (RA); BMJ Publishing Group Ltd.: London, UK, 2016. [Google Scholar]
- Behrens, F.; Tak, P.P.; Østergaard, M.; Stoilov, R.; Wiland, P.; Huizinga, T.W.; Berenfus, V.Y.; Vladeva, S.; Rech, J.; Rubbert-Roth, A.; et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: Results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 2015, 74, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Weinblatt, M.E.; McInnes, I.B.; Porter, D.; Barbarash, O.; Vatutin, M.; Szombati, I.; Esfandiari, E.; Sleeman, M.A.; Kane, C.D.; et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1445–1452. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Korkosz, M.; Vencovsky, J.; Rubbert-Roth, A.; Mysler, E.; Sleeman, M.A.; Godwood, A.; et al. Efficacy and safety of mavrilimumab, A fully human Gm–CSFR-Alpha monoclonal antibody in patients with rheumatoid arthritis: Primary results from the Earth Explorer 1 Study. Ann. Rheum. Dis. 2015, 74, S78. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.; Miranda, P.; Korkosz, M.; Vencovsky, J.; Rubbert-Roth, A.; Mysler, E.; Sleeman, M.A.; Godwood, A.; et al. A randomised phase IIb study of mavrilimumab, a novel GM-CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.C.; Wu, C.Y.; Roskos, L.K.; Godwood, A.; Close, D.; Wang, B. AB0445 Exposure–Efficacy Analysis of Mavrilimumab in Rheumatoid Arthritis: Modeling and Simulation of Phase II Clinical Data. Ann. Rheum. Dis. 2015, 74, 1043. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Vencovský, J.; Godwood, A.; Albulescu, M.; Michaels, M.A.; Guo, X.; Close, D.; et al. Mavrilimumab, a Fully Human Granulocyte-Macrophage Colony-Stimulating Factor Receptor α Monoclonal Antibody: Long-Term Safety and Efficacy in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 679–689. [Google Scholar] [CrossRef]
- Weinblatt, M.; McInnes, I.; Kremer, J.; Miranda, P.; Vencovský, J.; Godwood, A.; Albulescu, M.; Close, D.; Burmester, G. Earth explorer 2, a phase IIb exploratory study evaluating efficacy and safety of mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor-alpha monoclonal antibody, and the tumor necrosis factor antagonist golimumab in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, S717–S718. [Google Scholar]
- Chen, Y.; Li, F.; Hua, M.; Liang, M.; Song, C. Role of GM-CSF in lung balance and disease. Front. Immunol. 2023, 14, 1158859. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; Pitts, K.; Wang, T.; Lee, E.; Buchbinder, E.; Dougan, M.; Armstrong, D.G.; Paine, R., 3rd; Ragsdale, C.E.; Boyd, T.; et al. Recombinant GM-CSF for diseases of GM-CSF insufficiency: Correcting dysfunctional mononuclear phagocyte disorders. Front. Immunol. 2023, 13, 1069444. [Google Scholar] [CrossRef]
- McCormick, T.S.; Hejal, R.B.; Leal, L.O.; Ghannoum, M.A. GM-CSF: Orchestrating the Pulmonary Response to Infection. Front. Pharmacol. 2022, 12, 735443. [Google Scholar] [CrossRef]
- Wishart, A.L.; Pechacek, J.; Rosen, L.B.; Desai, J.V.; Zarakas, M.A.; Webb, T.; Pittaluga, S.; Seyedmousavi, A.; Hohl, T.M.; Kuhns, D.B.; et al. Neutralizing GM-CSF autoantibodies impair neutrophil antifungal effector function in a patient with aspergillosis. J. Infect. 2025, 91, 106588. [Google Scholar] [CrossRef]
- Ataya, A.; Knight, V.; Carey, B.C.; Lee, E.; Tarling, E.J.; Wang, T. The Role of GM-CSF Autoantibodies in Infection and Autoimmune Pulmonary Alveolar Proteinosis: A Concise Review. Front. Immunol. 2021, 12, 752856. [Google Scholar] [CrossRef]
- Campo, I.; Carey, B.C.; Paracchini, E.; Kadija, Z.; De Silvestri, A.; Rodi, G.; De Amici, M.; Torre, C.; Zorzetto, M.; Griese, M.; et al. Inhaled recombinant GM-CSF reduces the need for whole lung lavage and improves gas exchange in autoimmune pulmonary alveolar proteinosis patients. Eur. Respir. J. 2024, 63, 2301233. [Google Scholar] [CrossRef]
- Bonfield, T.L.; Kavuru, M.S.; Thomassen, M.J. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin. Immunol. 2002, 105, 342–350. [Google Scholar] [CrossRef]
- Campo, I.; Meloni, F.; Gahlemann, M.; Sauter, W.; Ittrich, C.; Schoelch, C.; Trapnell, B.C.; Gupta, A. An exploratory study investigating biomarkers associated with autoimmune pulmonary alveolar proteinosis (aPAP). Sci. Rep. 2022, 12, 8708. [Google Scholar] [CrossRef]
- Chen, W.; Feng, X.; Yao, L.K.; Li, X.; Yang, Z.M.; Qin, X.Y.; Li, Y.; Qiu, Y. Exogenous GM-CSF therapy for autoimmune pulmonary alveolar proteinosis: A systematic literature review. Front. Med. 2025, 12, 1552566. [Google Scholar]
- Chen, H.; Gao, N.; Fan, D.; Wu, J.; Zhu, J.; Li, J.; Wang, J.; Chen, Y.; An, J. Suppressive effects on the immune response and protective immunity to a JEV DNA vaccine by co-administration of a GM-CSF-expressing plasmid in mice. PLoS ONE 2012, 7, e34602. [Google Scholar] [CrossRef] [PubMed]
- Parmiani, G.; Castelli, C.; Pilla, L.; Santinami, M.; Colombo, M.P.; Rivoltini, L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann. Oncol. 2007, 18, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.W.; Chueh, H.Y.; Tsai, C.C.; Lin, C.T.; Qiu, J.T. Novel GM-CSF-based vaccines: One small step in GM-CSF gene optimization, one giant leap for human vaccines. Hum. Vaccin. Immunother. 2016, 12, 3020–3028. [Google Scholar] [CrossRef]
- Henrickson, S.E.; Ruffner, M.A.; Kwan, M. Unintended Immunological Consequences of Biologic Therapy. Curr. Allergy Asthma Rep. 2016, 16, 46. [Google Scholar] [CrossRef]
- Zhang, F.; Jonsson, A.H.; Nathan, A.; Millard, N.; Curtis, M.; Xiao, Q.; Gutierrez-Arcelus, M.; Apruzzese, W.; Watts, G.F.M.; Weisenfeld, D.; et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 2023, 623, 616–624. [Google Scholar] [CrossRef]
- Hanlon, M.M.; Smith, C.M.; Canavan, M.; Neto, N.G.B.; Song, Q.; Lewis, M.J.; O’Rourke, A.M.; Tynan, O.; Barker, B.E.; Gallagher, P.; et al. Loss of synovial tissue macrophage homeostasis precedes rheumatoid arthritis clinical onset. Sci. Adv. 2024, 10, eadj1252. [Google Scholar] [CrossRef]
- Achuthan, A.; Cook, A.D.; Lee, M.C.; Saleh, R.; Khiew, H.W.; Chang, M.W.; Louis, C.; Fleetwood, A.J.; Lacey, D.C.; Christensen, A.D.; et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J. Clin. Investig. 2016, 126, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Bluett, J. Towards Personalized Medicine in Rheumatoid Arthritis. Open Access Rheumatol. 2024, 16, 89–114. [Google Scholar] [CrossRef]
- Momoi, Y.; Kumagai, S.; Nishikawa, H. Immunogenomic precision medicine: A personalized approach based on immunogenomic cancer evolution. Int. Immunol. 2025, 37, 517–537. [Google Scholar] [CrossRef]
- Silva, R.C.M.C.; Travassos, L.H.; Dutra, F.F. The dichotomic role of single cytokines: Fine-tuning immune responses. Cytokine 2024, 173, 156408. [Google Scholar] [CrossRef]
- Chang, C.J.; Chen, Y.H.; Huang, K.W.; Cheng, H.W.; Chan, S.F.; Tai, K.F.; Hwang, L.H. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology 2007, 45, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, Y.; Li, Q.; Luo, L.; Li, X.; Luo, Z.; Lu, Y.; Zhang, J.; Jiang, M.; Qin, B.; et al. Rational administration sequencing of immunochemotherapy elicits powerful anti-tumor effect. J. Control Release 2022, 341, 769–781. [Google Scholar] [CrossRef]
- Kwon, O.C.; Lee, E.J.; Chang, E.J.; Youn, J.; Ghang, B.; Hong, S.; Lee, C.K.; Yoo, B.; Kim, Y.G. IL-17A+GM-CSF+ Neutrophils Are the Major Infiltrating Cells in Interstitial Lung Disease in an Autoimmune Arthritis Model. Front. Immunol. 2018, 9, 1544. [Google Scholar] [CrossRef]
- O’Rielly, D.D.; Rahman, P. Pharmacogenetics of rheumatoid arthritis: Potential targets from susceptibility genes and present therapies. Pharmgenom. Pers. Med. 2010, 3, 15–31. [Google Scholar]
- Gent, Y.Y.; Voskuyl, A.E.; Kloet, R.W.; van Schaardenburg, D.; Hoekstra, O.S.; Dijkmans, B.A.; Lammertsma, A.A.; van der Laken, C.J. Macrophage positron emission tomography imaging as a biomarker for preclinical rheumatoid arthritis: Findings of a prospective pilot study. Arthritis Rheum. 2012, 64, 62–66. [Google Scholar] [PubMed]
- Bruijnen, S.T.G.; Verweij, N.J.F.; Gent, Y.Y.J.; Huisman, M.C.; Windhorst, A.D.; Kassiou, M.; van de Ven, P.M.; Lammertsma, A.A.; Hoekstra, O.S.; Voskuyl, A.E.; et al. Imaging disease activity of rheumatoid arthritis by macrophage targeting using second generation translocator protein positron emission tomography tracers. PLoS ONE 2019, 14, e0222844. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; van der Helm-van Mil, A.H.; Knevel, R.; Huizinga, T.W.; Haney, D.J.; Shen, Y.; Ramanujan, S.; Cavet, G.; Centola, M.; Hesterberg, L.K.; et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res. 2012, 64, 1794–1803. [Google Scholar] [CrossRef]
- Syversen, S.W.; Landewe, R.; van der Heijde, D.; Bathon, J.M.; Boers, M.; Bykerk, V.P.; Fitzgerald, O.; Gladman, D.D.; Garnero, P.; Geusens, P.; et al. Testing of the OMERACT 8 draft validation criteria for a soluble biomarker reflecting structural damage in rheumatoid arthritis: A systematic literature search on 5 candidate biomarkers. J. Rheumatol. 2009, 36, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.; Pesenacker, A.M.; Bending, D.; Thirugnanabalan, B.; Varsani, H.; Wedderburn, L.R.; Nistala, K. T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol. 2014, 66, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Malengier-Devlies, B.; Bernaerts, E.; Ahmadzadeh, K.; Filtjens, J.; Vandenhaute, J.; Boeckx, B.; Burton, O.; De Visscher, A.; Mitera, T.; Berghmans, N.; et al. Role for Granulocyte Colony-Stimulating Factor in Neutrophilic Extramedullary Myelopoiesis in a Murine Model of Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2022, 74, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
| Antibody Name | Target | Preclinical Model | Key Findings | References |
|---|---|---|---|---|
| CAM-3003 | GM-CSFR | Mouse CIA model | Anti-GM-CSF mAb treatment reduced arthritis severity and progression, decreased synovial inflammation and cartilage damage, and lowered TNF-α and IL-1β levels in joint tissue | [131] |
| GM-CSF blockade reduced CIA severity, circulating Ly-6Chigh monocytes, and synovial immune cell infiltration | [132] | |||
| Arthritis severity was reduced in a dose-dependent manner, with concomitant decreases in F4/80+ synovial macrophages, joint inflammation, and cartilage and bone damage | [133] | |||
| Protein-engineered anti-GM-CSFRα (574D04) | GM-CSFR | Cynomolgus monkey | Pretreatment with 574D04 dose-dependently inhibited GM-CSF-induced hematologic responses. GM-CSF triggered acute leukocyte margination and subsequent leukocytosis in controls, both of which were significantly suppressed by 574D04 at 1–10 mg/kg | [134] |
| Antibody Name | Target | Key Findings | Bibliographic Reference | Clinical Trial No. |
|---|---|---|---|---|
| Otilimab (GSK3196165) | GM-CSF | In the phase III ContRAst 3 trial of refractory RA patients (549 patients), otilimab at 90 mg and 150 mg doses did not achieve a statistically significant ACR20 response compared with placebo at week 12, with response rates of 45% (p = 0.29; OR 1.38; 95% CI 0.76–2.48) and 51% (p = 0.06; OR 1.75; 95% CI 0.98–3.15), respectively, vs. 38% for placebo. Sarilumab 200 mg showed superior efficacy with a 57.5% ACR20 response (p = 0.005; OR 2.34; 95% CI 1.29–4.23). No significant improvements were observed in secondary endpoints for otilimab, and safety profiles were comparable across groups | [28] | [NCT04134728] |
| In the contRAst X Phase III long-term extension trial of approximately 3000 RA patients treated with otilimab, the safety profile was sustained for up to 4 years with predominantly mild to moderate adverse events and no cases of pulmonary alveolar proteinosis, tuberculosis reactivation, or serious hypersensitivity. Adverse event rates were similar between the 90 mg and 150 mg doses, with AE incidences of 62% and 64%, respectively, and serious adverse events at 8% for both doses. The CDAI low disease activity response was maintained over time. No new safety signals were observed during long-term treatment | [126] | [NCT04333147] | ||
| In the Phase III contRAst 1 (n = 1537) and contRAst 2 (n = 1625) trials in RA patients with inadequate response to methotrexate or bDMARDs, otilimab met the primary endpoint with significantly greater ACR20 response at 12 weeks, with contRAst 1 showing 54.7% response at 90 mg (p = 0.0023) and 50.9% at 150 mg (p = 0.0362) vs. 41.7% in placebo group. In the contRAst 2 trial, response rates were 54.9% and 54.5% for the 90 mg and 150 mg doses, respectively, compared with 32.5% for placebo (both p < 0.0001). Secondary endpoints (CDAI and HAQ-DI) improved but were consistently inferior to tofacitinib and the safety profile was generally well tolerated with no new safety signals reported | [135] | [NCT03980483] [NCT03970837] | ||
| A Phase IIa clinical trial (39 patients) evaluated the effects of weekly s.c. administration of otilimab 180 mg. At week 12, otilimab produced greater reductions in RAMRIS (−1.3 ± 0.6 vs. 0.8 ± 1.2) and RAMRIQ (−1417.0 μL ± 671.5 vs. −912.3 μL ± 1405.8) synovitis scores compared with placebo, but these differences were not statistically significant for synovitis, osteitis, or bone erosion. Adverse events occurred in 39% of otilimab-treated and 36% of placebo-treated patients, most frequently cough with otilimab and extremity pain or RA symptoms with placebo. No serious adverse events or deaths were reported | [136] | [NCT02799472] | ||
| Namilumab (MT203) (AMG203) | GM-CSF | A Phase II trial (108 patients) showed that s.c. namilumab 150 mg significantly reduced disease activity in patients with moderate-to-severe RA refractory to prior therapies. At week 12, namilumab 150 mg showed a statistically significant improvement in DAS28-CRP vs. placebo (p = 0.005), with separation evident from week 2 (p < 0.05) and higher ACR50 and overall response rates. Namilumab was well tolerated with no serious safety concerns, supporting further clinical development | [137] | [NCT02379091] |
| A Phase II randomized, double-blind clinical trial in 36 patients with moderate-to-severe RA receiving methotrexate demonstrated that s.c. administration of namilumab (150 mg every 2–4 weeks) significantly reduced synovitis, bone erosion, and bone marrow edema at week 24 according to OMERACT criteria. Clinical outcomes indicated improved disease activity based on DAS28-CRP (day 43, p = 0.0117; day 99, p = 0.0154) and ACR20/50/70 responses, while dynamic contrast-enhanced MRI revealed decreased synovial vascular perfusion. The treatment was well tolerated, with no safety concerns reported | [NCT02393378] | |||
| Gimsilumab (MORAb-022) | GM-CSF | Phase I (20 patients) results showed gimsilumab was well tolerated in healthy subjects and RA patients, with linear pharmacokinetics and a half-life of 9–13 days, supporting further development | [138] | [NCT01357759] |
| Plonmarlimab (TJ003234) | GM-CSF | Plonmarlimab has completed a Phase I study (32 patients), with results not yet published | [NCT03794180] | |
| MOR103 | GM-CSF | MOR103 was assessed in a Phase I trial (96 patients), providing initial safety and efficacy data to support larger clinical studies in active RA patients | [139] | [NCT01023256] |
| Mavrilimumab (CAM-3003) | GM-CSFR | In a Phase I study (32 patients), mavrilimumab showed a favorable safety and tolerability profile with single escalating intravenous doses (0.01 to 10 mg/kg) in 32 RA patients | [129] | [NCT00771420] |
| EARTH phase IIa trial (233 RA patients) showed dose-dependent improvement in DAS28-CRP at 12 weeks vs. placebo. A ≥1.2 reduction in DAS28-CRP was achieved by 55.7% of patients receiving mavrilimumab vs. 34.7% in the placebo group (p = 0.003). The 100 mg subcutaneous biweekly cohort exhibited superior ACR response rates relative to placebo (ACR20: 69.2% vs. 40.0%, p = 0.005; ACR50: 30.8% vs. 12.0%, p = 0.021; ACR70: 17.9% vs. 4.0%, p = 0.03). DAS28-CRP remission (<2.6) was also significantly higher in the 100 mg group (23.1% vs. 6.7%, p = 0.016). Clinical improvement was evident as early as week 2 (p = 0.003). Mavrilimumab exhibited a favorable safety profile, with no serious pulmonary adverse events reported | [140] | [NCT01050998] | ||
| The EARTH EXPLORER 1 phase IIb trial (233 patients) demonstrated statistically significant efficacy of mavrilimumab 150 mg s.c. biweekly. Compared to placebo, the 150 mg group had ACR20, ACR50, and ACR70 response rates of 73.4% (p < 0.001), 40.5% (p < 0.001), and 13.9% (p = 0.026). The drug significantly reduced DAS28-CRP scores from baseline (−1.90 vs. −0.68 for placebo; p < 0.001). Safety was consistent with prior studies with no unexpected serious adverse events or pulmonary toxicity reported | [141,142] | [NCT01706926] | ||
| EARTH EXPLORER 2 study (138 patients) compared mavrilimumab 100 mg s.c. biweekly plus methotrexate vs. golimumab 50 mg s.c. every 4 weeks in 138 RA patients refractory to at least one biologic or synthetic DMARDs. Safety acceptable | [145] | [NCT01715896] | ||
| Pharmacokinetic and exposure-efficacy modeling analyses from a Phase II study (409 patients) indicated mavrilimumab exhibits dose-proportional kinetics and an effective half-life of approximately 13 days. Efficacy was dose-dependent, with statistically significant improvements in disease activity score DAS28-CRP from baseline to week 12 across doses (150 mg: −1.90 ± 0.14; 100 mg: −1.64 ± 0.13, 30 mg: −1.37 ± 0.14; placebo: −0.68 ± 0.14; p < 0.001). Moreover, the proportion of patients achieving ACR20 response at week 24 was significantly higher in mavrilimumab groups (150 mg: 73.4%; 100 mg: 61.2%; 30 mg: 50.6%) vs. placebo (24.7%; p < 0.001). | [143] | |||
| Long-term open-label extension (up to 3.3 years) involving 442 RA patients on mavrilimumab plus methotrexate reported sustained reductions in DAS28-CRP scores, with 65.0% of patients achieving DAS28-CRP < 3.2 and 40.6% < 2.6 at week 122. Safety was consistent with short-term studies; adverse events were predominantly mild/moderate with no reported cases of pulmonary alveolar proteinosis or serious pulmonary toxicity | [144] | [NCT01712399] | ||
| TJ003234 | GM-CSF | In a phase I/II, multicenter, randomized, double-blind, placebo-controlled trial (63 patients), single and multiple ascending doses were evaluated in adults with established RA per ACR/EULAR criteria, assessing safety, tolerability, and pharmacokinetics. Preliminary results indicate acceptable safety, predominantly mild side events, and effective GM-CSF pathway inhibition. Confirmatory efficacy and biomarker analyses are ongoing | [NCT04457856] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. Targeting GM-CSF in Rheumatoid Arthritis: Advances in Cytokine-Directed Immunotherapy and Clinical Implications. Life 2025, 15, 1737. https://doi.org/10.3390/life15111737
García-Domínguez M. Targeting GM-CSF in Rheumatoid Arthritis: Advances in Cytokine-Directed Immunotherapy and Clinical Implications. Life. 2025; 15(11):1737. https://doi.org/10.3390/life15111737
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2025. "Targeting GM-CSF in Rheumatoid Arthritis: Advances in Cytokine-Directed Immunotherapy and Clinical Implications" Life 15, no. 11: 1737. https://doi.org/10.3390/life15111737
APA StyleGarcía-Domínguez, M. (2025). Targeting GM-CSF in Rheumatoid Arthritis: Advances in Cytokine-Directed Immunotherapy and Clinical Implications. Life, 15(11), 1737. https://doi.org/10.3390/life15111737




