Serotonin Signaling Pathway Modulation Affects Retinal Neuron Survival in Experimental Model of Retinal Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Anesthesia, and Euthanasia
2.2. Oral Administration of SSRI
2.3. Retinal Ischemia Model (Episcleral Vein Cauterization)
2.4. Intraocular Pressure Measurements
2.5. Retrobulbar Injections of Meclofenamic Acid
2.6. Electroretinography (ERG)
2.7. Immunofluorescence
2.8. Cell Counting
2.9. Western Blotting
2.10. Data Analysis
3. Results
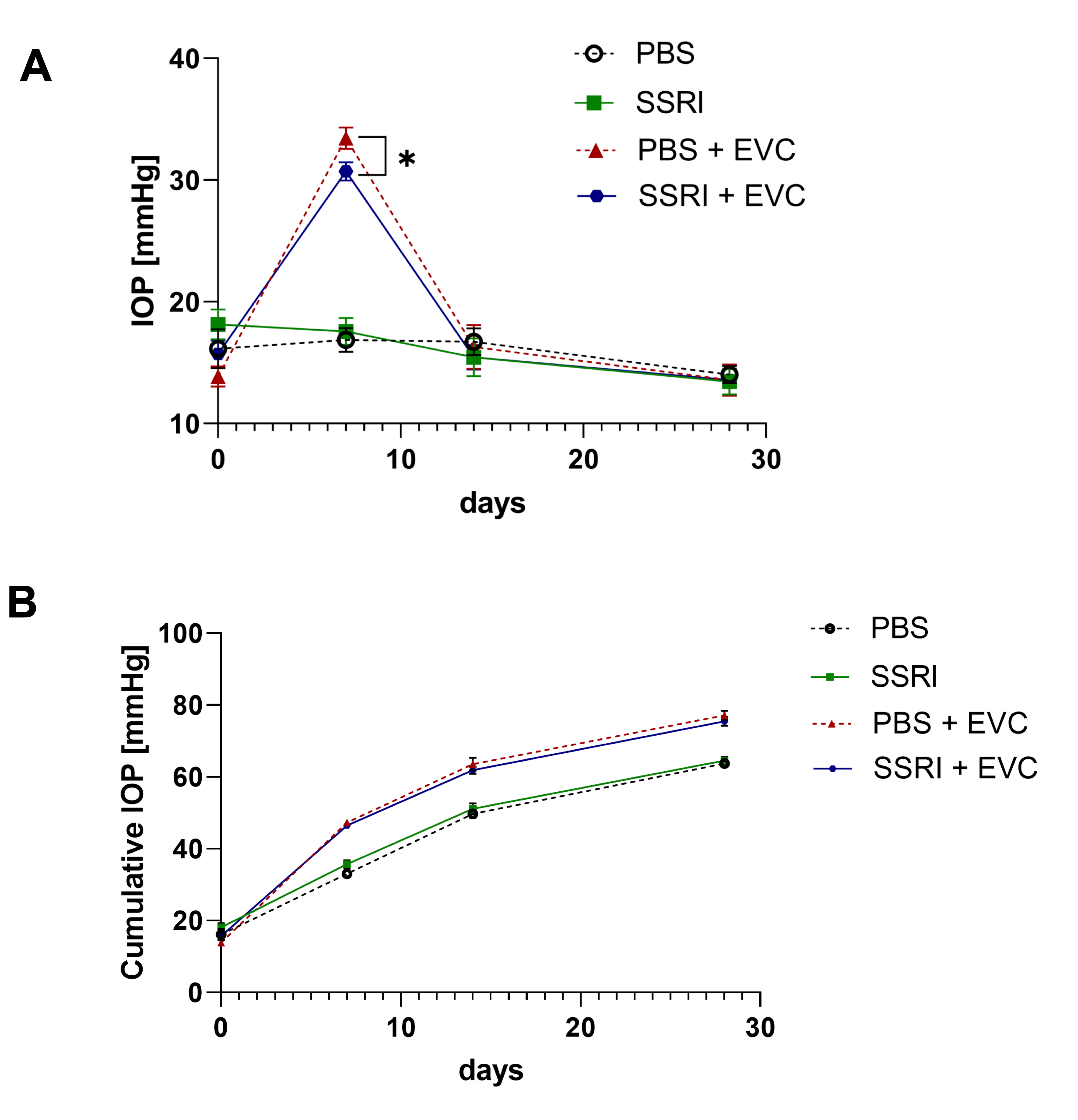
3.1. Cauterization of Episcleral Veins Effectively Increases Intraocular Pressure
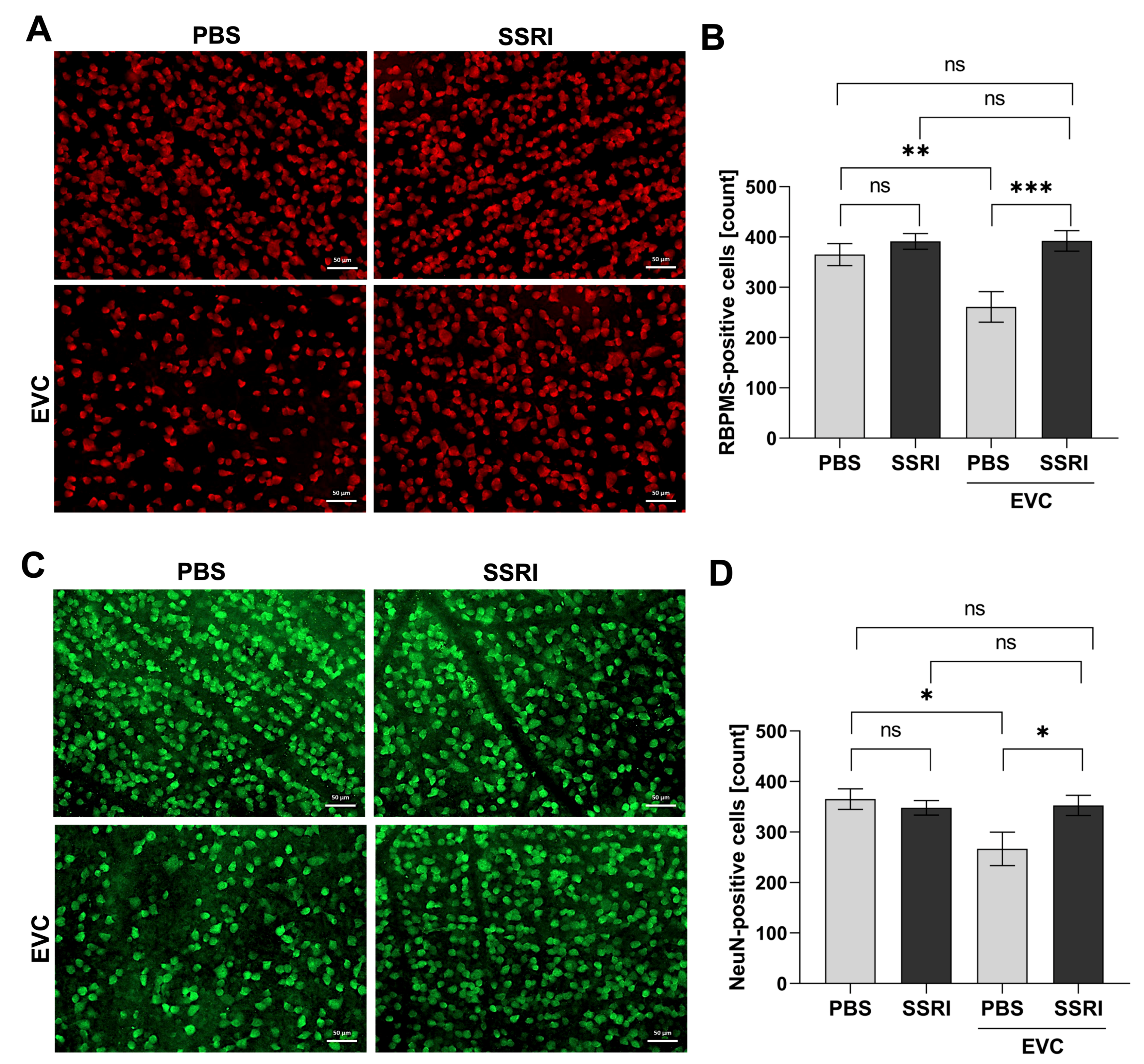
3.2. Escitalopram Protects Retinal Neuronal Cells from the Deleterious Effect of Cauterization of Episcleral Veins
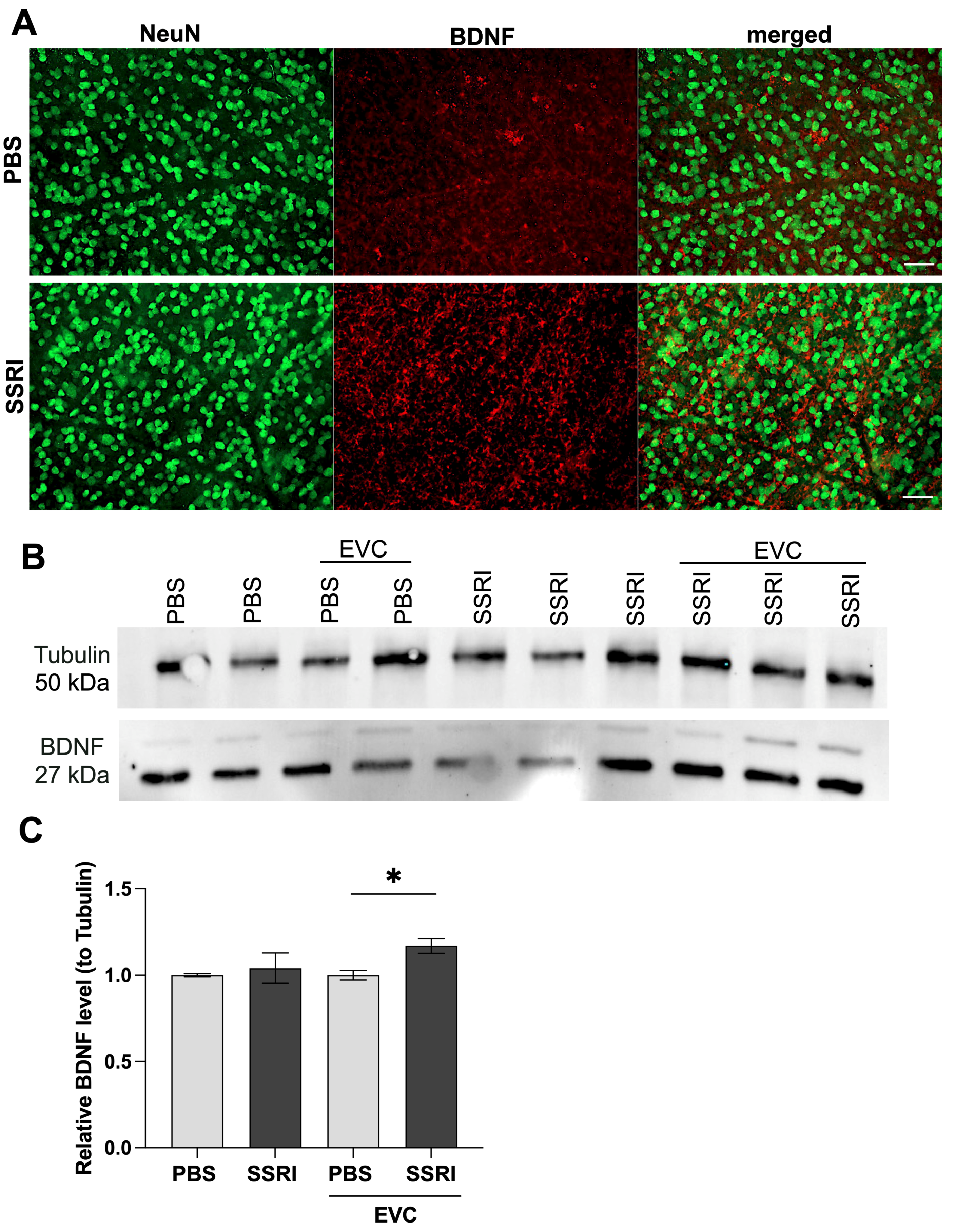
3.3. Escitalopram Treatment Significantly Elevated BDNF Content in the Retina
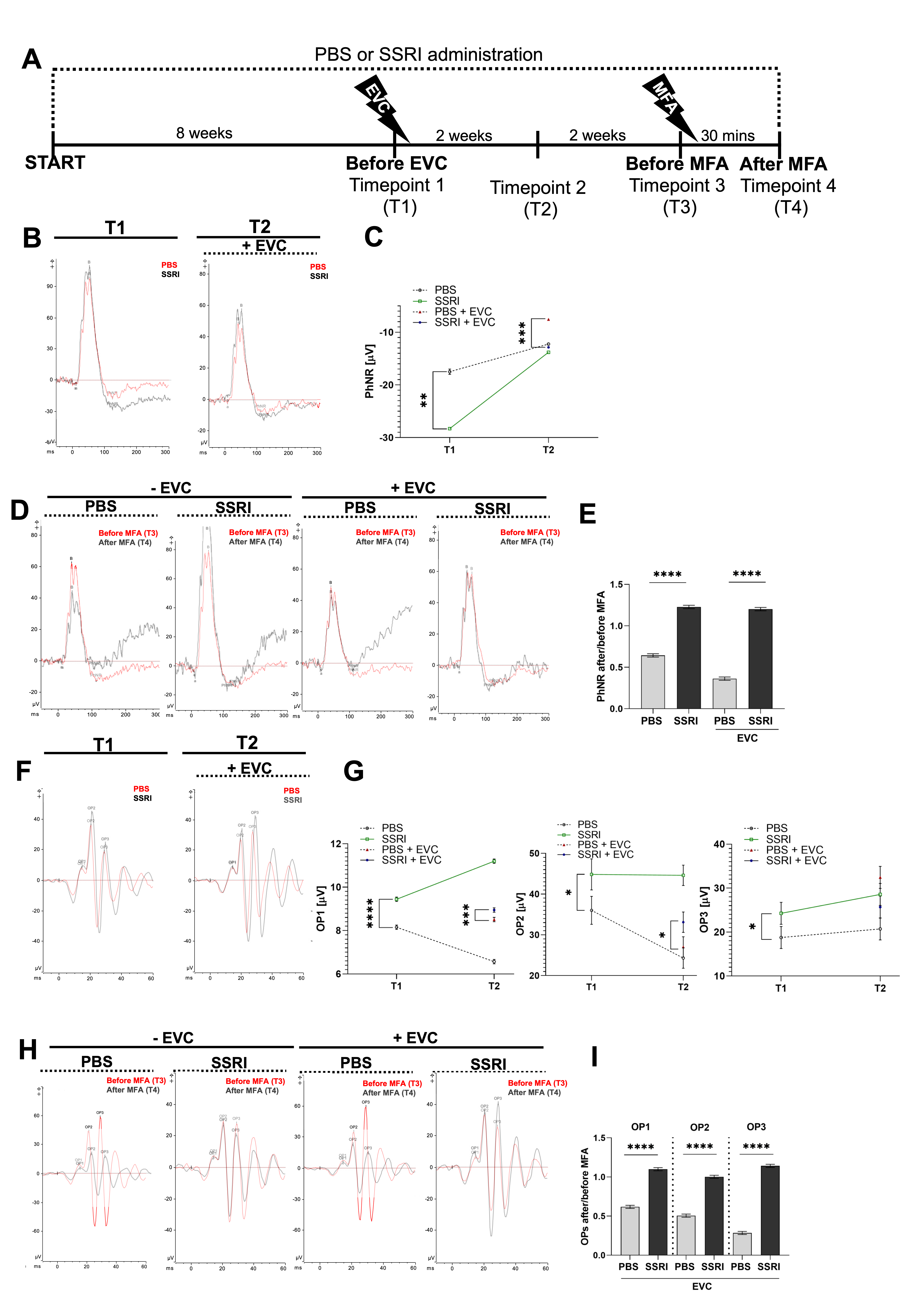
3.4. Escitalopram Treatment Promotes Functional Recovery of the Retina
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| CXs | Connexins |
| ERG | Electroretinography |
| EVC | Episcleral vein cauterization |
| GCL | Ganglion cell layer |
| IOP | Intraocular pressure |
| MFA | Meclofenamic acid |
| NeuN | Neuronal-Nuclei |
| OPs | Oscillatory potentials |
| PhNR | Photopic negative response |
| RBPMS | RNA-binding protein with multiple splicing |
| RGCs | Retinal ganglion cells |
| SSRIs | Selective serotonin reuptake inhibitors |
References
- Costa, M.; Furness, J.B. Neuronal Peptides in the Intestine. Br. Med. Bull. 1982, 38, 247–252. [Google Scholar] [CrossRef]
- Jonnakuty, C.; Gragnoli, C. What Do We Know about Serotonin? J. Cell. Physiol. 2008, 217, 301–306. [Google Scholar] [CrossRef]
- Ghai, K.; Zelinka, C.; Fischer, A.J. Serotonin Released from Amacrine Neurons Is Scavenged and Degraded in Bipolar Neurons in the Retina. J. Neurochem. 2009, 111, 1–14. [Google Scholar] [CrossRef]
- Mitchell, C.K.; Redburn, D.A. Analysis of Pre- and Postsynaptic Factors of the Serotonin System in Rabbit Retina. J. Cell Biol. 1985, 100, 64–73. [Google Scholar] [CrossRef]
- Pootanakit, K.; Brunken, W.J. 5-HT(1A) and 5-HT(7) Receptor Expression in the Mammalian Retina. Brain Res. 2000, 875, 152–156. [Google Scholar] [CrossRef]
- Pootanakit, K.; Prior, K.J.; Hunter, D.D.; Brunken, W.J. 5-HT2a Receptors in the Rabbit Retina: Potential Presynaptic Modulators. Vis. Neurosci. 1999, 16, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A.; Senchyna, M. Serotonin Receptor Subtype MRNA Expression in Human Ocular Tissues, Determined by RT-PCR. Mol. Vis. 2006, 12, 1040–1047. [Google Scholar] [PubMed]
- Mangel, S.C.; Brunken, W.J. The Effects of Serotonin Drugs on Horizontal and Ganglion Cells in the Rabbit Retina. Vis. Neurosci. 1992, 8, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Brunken, W.J.; Jin, X.T.; Pis-Lopez, A.M. Chapter 4 The Properties of the Serotoninergic System in the Retina. Prog. Retin. Res. 1993, 12, 75–99. [Google Scholar] [CrossRef]
- Costagliola, C.; Parmeggiani, F.; Sebastiani, A. SSRIs and Intraocular Pressure Modifications: Evidence, Therapeutic Implications and Possible Mechanisms. CNS Drugs 2004, 18, 475–484. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Piegors, D.J.; Heistad, D.D. Serotonin-Induced Constriction of Ocular Arteries in Atherosclerotic Monkeys. Implications for Ischemic Disorders of the Retina and Optic Nerve Head. Arch. Ophthalmol. 1997, 115, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Patel, Y.; Martin, E.A.; Dembinska, O.; Hellberg, M.; Krueger, D.S.; Kapin, M.A.; Romano, C. Agonists at the Serotonin Receptor (5-HT(1A)) Protect the Retina from Severe Photo-Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2118–2126. [Google Scholar] [CrossRef]
- Costagliola, C.; Parmeggiani, F.; Semeraro, F.; Sebastiani, A. Selective Serotonin Reuptake Inhibitors: A Review of Its Effects on Intraocular Pressure. Curr. Neuropharmacol. 2008, 6, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.; Köhler-Forsberg, K.; Henriksen, T.; Weimann, A.; Brandslund, I.; Ellervik, C.; Poulsen, H.E.; Knudsen, G.M.; Frokjaer, V.G.; Jorgensen, M.B. Systemic DNA and RNA Damage from Oxidation after Serotonergic Treatment of Unipolar Depression. Transl. Psychiatry 2022, 12, 204. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Liu, L.; Qiao, D.; Baldwin, D.S.; Hou, R. Effects of SSRIs on Peripheral Inflammatory Markers in Patients with Major Depressive Disorder: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2019, 79, 24–38. [Google Scholar] [CrossRef]
- Hanson, N.D.; Owens, M.J.; Nemeroff, C.B. Depression, Antidepressants, and Neurogenesis: A Critical Reappraisal. Neuropsychopharmacology 2011, 36, 2589–2602. [Google Scholar] [CrossRef]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased Hippocampal BDNF Immunoreactivity in Subjects Treated with Antidepressant Medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Mosiołek, A.; Mosiołek, J.; Jakima, S.; Pięta, A.; Szulc, A. Effects of Antidepressant Treatment on Neurotrophic Factors (BDNF and IGF-1) in Patients with Major Depressive Disorder (MDD). J. Clin. Med. 2021, 10, 3377. [Google Scholar] [CrossRef]
- Jeanson, T.; Pondaven, A.; Ezan, P.; Mouthon, F.; Charvériat, M.; Giaume, C. Antidepressants Impact Connexin 43 Channel Functions in Astrocytes. Front. Cell. Neurosci. 2016, 9, 495. [Google Scholar] [CrossRef]
- Charvériat, M.; Naus, C.C.; Leybaert, L.; Sáez, J.C.; Giaume, C. Connexin-Dependent Neuroglial Networking as a New Therapeutic Target. Front. Cell. Neurosci. 2017, 11, 174. [Google Scholar] [CrossRef]
- Pacwa, A.; Machowicz, J.; Akhtar, S.; Rodak, P.; Liu, X.; Pietrucha-Dutczak, M.; Lewin-Kowalik, J.; Amadio, M.; Smedowski, A. Deficiency of the RNA-Binding Protein ELAVL1/HuR Leads to the Failure of Endogenous and Exogenous Neuroprotection of Retinal Ganglion Cells. Front. Cell. Neurosci. 2023, 17, 1131356. [Google Scholar] [CrossRef]
- Olakowska, E.; Rodak, P.; Pacwa, A.; Machowicz, J.; Machna, B.; Lewin-Kowalik, J.; Smedowski, A. Surgical Menopause Impairs Retinal Conductivity and Worsens Prognosis in an Acute Model of Rat Optic Neuropathy. Cells 2022, 11, 3062. [Google Scholar] [CrossRef]
- Kaneko, M.; Maeda, H.; Wang, M.; Zhou, W.; Frishman, L.J. Origins of Oscillatory Potentials in the Photopic Flash ERG of the Mouse. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2257. [Google Scholar]
- Tan, B.; MacLellan, B.; Mason, E.; Bizheva, K. Structural, Functional and Blood Perfusion Changes in the Rat Retina Associated with Elevated Intraocular Pressure, Measured Simultaneously with a Combined OCT+ERG System. PLoS ONE 2018, 13, e0193592. [Google Scholar] [CrossRef]
- Zhi, Z.; Cepurna, W.; Johnson, E.; Jayaram, H.; Morrison, J.; Wang, R.K. Evaluation of the Effect of Elevated Intraocular Pressure and Reduced Ocular Perfusion Pressure on Retinal Capillary Bed Filling and Total Retinal Blood Flow in Rats by OMAG/OCT. Microvasc. Res. 2015, 101, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; de Sevilla Müller, L.P.; Brecha, N.C. The RNA Binding Protein RBPMS Is a Selective Marker of Ganglion Cells in the Mammalian Retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef] [PubMed]
- Gusel’nikova, V.V.; Korzhevskiy, D.E. NeuN as a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Naturae 2015, 7, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.L.; Blumenfeld, Z.; Luebbert, L.; Knox, H.J.; Muthusamy, A.K.; Marvin, J.S.; Kim, C.H.; Grant, S.N.; Walton, D.P.; Cohen, B.N.; et al. Selective Serotonin Reuptake Inhibitors within Cells: Temporal Resolution in Cytoplasm, Endoplasmic Reticulum, and Membrane. J. Neurosci. 2023, 43, 2222–2241. [Google Scholar] [CrossRef]
- Smedowski, A.; Akhtar, S.; Liu, X.; Pietrucha-Dutczak, M.; Podracka, L.; Toropainen, E.; Alkanaan, A.; Ruponen, M.; Urtti, A.; Varjosalo, M.; et al. Electrical Synapses Interconnecting Axons Revealed in the Optic Nerve Head—A Novel Model of Gap Junctions’ Involvement in Optic Nerve Function. Acta Ophthalmol. 2019, 98, 408. [Google Scholar] [CrossRef]
- Ning, N.; Wen, Y.; Li, Y.; Li, J. Meclofenamic Acid Blocks the Gap Junction Communication between the Retinal Pigment Epithelial Cells. Hum. Exp. Toxicol. 2013, 32, 1164–1169. [Google Scholar] [CrossRef]
- Kazdin, A.E.; Wu, C.-S.; Hwang, I.; Puac-Polanco, V.; Sampson, N.A.; Al-Hamzawi, A.; Alonso, J.; Andrade, L.H.; Benjet, C.; Caldas-de-Almeida, J.-M.; et al. Antidepressant Use in Low- Middle- and High-Income Countries: A World Mental Health Surveys Report. Psychol. Med. 2023, 53, 1583–1591. [Google Scholar] [CrossRef]
- Sacre, S.; Medghalchi, M.; Gregory, B.; Brennan, F.; Williams, R. Fluoxetine and Citalopram Exhibit Potent Antiinflammatory Activity in Human and Murine Models of Rheumatoid Arthritis and Inhibit Toll-like Receptors. Arthritis Rheum. 2010, 62, 683–693. [Google Scholar] [CrossRef]
- Meikle, C.K.S.; Creeden, J.F.; McCullumsmith, C.; Worth, R.G. SSRIs: Applications in Inflammatory Lung Disease and Implications for COVID-19. Neuropsychopharmacol. Rep. 2021, 41, 325–335. [Google Scholar] [CrossRef]
- Stamoula, E.; Siafis, S.; Dardalas, I.; Ainatzoglou, A.; Matsas, A.; Athanasiadis, T.; Sardeli, C.; Stamoulas, K.; Papazisis, G. Antidepressants on Multiple Sclerosis: A Review of In Vitro and In Vivo Models. Front. Immunol. 2021, 12, 677879. [Google Scholar] [CrossRef]
- Ovlyakulov, B.; Hu, B.-L.; Kan, H.-Y.; Guo, Q.; Li, X.-F.; Fan, H.-H.; Wu, H.-M.; Wang, J.-Y.; Zhang, X.; Zhu, J.-H. Escitalopram Moderately Outperforms Citalopram towards Anti-Neuroinflammation and Neuroprotection in 6-Hydroxydopamine-Induced Mouse Model of Parkinson’s Disease. Int. Immunopharmacol. 2024, 139, 112715. [Google Scholar] [CrossRef]
- Shetty, S.; Hariharan, A.; Shirole, T.; Jagtap, A.G. Neuroprotective Potential of Escitalopram against Behavioral, Mitochondrial and Oxidative Dysfunction Induced by 3-Nitropropionic Acid. Ann. Neurosci. 2015, 22, 11–18. [Google Scholar] [CrossRef]
- Siepmann, T.; Penzlin, A.I.; Kepplinger, J.; Illigens, B.M.-W.; Weidner, K.; Reichmann, H.; Barlinn, K. Selective Serotonin Reuptake Inhibitors to Improve Outcome in Acute Ischemic Stroke: Possible Mechanisms and Clinical Evidence. Brain Behav. 2015, 5, e00373. [Google Scholar] [CrossRef]
- McCann, S.K.; Irvine, C.; Mead, G.E.; Sena, E.S.; Currie, G.L.; Egan, K.E.; Macleod, M.R.; Howells, D.W. Efficacy of Antidepressants in Animal Models of Ischemic Stroke: A Systematic Review and Meta-Analysis. Stroke 2014, 45, 3055–3063. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Trounce, I.A.; Crowston, J.G. Mechanisms of Retinal Ganglion Cell Injury in Aging and Glaucoma. Ophthalmic Res. 2010, 44, 173–178. [Google Scholar] [CrossRef]
- You, Y.; Gupta, V.K.; Li, J.C.; Klistorner, A.; Graham, S.L. Optic Neuropathies: Characteristic Features and Mechanisms of Retinal Ganglion Cell Loss. Rev. Neurosci. 2013, 24, 301–321. [Google Scholar] [CrossRef]
- Osborne, N.N.; Ugarte, M.; Chao, M.; Chidlow, G.; Bae, J.H.; Wood, J.P.; Nash, M.S. Neuroprotection in Relation to Retinal Ischemia and Relevance to Glaucoma. Surv. Ophthalmol. 1999, 43 (Suppl. 1), S102–S128. [Google Scholar] [CrossRef]
- Mohite, A.A.; Perais, J.A.; McCullough, P.; Lois, N. Retinal Ischaemia in Diabetic Retinopathy: Understanding and Overcoming a Therapeutic Challenge. J. Clin. Med. 2023, 12, 2406. [Google Scholar] [CrossRef]
- Stefánsson, E.; Geirsdóttir, A.; Sigurdsson, H. Metabolic Physiology in Age Related Macular Degeneration. Prog. Retin. Eye Res. 2011, 30, 72–80. [Google Scholar] [CrossRef]
- Terelak-Borys, B.; Skonieczna, K.; Grabska-Liberek, I. Ocular Ischemic Syndrome—A Systematic Review. Med. Sci. Monit. 2012, 18, RA138–RA144. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, Y.; Chen, Q.; Xu, J.; Sarunic, M.V.; Saragovi, U.H.; Zhuo, Y. Validation of Glaucoma-like Features in the Rat Episcleral Vein Cauterization Model. Chin. Med. J. 2014, 127, 359–364. [Google Scholar] [CrossRef]
- Danias, J.; Shen, F.; Kavalarakis, M.; Chen, B.; Goldblum, D.; Lee, K.; Zamora, M.-F.; Su, Y.; Brodie, S.E.; Podos, S.M.; et al. Characterization of Retinal Damage in the Episcleral Vein Cauterization Rat Glaucoma Model. Exp. Eye Res. 2006, 82, 219–228. [Google Scholar] [CrossRef]
- Zhi, Z.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Wang, R.K. Impact of Intraocular Pressure on Changes of Blood Flow in the Retina, Choroid, and Optic Nerve Head in Rats Investigated by Optical Microangiography. Biomed. Opt. Express 2012, 3, 2220–2233. [Google Scholar] [CrossRef]
- Gündüz, G.U.; Parmak Yener, N.; Kılınçel, O.; Gündüz, C. Effects of Selective Serotonin Reuptake Inhibitors on Intraocular Pressure and Anterior Segment Parameters in Open Angle Eyes. Cutan. Ocul. Toxicol. 2018, 37, 36–40. [Google Scholar] [CrossRef]
- Kergoat, H.; Hérard, M.-E.; Lemay, M. RGC Sensitivity to Mild Systemic Hypoxia. Invest. Ophthalmol. Vis. Sci. 2006, 47, 5423–5427. [Google Scholar] [CrossRef]
- Schmid, H.; Renner, M.; Dick, H.B.; Joachim, S.C. Loss of Inner Retinal Neurons after Retinal Ischemia in Rats. Invest. Ophthalmol. Vis. Sci. 2014, 55, 2777–2787. [Google Scholar] [CrossRef]
- Ames, A. 3rd Energy Requirements of CNS Cells as Related to Their Function and to Their Vulnerability to Ischemia: A Commentary Based on Studies on Retina. Can. J. Physiol. Pharmacol. 1992, 70, S158–S164. [Google Scholar] [CrossRef]
- He, Q.; Xiao, L.; Shi, Y.; Li, W.; Xin, X. Natural Products: Protective Effects against Ischemia-Induced Retinal Injury. Front. Pharmacol. 2023, 14, 1149708. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three Distinct Mechanisms Generate Oxygen Free Radicals in Neurons and Contribute to Cell Death during Anoxia and Reoxygenation. J. Neurosci. 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Wagner, N.; Reinehr, S.; Palmhof, M.; Schuschel, D.; Tsai, T.; Sommer, E.; Frank, V.; Stute, G.; Dick, H.B.; Joachim, S.C. Microglia Activation in Retinal Ischemia Triggers Cytokine and Toll-Like Receptor Response. J. Mol. Neurosci. 2021, 71, 527–544. [Google Scholar] [CrossRef]
- Hangai, M.; Yoshimura, N.; Hiroi, K.; Mandai, M.; Honda, Y. Inducible Nitric Oxide Synthase in Retinal Ischemia-Reperfusion Injury. Exp. Eye Res. 1996, 63, 501–509. [Google Scholar] [CrossRef]
- Kofod, J.; Elfving, B.; Nielsen, E.H.; Mors, O.; Köhler-Forsberg, O. Depression and Inflammation: Correlation between Changes in Inflammatory Markers with Antidepressant Response and Long-Term Prognosis. Eur. Neuropsychopharmacol. 2022, 54, 116–125. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, J.; Yao, W.; Ren, Q.; Yang, C.; Ma, M.; Han, M.; Saito, R.; Hashimoto, K. Effects of Escitalopram, R-Citalopram, and Reboxetine on Serum Levels of Tumor Necrosis Factor-α, Interleukin-10, and Depression-like Behavior in Mice after Lipopolysaccharide Administration. Pharmacol. Biochem. Behav. 2016, 144, 7–12. [Google Scholar] [CrossRef]
- Sokołowska, P.; Seweryn Karbownik, M.; Jóźwiak-Bębenista, M.; Dobielska, M.; Kowalczyk, E.; Wiktorowska-Owczarek, A. Antidepressant Mechanisms of Ketamine’s Action: NF-ΚB in the Spotlight. Biochem. Pharmacol. 2023, 218, 115918. [Google Scholar] [CrossRef]
- Li, J.-M.; Liu, L.-L.; Su, W.-J.; Wang, B.; Zhang, T.; Zhang, Y.; Jiang, C.-L. Ketamine May Exert Antidepressant Effects via Suppressing NLRP3 Inflammasome to Upregulate AMPA Receptors. Neuropharmacology 2019, 146, 149–153. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Morsy, S.M.Y.; Sleem, A.A. The Effect of Different Antidepressant Drugs on Oxidative Stress after Lipopolysaccharide Administration in Mice. EXCLI J. 2011, 10, 290–302. [Google Scholar]
- Crespi, F. The Selective Serotonin Reuptake Inhibitor Fluoxetine Reduces Striatal in vivo Levels of Voltammetric Nitric Oxide (NO): A Feature of Its Antidepressant Activity? Neurosci. Lett. 2010, 470, 95–99. [Google Scholar] [CrossRef]
- Halaris, A.; Myint, A.-M.; Savant, V.; Meresh, E.; Lim, E.; Guillemin, G.; Hoppensteadt, D.; Fareed, J.; Sinacore, J. Does Escitalopram Reduce Neurotoxicity in Major Depression? J. Psychiatr. Res. 2015, 66, 118–126. [Google Scholar] [CrossRef]
- Borsini, A.; Alboni, S.; Horowitz, M.A.; Tojo, L.M.; Cannazza, G.; Su, K.-P.; Pariante, C.M.; Zunszain, P.A. Rescue of IL-1β-Induced Reduction of Human Neurogenesis by Omega-3 Fatty Acids and Antidepressants. Brain Behav. Immun. 2017, 65, 230–238. [Google Scholar] [CrossRef]
- Kroeze, Y.; Zhou, H.; Homberg, J.R. The Genetics of Selective Serotonin Reuptake Inhibitors. Pharmacol. Ther. 2012, 136, 375–400. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, S.I.; Oh, J.H.; Kim, S.M.; Jeong, C.H.; Jun, J.A.; Lee, K.-S.; Oh, W.; Lee, J.-K.; Jeun, S.-S. Brain-Derived Neurotrophic Factor Stimulates the Neural Differentiation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Survival of Differentiated Cells through MAPK/ERK and PI3K/Akt-Dependent Signaling Pathways. J. Neurosci. Res. 2008, 86, 2168–2178. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, J.H.; Yoo, K.-Y.; Choi, J.H.; Hwang, I.K.; Ryu, P.D.; Kim, D.-H.; Kwon, Y.-G.; Kim, Y.-M.; Won, M.-H. Pre- and Post-Treatments with Escitalopram Protect against Experimental Ischemic Neuronal Damage via Regulation of BDNF Expression and Oxidative Stress. Exp. Neurol. 2011, 229, 450–459. [Google Scholar] [CrossRef]
- Dionisie, V.; Ciobanu, A.M.; Toma, V.A.; Manea, M.C.; Baldea, I.; Olteanu, D.; Sevastre-Berghian, A.; Clichici, S.; Manea, M.; Riga, S.; et al. Escitalopram Targets Oxidative Stress, Caspase-3, BDNF and MeCP2 in the Hippocampus and Frontal Cortex of a Rat Model of Depression Induced by Chronic Unpredictable Mild Stress. Int. J. Mol. Sci. 2021, 22, 7483. [Google Scholar] [CrossRef]
- Ivanova, E.; Yee, C.W.; Sagdullaev, B.T. Increased Phosphorylation of Cx36 Gap Junctions in the AII Amacrine Cells of RD Retina. Front. Cell. Neurosci. 2015, 9, 390. [Google Scholar] [CrossRef]
- Hosseinirezaabad, S.; Motaghi, S.; Dogani, M.; Teimouri, M. Antidepressant Effects of Fluoxetine: Upregulation of Connexin 36 and 43 in the Hippocampus, Prefrontal Cortex, and Amygdala. Mol. Biol. Rep. 2025, 52, 581. [Google Scholar] [CrossRef]
- Oguro, K.; Jover, T.; Tanaka, H.; Lin, Y.; Kojima, T.; Oguro, N.; Grooms, S.Y.; Bennett, M.V.; Zukin, R.S. Global Ischemia-Induced Increases in the Gap Junctional Proteins Connexin 32 (Cx32) and Cx36 in Hippocampus and Enhanced Vulnerability of Cx32 Knock-out Mice. J. Neurosci. 2001, 21, 7534–7542. [Google Scholar] [CrossRef]
- Moulard, M.; Cosker, E.; Angioi-Duprez, K.; Laprévote, V.; Schwan, R.; Schwitzer, T. Retinal Markers of Therapeutic Responses in Major Depressive Disorder: Effects of Antidepressants on Retinal Function. J. Psychiatr. Res. 2022, 154, 71–79. [Google Scholar] [CrossRef]
- de Deus, M.; Petit, C.; Schwitzer, T. ElectroRetinoGraphy toward an Exploration of the Therapeutic Potential of Antidepressants in Patients with Major Depressive Disorder: A Scoping Review of the Literature. Neurosci. Biobehav. Rev. 2024, 164, 105833. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wen, Y.; Ning, N.; An, J.; Li, J. Retinal Toxicity Associated with High Dose of Meclofenamic Acid. Drug Chem. Toxicol. 2013, 36, 461–465. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machowicz, J.; Mróz, K.; Pacwa, A.; Gąsiorek, A.; Rodak, P.; Lewin-Kowalik, J.; Amadio, M.; Smędowski, A. Serotonin Signaling Pathway Modulation Affects Retinal Neuron Survival in Experimental Model of Retinal Ischemia. Life 2025, 15, 1726. https://doi.org/10.3390/life15111726
Machowicz J, Mróz K, Pacwa A, Gąsiorek A, Rodak P, Lewin-Kowalik J, Amadio M, Smędowski A. Serotonin Signaling Pathway Modulation Affects Retinal Neuron Survival in Experimental Model of Retinal Ischemia. Life. 2025; 15(11):1726. https://doi.org/10.3390/life15111726
Chicago/Turabian StyleMachowicz, Joanna, Klaudia Mróz, Anna Pacwa, Anna Gąsiorek, Piotr Rodak, Joanna Lewin-Kowalik, Marialaura Amadio, and Adrian Smędowski. 2025. "Serotonin Signaling Pathway Modulation Affects Retinal Neuron Survival in Experimental Model of Retinal Ischemia" Life 15, no. 11: 1726. https://doi.org/10.3390/life15111726
APA StyleMachowicz, J., Mróz, K., Pacwa, A., Gąsiorek, A., Rodak, P., Lewin-Kowalik, J., Amadio, M., & Smędowski, A. (2025). Serotonin Signaling Pathway Modulation Affects Retinal Neuron Survival in Experimental Model of Retinal Ischemia. Life, 15(11), 1726. https://doi.org/10.3390/life15111726






