Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) and atrial fibrillation (AF) are two highly prevalent conditions that share overlapping cardiometabolic risk factors, including obesity, type 2 diabetes, hypertension, and dyslipidemia. Growing evidence suggests that these two disease entities are pathophysiologically linked through systemic inflammation, oxidative stress, and structural remodeling. Population-based studies and meta-analyses report an association between steatotic liver disease and both incident and recurrent AF. While several analyses observe persistence of this association after adjustment for cardiometabolic risk factors, residual confounding and limitations of observational designs preclude firm causal inference. Conversely, heart rhythm disturbances may exacerbate hepatic fibrosis and dysfunction. Lifestyle interventions—particularly sustained weight loss—have demonstrated significant benefits in both conditions. Emerging pharmacological options, including incretin mimetics, flozins, statins, and thiazolidinediones, show promise in addressing the liver–heart axis, while appropriate anticoagulation remains essential in AF management. This review summarizes current epidemiological data, mechanistic insights, diagnostic approaches, and therapeutic strategies related to the coexistence of MASLD and AF. Emphasis is placed on shared pathogenic pathways, non-invasive diagnostic tools, and integrated management options.
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic liver disease associated with metabolic syndrome and represents a threat to global and public health due to its complications. MASLD is the most common liver impairment in the general population and, furthermore, the leading cause of mortality and morbidity related to liver diseases [1]. MASLD is a fatty liver condition characterized by the presence of one or more cardiometabolic risk factors but without harmful effects exerted by alcohol intake; consequently, these individuals face a higher risk of cardiovascular disease (CVD) [2]. Recently, MASLD has replaced the term non-alcoholic fatty liver disease (NAFLD), which was used as previous name of this pathological condition [3]. To maintain consistent terminology, this review utilizes the MASLD term, with exception for studies and trials based on legacy cohorts, where the NAFLD term was retained. The prevalence of MASLD is steadily increasing, with an estimated 30% of the world’s adult population currently affected [1]. Moreover, around 75% of individuals with excess body weight develop MASLD, linking its prevalence to global obesity trends [4]. The pathogenesis of MASLD is complex. Among the risk factors of this condition are dyslipidemia, obesity, hypertension (HA), insulin resistance (IR), and hypertriglyceridemia [5,6]. The development of MASLD and its progression to metabolic-associated steatohepatitis (MASH) can be explained by the interplay of lipid metabolism pathways [7]. Its complications encompass MASH, cirrhosis, and hepatocellular carcinoma [8,9]. Moreover, MASLD increases cardiovascular risk and the associated consequences [10]. Indeed, CVD incidence is elevated among MASLD patients, and epidemiological statistics acknowledge it as the leading cause of mortality in this group [11,12]. Conversely, some studies have suggested that MASLD per se does not increase mortality risk in individuals with cardiometabolic risk factors, except among those with higher alcohol consumption [13].
Atrial fibrillation (AF) is a highly prevalent age-related cardiac arrhythmia, and its occurrence is associated with increased morbidity and mortality [14]. According to the 2024 ESC Guidelines, AF is one of the most common heart rhythm disorders. Mechanistically, this disease is characterized by disorganized electrical impulses in the atria, which result in ineffective and rapid atrial contractions [15,16]. As a supraventricular arrhythmia with uncoordinated atrial activation, AF results in a loss of effective atrial contraction. The distinctive features of surface electrocardiogram (ECG) in this condition include the absence of discernible and regular P waves as well as irregular activation of the ventricles. This results in no specific pattern to RR intervals in the absence of an atrioventricular block [15,16]. The irregular rhythm generated in AF leads to disrupted and turbulent blood flow within the heart, creating favorable conditions for the formation of thrombi (blood clots). These clots may subsequently embolize and result in a stroke. AF is recognized as the primary cardiac-related cause of ischemic stroke. AF is also a major risk factor for other forms of CVD, significantly elevating the odds of heart failure (HF), sudden cardiac death, and overall cardiovascular mortality [17]. Current practice guidelines identify modifiable risk factors for AF as obesity, HA, sedentary lifestyle, sleep apnea, smoking, IR, and type 2 diabetes mellitus (T2DM). As referenced above, similar conditions also contribute to the development of MASLD. Moreover, AF is characterized by disorganized atrial electrical activity, which leads to persistent hemodynamic fluctuations and structural remodeling of the heart; these processes may be exacerbated by metabolic dysfunction, such as MASLD [17,18].
Given the increasing burden of MASLD and the shared risk factors with AF, this study aims to characterize the coexistence of MASLD and AF from a clinical perspective, focusing on epidemiological patterns, diagnostic pathways, and multidisciplinary treatment options, considering the benefits and risks of drug use. The overarching aim is to translate these insights into actionable clinical recommendations.
2. Epidemiological Data on Coexistence of MASLD and AF
2.1. Risk of AF Development in MASLD-Affected Individuals
MASLD is currently estimated to affect approximately 38% of the global population [19,20]. Worldwide MASLD prevalence has risen by over 50% in the last three decades, increasing from 25.3% (1990–2006) to 38.0% (2016–2019) according to a recent systematic review, highlighting MASLD’s expanding public health burden and the need for proactive clinical and research responses [21]. The global rise in MASLD occurrence mirrors trends in obesity and T2DM, underscoring their shared metabolic origins [19,22].
The global prevalence of AF has risen significantly over the past 30 years, now affecting an estimated 60 million people worldwide. The lifetime risk of AF is around 33%, with individual estimates influenced by factors such as age, sex, race, and the presence of clinical risk factors [23]. In a prospective multicenter cohort study including patients with nonvalvular AF, NAFLD was diagnosed in 732 of 1735 (42.2%) participants. Patients with NAFLD were younger, less frequently women, and more likely to be treated with non-vitamin K oral anticoagulants (NOACs) or to have obesity, dyslipidemia, and persistent/permanent AF [24]. In a similar analysis, subjects diagnosed with MASLD were more likely than controls to present with T2DM, obesity, HA, dyslipidemia, and chronic kidney disease (CKD). Coexistence of T2DM markedly amplified the risk of cardiovascular events, malignancy, and liver-related complications in individuals with MASLD [25]. Moreover, they exhibited a modest but statistically significant increase in the incidence of AF compared with controls (difference: 0.9 per 1000 person-years) [26]. Another assessment revealed that among patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH), the prevalence of AF was roughly double that of the general population [27]. Screening of 6293 individuals, suffering from NAFLD, allowed the detection AF in 59 patients (0.9%). Compared with those without AF, affected subjects were significantly older (64.6 vs. 52.0 years, p < 0.001), had a higher body mass index (BMI) (26.6 vs. 25.2 kg/m2, p < 0.001), and a larger waist circumference (89.9 vs. 84.0 cm, p < 0.001). Notably, AF was independently associated with advanced liver fibrosis in this cohort. Using the NAFLD fibrosis score (NFS), AF remained a significant predictor in both the low cut-off value (COV) group (adjusted odds ratio (aOR) = 2.85, p = 0.004) and high-COV group (aOR = 12.29, p < 0.001). Similarly, when assessed by the fibrosis-4 (FIB-4) index, AF was independently associated with advanced fibrosis in both the low-COV (aOR = 2.49, p < 0.001) and high-COV groups (aOR = 3.84, p = 0.016). These findings highlight a significant correlation between AF and fibrosis severity in patients with MASLD [17]. Kim et al. in a nationwide population-based study showed that over a median follow-up of 9.6 years, AF was newly diagnosed in 5335 participants, corresponding to an incidence rate of 2.74 per 1000 person-years. The risk of AF was significantly elevated among patients with MASLD who abstained from alcohol (adjusted hazard ratio (aHR) 1.32, p < 0.001), as well as in those with MASLD and concomitant alcohol consumption (aHR 1.48, p < 0.001). Notably, when compared with all other alcohol consumers regardless of steatotic liver disease status, nondrinking patients with MASLD demonstrated a persistently higher risk of AF (aHR 1.11, p = 0.011) [27]. Cho et al. presented a nationwide study, in which participants with MASLD exhibited an elevated risk of new-onset AF (aHR 1.10). Those with MASLD combined with additional metabolic or clinical comorbidities demonstrated an even higher risk of AF development (aHR 1.22) [28]. Consistent findings come from meta-analyses of large cohort studies. Evaluation of nine cross-sectional and longitudinal studies, including over 360,000 subjects revealed a strong correlation: NAFLD doubles the odds of prevalent AF (OR ~2.07) [29]. An even larger examination, comprising data gathered from 18 million participants, reported a risk ratio (RR) of 1.42 for AF, HF, stroke, and cardiovascular mortality among NAFLD patients [30]. A meta-analysis conducted by Mantovani et al. in 2024, after identifying 16 retrospective cohort studies with aggregate data on ~19.5 million individuals followed for a median of 7.2 years, showed that MASLD was significantly associated with increased odds of developing incident AF (random-effects hazard ratio (HRRE) 1.20). This correlation did not appear to increase further with the severity of liver fibrosis. The risk of AF remained significant even after adjusting for age, sex, BMI, HA, T2DM, or other cardiometabolic risk factors. Sensitivity analyses did not modify these findings. However, the collective data regarding this particular association come from observational studies, with important limitations resulting from their design as well as heterogeneity of examined populations. Therefore, the observations that MASLD exacerbates AF risk independently from fundamental cardiometabolic factors shall be taken with caution [31]. Moreover, in one study, the presence of MASLD also significantly increased the risk of AF recurrence after cryoballoon ablation, underscoring the importance of targeted interventions for MASLD in the periprocedural management of AF [32].
2.2. Controversies and Evidence Appraisal
Across cohorts, effect estimates vary by disease definitions (NAFLD vs. MASLD), exposure ascertainment (enzymes vs. imaging vs. histology), and AF endpoints (incident vs. prevalent vs. post-ablation recurrence). Associations typically attenuate with broader adjustment for adiposity and diabetes, and residual confounding and reverse causality remain possible. Prior meta-analyses differ in inclusion criteria and adjustment sets; a side-by-side summary is provided in Table 1. Collectively, current data support an association but not definitive independence or causality.

Table 1.
Comparison of methodological, populational and outcome differences in studies assessing coexistence of MASLD and AF.
Across meta-analyses [30,31], MASLD/NAFLD is associated with a modestly increased risk of incident AF (11–20% after full adjustment), converging with biopsy-based evidence [25,26]. Estimates diverge in international classification of diseases (ICD)-only cohorts or when AF is not a primary endpoint. Adjustments for adiposity, T2DM, and HA attenuate, but do not abolish, the association; fibrosis severity yields higher risks in some cohorts (e.g., cirrhosis), while ablation populations show stronger effects for recurrence [32].
3. Pathophysiological Mechanisms Contributing to Development and Progression of MASLD and AF
Several population-based cohort studies, which utilized mildly elevated serum liver enzyme levels as surrogate indicators of MASLD, a method that warrants cautious interpretation, demonstrated that this disorder was independently associated with an increased long-term risk of developing AF [34]. Moreover, advanced MASLD significantly contributed to occurrence of AF compared with no-MASLD [35]. Concurrent MASLD and AF independently and additively elevate cardiovascular risk. Moreover, AF in MASLD patients is linked to an increased incidence of HF, stroke, and CVD-associated death [36]. CVD, including AF and HF, is the leading cause of death in patients with MASLD, followed sequentially by extrahepatic malignancies and complications directly related to hepatic dysfunction [11,13,37,38,39]. This section summarizes data regarding pathophysiological pathways and molecular alterations that promote development and progression of MASLD and AF. Most essential pathophysiological and biochemical mechanisms linking development of MASLD and AF have been summarized in Figure 1.
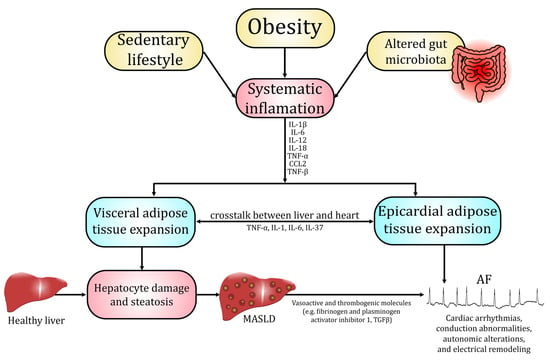
Figure 1.
Pathophysiological and biochemical mechanisms linking MASLD and AF.
3.1. Inflammatory and Oxidative-Stress Pathways
The development of MASLD is believed to be influenced by a range of interconnected physiological disturbances, with chronic systemic inflammation and oxidative stress as key factors. Excessive lipid accumulation in hepatocytes enhances mitochondrial β-oxidation and cytochrome P450 activity, leading to the overproduction of ROS, which overwhelm antioxidant defense systems such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) [6,40]. The ensuing oxidative imbalance further induces lipid peroxidation, protein oxidation, and mitochondrial dysfunction, activating stress-responsive pathways including nuclear factor kappa β (NF-κβ) and c-Jun N-terminal kinase (JNK) that promote the transcription of tumor necrosis factor α (TNF-α), interleukin (IL)-6, and IL-1β [41,42]. These cytokines amplify hepatic inflammation by recruiting immune cells and stimulating Kupffer cell activation, while simultaneously triggering hepatic stellate cell activation and fibrogenesis [41]. Antioxidant dysregulation—particularly impairment of the nuclear factor erythroid 2-related factor 2 (Nrf2)–GPX4 axis—further exacerbates oxidative injury and lipid peroxidation, contributing to hepatocellular damage and disease progression [6,40]. Persistent oxidative and inflammatory signaling thus drives the transition from simple steatosis to MASH, ultimately predisposing to cirrhosis and hepatocellular carcinoma [6,41,43]. Oxidative stress and chronic, low-grade inflammation in MASLD not only drive hepatic injury but also generate systemic mediators that promote atrial structural and electrical remodeling conducive to AF [44,45]. Circulating oxidative byproducts and impaired systemic antioxidant capacity exert unfavorable alterations in the myocardium and provoke atrial myocyte oxidative damage, mitochondrial dysfunction, and abnormal Ca2+ handling—all established triggers of AF [44,45]. Concurrently cytokines such as IL-1β, IL-6, and TNF-α induce endocrine and paracrine effects on the heart, which result in atrial fibroblast activation and extracellular matrix deposition. Induction of the NLR family pyrin domain containing 3 (NLRP3)/caspase-1 axis also contributes directly to cardiomyocyte electrical instability by altering sarcoplasmic reticulum Ca2+ release and promoting spontaneous discharges [46,47,48].
3.2. Adipose Tissue and Epicardial Fat Signaling
Dysfunctional white adipose tissue acts as an endocrine organ that drives MASLD progression through several coordinated alterations. Hypertrophic adipocytes and insulin resistance increase lipolysis and the release of free fatty acids to the liver. Simultaneously, an altered secretome (decreased hepatoprotective adiponectin and omentin and increased pro-inflammatory adipokines such as leptin, resistin, chemerin, visfatin, and elevated chemokines (mostly monocyte chemoattractant protein 1 (MCP-1))) promotes hepatic steatosis, further impairs sensitivity to insulin, as well as induces Kupffer cell activation and fibrogenesis [49,50,51,52]. Chronic adipose inflammation with macrophage infiltration and increased lipolytic flux not only supplies hepatotoxic lipid species (diacylglycerols, ceramides) but also reduces systemic antioxidant capacity and raises circulating cytokines (IL-6, TNF-α, IL-1β) that exacerbate dysfunction of hepatic tissue [53,54]. These adipose-derived pathways extend to the heart through visceral and ectopic depots, most notably epicardial adipose tissue (EAT), which—by virtue of its anatomic contiguity and paracrine secretory activity—conveys adipokines, cytokines, free fatty acids, and extracellular vesicles directly to the atrial myocardium [55,56]. In obese and metabolically dysfunctional states, EAT becomes inflamed and profibrotic (higher production of local leptin, chemerin, IL-6, IL-1β, transforming growth factor β (TGF-β), and matrix-remodeling proteases), driving atrial fibroblast activation, extracellular-matrix (ECM) deposition, and fibrosis [55,57,58]. It also perturbs cardiomyocyte electrophysiology through oxidative stress, altered Ca2+ handling, and ion-channel modulation, thereby increasing atrial ectopy and promoting the development of AF [55,56,57]. Clinical and imaging studies show that increased EAT volume and altered EAT phenotype correlate with MASLD severity and with higher incidence and recurrence of AF after ablation, supporting a liver/adipose tissue/heart axis in which adipose endocrine dysfunction links MASLD pathobiology to arrhythmogenesis [50,56,58].
3.3. Fibrosis and Structural Remodeling (Atrial Cardiomiopathy)
Progression of MASLD to advanced fibrosis and cirrhosis is driven by a sustained wound-healing response in which chronic hepatocellular injury, inflammation, and metabolic stress activate hepatic stellate cells and portal fibroblasts to produce excess ECM components, principally fibrillar collagens (type I and III), fibronectin, and proteoglycans, while the balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases inhibitors (TIMPs) shifts toward decreased matrix degradation, causing ECM accumulation and increased tissue stiffness [59,60,61]. This profibrotic environment is orchestrated by paracrine mediators (notably TGF-β and platelet-derived growth factor (PDGF)), macrophage-derived signals, and hepatocyte senescence/damage-associated molecular pattern (DAMP) signaling that sustain hepatic stellate cell activation and myofibroblast transdifferentiation [59,60]. Concurrently, altered ECM composition and crosslinking, including the upregulation of lipoxygenase (LOX) family enzymes and galectin-3–associated pathways, create a mechanically and biochemically abnormal niche that reinforces fibrogenesis and impairs liver function [59,60,62]. The same systemic and paracrine profibrotic mechanisms that remodel the liver can promote structural remodeling of the heart and contribute to atrial cardiomyopathy [63,64]. Chronic hepatic fibrosis is associated with persistent systemic inflammation and elevated circulating profibrotic mediators (TGF-β, galectin-3) and ECM fragments that can reach the myocardium, where they promote fibroblast activation and shift cardiac MMP/TIMP balance toward ECM deposition. In the atria, this leads to interstitial fibrosis, conduction heterogeneity, and impaired contractile function—hallmarks of atrial cardiomyopathy [44,60,62,63,64]. These alterations combined strongly contribute to development and aggravation of AF. Recent mechanistic and clinical studies link higher liver fibrosis burden or fibrosis biomarkers with greater prevalence and incidence of AF as well as with poorer outcomes of this disease entity [63,64,65]. This conceptual integration reframes AF not merely as an electrical disorder but as a manifestation of multisystem fibrotic remodeling consistent with the atrial cardiomyopathy paradigm [63,64].
3.4. Genetic and Molecular Mediators
Genetic variation and molecular regulatory mechanisms are major determinants of the susceptibility to and progression of MASLD. Common single-nucleotide polymorphisms in genes controlling hepatic lipid and retinol metabolism, notably PNPLA3, TM6SF2, GCKR, and MBOAT7, increase fat retention in liver, promote lipotoxicity, and accelerate inflammation-driven fibrosis. Loss-of-function variants in HSD17B13 are protective against progression to steatohepatitis and fibrosis [66,67,68]. Large genome-wide association studies (GWASs) and multi-omics studies further show that these loci influence hepatic gene expression, lipid and retinoid pathways, as well as mitochondrial/metabolic networks that determine whether simple steatosis progresses to inflammatory, fibrotic disease [66,68]. Beyond inherited variation, epigenetic remodeling (DNA methylation, histone modifications) and noncoding RNAs—especially extracellular-vesicle–packaged miRNAs that regulate inflammation, insulin signaling, and fibrogenic programs—act as dynamic molecular mediators linking environmental exposures (diet, obesity) to MASLD phenotypes [69,70]. These genetic and molecular mechanisms plausibly increase the risk of AF through shared downstream biology rather than by simple gene-for-gene causation. The PNPLA3/TM6SF2-driven propensity to hepatic lipid accumulation, mitochondrial dysfunction, and inflammation promotes systemic metabolic derangement, chronic low-grade inflammation, and profibrotic circulating mediators, including TGF-β and ECM fragments, which can reach the myocardium and foster atrial fibrosis and electrical remodeling—core components of atrial cardiomyopathy [64]. Observational cohorts show that MASLD severity predicts a higher incidence of new-onset AF [44]. By contrast, GWAS of AF have identified largely distinct cardiac loci, primarily involving genes related to ion-channel regulation (PITX2, KCNN3), cardiac structural proteins (TTN, MYH6), and transcriptional networks of atrial development [71]. Importantly, variants referenced above, strongly associated with hepatic lipid metabolism and MASLD susceptibility, do not significantly overlap with these AF risk loci, indicating that the genetic architecture of AF is primarily cardiac, whereas MASLD increases AF risk through systemic metabolic and inflammatory pathways rather than direct shared heritability [71,72].
4. Diagnostic Measures for Detection of MASLD and AF
The gold standard for diagnosing MASLD remains liver biopsy, which enables detection of increased fat accumulation within hepatic tissue. Results of histopathological assessment combined with the presence of at least one of five cardiometabolic risk factors—obesity, hyperglycemia, HA, hypertriglyceridemia, or hypoalphalipoproteinemia—and ruling out excessive consumption of alcohol and other chronic liver diseases allow the fulfillment of the diagnostic criteria and confirmation of the presence of this metabolic disorder [73]. However, due to biopsy’s invasive nature and associated discomfort, it is often not well tolerated by patients. As a result, non-invasive imaging techniques such as magnetic resonance imaging–proton density fat fraction (MRI-PDFF) and transient elastography (TE) have become widely utilized for the detection of hepatic steatosis and steatohepatitis. Among these, TE is gaining prominence owing to its high sensitivity and specificity in identifying MASLD, coupled with a high rate of patient acceptance. Diagnosis based on combination of biopsy and radiological imaging is limited in availability and costly [74]. To address this challenge, researchers have introduced a simple, non-invasive testing (NIT) approach that relies on biochemical blood tests and scoring systems [65]. FIB-4 index, calculated from aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet count, and patient age has emerged as a practical first-line tool in primary care for identifying advanced fibrosis in individuals with MASLD. Its simplicity, low cost, and ability to stratify risk make it a gateway test that can determine which patients require more specialized evaluation [75]. Another relatively simple and therefore widely used blood-based test is NFS. It allows the estimation of the likelihood of advanced fibrosis using parameters like BMI, fasting blood glucose, and liver enzymes. Recently AST/platelet ratio index (APRI), initially employed for detecting advanced fibrosis in patients with chronic hepatitis C virus, has also become more commonly used among individuals with MASLD. However, it should be noted that its usefulness as a standalone diagnostic measure is limited, and it is considered unsuitable for identifying significant fibrosis alone. A more complex and advanced approach utilizes the enhanced liver fibrosis (ELF) test. This diagnostic tool estimates the severity of liver fibrosis by measuring hyaluronic acid, procollagen III amino-terminal peptide (PIIINP), and TIMP-1—serum markers of changes occurring in the extracellular matrix during the fibrogenesis process. Other blood-derived indicators of fibrosis progression are type IV collagen 7s domain and Mac-2 binding protein glycosylation isomer (M2BPGi), although they are not a part of the ELF test panel [75,76,77]. Apart from scoring systems developed to improve the diagnostic process, there are several additional biomarkers that may serve as accessory factors highlighting deterioration of organism’s metabolic condition and therefore, progression of liver disease. Among the most important ones are the triglyceride–glucose index (TyG), triglyceride–glucose–body mass index (TyG-BMI), atherogenic index of plasma (AIP), non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR), uric acid to albumin ratio (UAR), and uric acid to high-density lipoprotein cholesterol ratio (UHR) [65]. The monocyte-to-high-density lipoprotein cholesterol ratio (MHR), red cell distribution width-to-albumin ratio (RAR), neutrophil-to-lymphocyte Ratio (NLR), systemic inflammatory response index (SIRI), neutrophil-to-platelet ratio (NPAR), and prognostic nutritional index (PNI) are emerging biomarkers that reflect both inflammatory activity and nutritional status [78,79]. As excessive inflammatory activity and impaired carbohydrate or lipid metabolism negatively affect functionality of both liver and heart, many of the abovementioned markers and scoring systems may also be utilized as additional tools for predicting risk of cardiovascular events and disorders [65,80,81]. For instance, individuals with higher values achieved in FIB-4 or NFS indices are simultaneously at greater risk of developing AF and display higher all-cause mortality because of more frequent adverse outcomes like major bleeding or heart failure [80,81]. The fatty liver index (FLI) is the most well-validated tool for ruling in or ruling out hepatic steatosis in older adults and has also been shown to predict the incidence of AF [82]. Considering these findings, it may seem reasonable to screen patients with MASLD or other forms of metabolic disorders for the presence of different kinds of arrhythmias, especially AF.
In routine clinical practice, the diagnosis of AF does not pose a significant challenge, as it relies on simple and widely accessible methods, such as pulse palpation and ECG-based devices, including the standard 12-lead electrocardiogram and Holter monitoring (ranging from 24 h to a week or longer) [83]. Characteristic features of ECG in AF include lack of discernible and regular P waves and no specific pattern to RR intervals [16]. Figure 2 and Figure 3 present a diagnostic scheme for the detection of MASLD and AF as well as an overview of the most common diagnostic and therapeutic measures available in these disease entities.
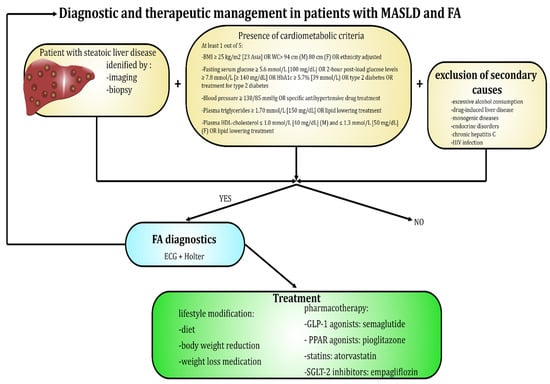
Figure 2.
Diagnostic and therapeutic options available in patients suffering from MASLD and AF.
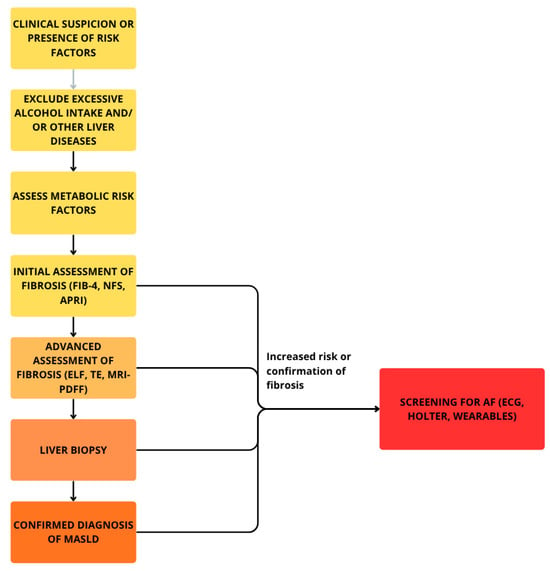
Figure 3.
Diagnostic scheme for detection of MASLD and accompanying AF.
5. Potential Therapeutic Strategies Targeting MASLD and Atrial Fibrillation
5.1. Interventions Affecting Lifestyle and Body Weight
Lifestyle interventions may provide a dual advantage, simultaneously attenuating the progression of MASLD while reducing the risk and burden of AF [84]. Sustained weight loss (~7–10%) improves steatosis, inflammation, and fibrosis [26]. Overall, the findings from Chenug et al. studies demonstrate significant positive associations between MASLD and AF, indicating that managing MASLD, traditionally considered a relatively benign condition, may play a preventive or therapeutic role in AF development. Despite some heterogeneity across studies, clinical trials consistently demonstrate that interventions focusing on weight loss are associated with improvement in hepatic biomarkers among individuals with MASLD in the short to intermediate term. However, data on long-term clinical outcomes remain limited. These findings underscore the importance of revising current clinical guidelines to incorporate structured weight management programs as a core component of MASLD management [85]. Lifestyle and risk factor modification (LRFM) has been increasingly recognized as a complementary pillar of AF management, standing alongside rhythm control, rate control, and anticoagulation [32]. On the subject of the AF, the Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort (LEGACY) study claims that sustained long-term weight reduction has been consistently linked to a marked decrease in AF burden and an improved likelihood of maintaining durable sinus rhythm [86]. Weight reduction achieved through a low-calorie diet and structured exercise has been shown to lower systemic inflammatory markers, including IL-6 and TNF-α [87]. In a meta-analysis of 116 studies, Askarpour et al. demonstrated that bariatric surgery in obese patients was associated with significant reduction in levels of inflammatory biomarkers, including CRP (p < 0.001), IL-6 (p < 0.001), and TNF-α (p = 0.031) [88]. As systemic inflammatory state is an important condition contributing to development and progression of both MASLD and AF, the interventions focused on its alleviation emerge as effective elements of wide-ranging prophylaxis and treatment in these two disease entities [87,88].
5.2. Pharmacotherapeutic Options—Where Are We Today?
Currently, no pharmacological agents have been approved specifically for the treatment of MASLD/NAFLD [82]. Various drugs are being investigated, including incretin mimetics, SGLT-2 inhibitors, pioglitazone, and statins. These agents may also confer benefits in the treatment or prevention of AF.
5.2.1. GLP-1 Agonists
Some of the available treatment options utilize the beneficial potential of incretins and the gut–liver axis [89]. Gut-associated signals encompass a broad spectrum of factors, including dietary nutrients, enteroendocrine hormones, bile acids, microbial metabolites, and antigens. These signals play a pivotal role in modulating immune responses and systemic metabolic processes, underscoring the gut’s function as a key integrative organ, regulating metabolism, and immunity [90]. Incretins, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are metabolic hormones released postprandially by specialized enteroendocrine cells within the gastrointestinal mucosa. These hormones play a crucial role in glycemic regulation by enhancing insulin secretion and suppressing glucagon release from pancreatic alpha cells. Additionally, they contribute to delayed gastric emptying and are thought to promote central glucose clearance, potentially leading to reduced appetite and food intake. Pharmacological agents that mimic incretin activity or combine actions of multiple incretins have demonstrated significant metabolic benefits, including improved glycemic control, enhanced insulin sensitivity, reduction in hepatic steatosis, and meaningful weight loss [91]. GLP-1 receptor agonists such as dulaglutide, exenatide, liraglutide, or semaglutide have been licensed for the treatment of T2DM and, more recently, obesity, two major risk factors in AF [92]. GLP-1 receptor agonists, approved for T2DM and obesity, were among the first agents tested in MASLD, with positive phase 2 results leading to phase 3 trials [36]. In the Liraglutide Safety and Efficacy in Patients with Non-alcoholic Steatohepatitis (LEAN) trial (NCT01237119), liraglutide achieved histological resolution of MASH without worsening fibrosis. These effects were accompanied by improvements in metabolic parameters, including enhanced insulin sensitivity and reduced lipotoxicity [93]. Similarly, semaglutide, an approved anti-diabetic agent, has shown promising MASH resolution but limited effects on fibrosis improvement [91]. GLP-1 receptor agonists reduce AF risk by stabilizing glucose metabolism, decreasing epicardial fat, controlling weight, regulating calcium levels, lowering oxidative stress, and modulating blood pressure. Weight loss is mediated via brown adipose tissue thermogenesis and neuropeptide Y (NPY)/agouti-related protein (AgRP) signaling, while oxidative stress is mitigated through connective tissue growth factor (CTGF) suppression and advanced glycation end products (AGE)/receptor of advanced glycation end products (RAGE) inhibition [94]. Meta-analysis of 10 randomized clinical trials demonstrated that semaglutide significantly reduced the incidence of AF by 42% compared with placebo in individuals at high cardiovascular risk, predominantly those with T2DM. This effect was consistent regardless of the drug’s route of administration (oral or subcutaneous), the presence of T2DM, or BMI [95]. Utilization of GLP-1 receptor agonist was also associated with a reduced risk of AF recurrence in patients undergoing AF ablation [96]. Similar results, regarding reduced risk of AF, were also noted in a matched cohort of 14,566 GLP-1 receptor agonists (RAs) and dipeptidyl peptidase 4 (DPP-4) inhibitor pairs, followed for a median of 3.8 years. However, the protective effect was significant only for participants receiving GLP-1RA [97]. Table 2 presents summarized results from studies regarding GLP-1RA effects in MASLD and AF.

Table 2.
Summarized results of studies regarding GLP-1 receptor agonists effects in MASLD and AF.
5.2.2. PPAR Agonists—Thiazolidinediones
In a randomized, double-blind, placebo-controlled trial investigating the effects of pioglitazone, patients were assigned to receive either pioglitazone or a placebo over a 6-month period. Liver biopsies were conducted both before and after treatment to assess histological changes. The study included 26 patients treated with pioglitazone (mean age 51 ± 7 years; 53.8% men) and 21 patients receiving a placebo (mean age 51 ± 10 years; 33% men). Compared with placebo, the pioglitazone group demonstrated significant improvements in key liver histological features, including steatosis (p = 0.003), ballooning necrosis (p = 0.02), and inflammation (p = 0.008). These findings suggest that pioglitazone may effectively reduce liver injury and inflammation in affected patients [98]. A meta-analysis of four randomized controlled trials, across three continents, demonstrated that pioglitazone significantly improved liver fibrosis in patients with NASH compared w controls (OR 1.7; 95% CI, 1.0–2.8). Moreover, thiazolidinediones, in general, markedly alleviated ballooning degeneration, lobular inflammation, steatosis, and combined necroinflammation in patients with NASH [99]. A meta-analysis by Zhang et al., including three randomized controlled trials (RCTs) and four observational studies with 130,854 patients, found that thiazolidinedione therapy was associated with a 30% lower risk of AF compared with controls (OR 0.73; 95% CI, 0.62–0.87) [100]. In a prospective observational cohort of 150 patients with drug-refractory paroxysmal atrial fibrillation (PAF) and T2DM undergoing catheter ablation, pioglitazone was associated with improved maintenance of sinus rhythm and a lower rate of repeat ablation [101]. Mechanistic explanation for pioglitazone-induced protection against AF comes from an in vivo study, which demonstrated that this drug significantly reversed β1-Aab-induced AF susceptibility, improved atrial structural remodeling, reduced systemic IR, and enhanced the expression of key glycolipid transport proteins: glucose transporter 1 (GLUT1), CD36, and carnitine palmitoyltransferase Ia (CPT1a). These findings suggest that pioglitazone effectively reduces AF vulnerability by restoring atrial metabolism and mitigating mitochondrial damage [102]. Long-term treatment with pioglitazone has been shown to be both effective and generally well tolerated in patients with NASH who also have prediabetes or T2DM, offering sustained improvements in liver histology and metabolic parameters such as reduced hepatic triglyceride content, improvement in individual histological scores, including the fibrosis score, and upregulation of insulin sensitivity in adipose tissue, liver, and skeletal muscles [103]. However, pioglitazone is associated with several adverse effects, including hypoglycemia, weight gain, fluid retention, increased risk of skeletal fractures, potential hepatotoxicity, concerns about bladder cancer, and an elevated risk of atherosclerotic cardiovascular events or HF [37]. Table 3 presents summarized results from studies regarding thiazolidinediones effects in MASLD and AF.

Table 3.
Summarized results of studies regarding thiazolidinediones effect in MASLD and AF.
5.2.3. Statins
Statins are a class of drugs known as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, which work by blocking the enzyme responsible for cholesterol synthesis in the liver, effectively lowering blood cholesterol levels and reducing the risk of CVD [104]. Off-label applications of this group of medications include MASLD. As treatment with statins decreases also the hepatic production of apolipoprotein B-100 (apoB-100) containing lipoproteins, which results in downregulation of both cholesterol and triglyceride concentrations, they are successful in counteracting excessive accumulation of triglycerides in the liver—a feature characteristic for MASLD [37]. In a retrospective cohort study of 1238 patients with MASLD (mean age 53.7 ± 14.2 years; 63.8% men), disease severity was evaluated using the FIB-4 score. Over a follow-up period of 3.3 years on average, statin therapy was introduced in nearly half of the patients (47%), while 18% progressed to a high fibrosis risk category. Cox regression analysis revealed that statin exposure (estimated with statin prescription intensity) was significantly associated with a reduced risk of advancing to a high-risk FIB-4 category. Moderate exposure to statins showed a hazard ratio (HR) of 0.60 (95% CI: 0.42–0.84) and high exposure to statins revealed an HR of 0.61 (95% CI: 0.42–0.88). These associations remained robust even after adjusting for multiple confounders, including sex, race, marital status, smoking, BMI, HA, T2DM, cardiovascular disease, hypothyroidism, and CKD [105]. Complementing these findings, a larger cohort study, involving 7988 patients (mean age 53.0 ± 13.7 years; 41.8% men) across 16 tertiary referral centers, demonstrated that statin use correlated with a substantially lower long-term risk of all-cause mortality (aHR: 0.233; 95% CI: 0.127–0.426) as well as reduced progression of liver stiffness (HR: 0.542; 95% CI: 0.389–0.755) [106]. Statins, widely used for metabolic syndrome, have also demonstrated beneficial effects in AF [87]. A trial involving 105 patients, supported by a meta-analysis of 13 studies, demonstrated that atorvastatin is highly effective in reducing severity of oxidative stress, concentrations of inflammatory markers: serum high-sensitivity C-reactive protein (hs-CRP) and total cholesterol (TC) levels, as well as lowering the incidence of AF [107]. Table 4 presents summarized results from studies regarding statins effects in MASLD and AF.

Table 4.
Summarized results from studies regarding statins effects in MASLD and AF.
5.2.4. SGLT-2 Inhibitors
Sodium–glucose cotransporter 2 (SGLT-2) inhibitors are a class of antihyperglycemic agents that lower blood glucose by selectively blocking renal glucose reabsorption, thereby enhancing urinary glucose excretion [108]. Representatives of this class of medications, including dapagliflozin and empagliflozin, are well established for their cardiometabolic benefits. Emerging data hint at a possible inhibitory effect on the accumulation of hepatic fat content; however, this reduction appears limited, and its influence on progression of hepatic fibrosis remains an open question [109]. Alongside empagliflozin, dapagliflozin has been shown to improve hepatic steatosis and reduce serum levels of ALT and γ-glutamyltranspeptidase (GGT). Notably, its effects appear more pronounced in patients with advanced fibrosis, where it may also contribute to attenuation of such state, suggesting a potential dual benefit in both metabolic and structural liver parameters [110]. Furthermore, dapagliflozin has been associated with a significant promotion of weight loss and improved glycemic control, underscoring its multifaceted metabolic benefits [111]. A meta-analysis of 31 randomized controlled trials demonstrated a 25% relative risk reduction in serious AF events among patients treated with SGLT-2 inhibitors compared with placebo or other glucose-lowering agents [108]. In the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial, which enrolled 17,160 high-risk patients with T2DM, dapagliflozin reduced the incidence of reported AF and atrial flutter events by 19%. This effect was consistent irrespective of prior AF, atherosclerotic CVD, or HF status [112]. Findings regarding such outcomes were further supported by a meta-analysis of 39 randomized controlled trials including 107,770 participants, which also confirmed that SGLT-2 inhibitors significantly reduced the risk of AF and atrial flutter compared with placebo (RR 0.86; 95% CI, 0.77–0.95; I2 = 0%; p = 0.003). Moreover, it revealed that no significant difference was observed between groups receiving high- and low-dose SGLT-2 inhibitors (RR 0.78; 95% CI, 0.60–1.02; I2 = 0%; p = 0.07) [113]. This class of drugs can also ameliorate outcomes of invasive treatment measures of AF, as a prospective study conducted in China, supported by a meta-analysis, demonstrated that use of SGLT-2 inhibitors led to reduced risk of AF recurrence in patients with diabetes following ablation [114]. Although SGLT-2 inhibitors are known to counteract occurrence of AF, reduce hepatic lipid accumulation and may attenuate fibrosis through various metabolic and anti-inflammatory pathways, the use of dapagliflozin has also been associated with a risk of renal-related adverse events, warranting careful patient selection and monitoring [115]. Table 5 presents summarized results from studies regarding SGLT-2 inhibitors effects in MASLD and AF.

Table 5.
Summarized results from studies regarding SGLT-2 inhibitors effects in MASLD and AF.
5.2.5. Anticoagulants
Uncontrolled hepatic fat accumulation triggers a profibrotic response that promotes a pro-thrombotic state, often preceding clinically overt liver fibrosis [116]. Warfarin, a vitamin K antagonist (VKA), is widely used for the treatment and prevention of coagulopathic and thromboembolic disorders [117]. Direct oral anticoagulants (DOACs) are classified as direct thrombin inhibitors (dabigatran) or direct factor Xa inhibitors (rivaroxaban, apixaban, edoxaban, betrixaban). Compared with VKA, they offer rapid onset, predictable pharmacokinetics and pharmacodynamics, and fixed dosing without routine coagulation monitoring [118]. A retrospective study with a health research network (TriNetX) has shown that in patients with AF, coexisting NAFLD was linked to a more than twofold higher risk of both composite cardiovascular events (HR 2.17; 95% CI, 2.12–2.22) and hemorrhagic complications (HR 2.15; 95% CI, 2.05–2.56) [119]. The management of anticoagulated patients with liver disease is challenging due to both an elevated bleeding risk, driven by impaired hepatic synthetic function, variceal lesions, thrombocytopenia, and increased odds of ischemic events as well [120]. A randomized, double-blind trial—the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation Thrombolysis in Myocardial Infarction Study 48 (ENGAGE AF-TIMI 48) trial, which enrolled 21,105 patients with AF, including 1083 participants (5.1%) with concomitant liver disease—found no significant difference in the risk of major bleeding or stroke between those treated with edoxaban and those receiving warfarin [121]. However, a meta-analysis of 3483 patients with AF and chronic liver disease demonstrated that DOAC therapy was associated with a significantly lower risk of major bleeding and a comparable risk of stroke relative to warfarin [122]. Another meta-analysis revealed that DOACs appeared to be associated with a relatively lower bleeding risk in patients with liver cirrhosis. It is worth noting that factors such as older age or class C of Child–Turcotte–Pugh scale of liver dysfunction negatively affected referenced phenomenon and increased risk of hemorrhage in DOAC-receiving subjects [123]. Table 6 presents summarized results from studies regarding anticoagulants effects in MASLD and AF.

Table 6.
Summarized results from studies regarding anticoagulants effects in MASLD and AF.
5.2.6. Resmetirom
The intricate interplay between dysregulated metabolism, chronic inflammation, and fibrogenesis in MASLD underscores the rationale for pharmacological interventions targeting multiple pathogenic pathways either through single agents with pleiotropic mechanisms of action or via combination therapies employing distinct compounds. The U.S. Food and Drug Administration (FDA) has recently granted approval for resmetirom, a thyroid hormone receptor β-selective agonist, marking it as the first pharmacological agent authorized for the treatment of MASH and associated liver fibrosis [124].
In a phase 3 trial (n = 966), 25.9–29.9% of patients treated with this medication achieved MASH resolution without worsening fibrosis, versus 9.7% in placebo [37]. Resmetirom has shown promising efficacy in reducing hepatic fat content and improving lipid parameters, while maintaining a favorable safety profile with generally mild side effects [36]. Thorough analyses of its pharmaceutical properties reveal that it exerts beneficial effects towards metabolic health in various mechanisms, including enhancement of hepatic mitochondrial β-oxidation, suppression of lipogenesis by affecting various intracellular signaling proteins and enzymes SREBP-1c, fatty acid synthase (FASN), and acetyl-CoA carboxylase 1 (ACC1), downregulation of low density lipoprotein (LDL), and reduction in fibrotic mediators such as TGF-β [36]. Figure 4 highlights resmetirom’s mechanism of action in treatment of MASH.
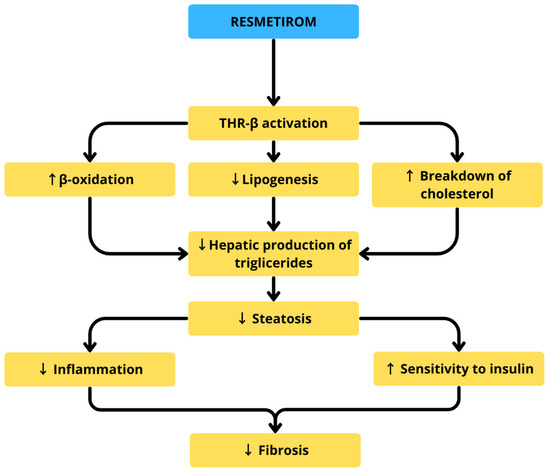
Figure 4.
Resmetirom’s mechanism of action in treatment of MASH.
5.3. Steatosis Due to Amiodarone or Other Antiarrhythmic Drugs
Amiodarone, a widely used antiarrhythmic drug and one of the possible therapeutic options for patients with AF, is one of the best-documented agents associated with drug-induced hepatic steatosis. Its cationic amphiphilic structure enables it to accumulate in mitochondria, where it disrupts β-oxidation of fatty acids and the electron transport chain, leading to both triglyceride accumulation and ROS generation. These mechanisms not only trigger macro- and micro-vesicular steatosis but can also progress to drug-induced steatohepatitis (DISH), fibrosis, and even cirrhosis with prolonged use [125]. Clinically, amiodarone-induced liver injury may manifest as asymptomatic transaminase elevations, jaundice, or, in rare cases, severe acute hepatitis. Other antiarrhythmic agents demonstrate similar steatogenic potential through mitochondrial toxicity. For example, dronedarone, a second-generation derivative, inhibits fatty acid β-oxidation but causes less electron transport disruption due to its shorter half-life, making severe mitochondrial injury less common. Perhexiline and propranolol have also been implicated in DISH via mechanisms involving mitochondrial dysfunction and oxidative stress [126]. Interestingly, some antiarrhythmic drugs such as verapamil may exert opposite effects: experimental models suggest verapamil can reduce lipid droplet accumulation and improve hepatic regeneration by enhancing autophagy, thus counteracting steatosis [127]. Collectively, while amiodarone and related compounds tend to promote hepatic fat accumulation through mitochondrial injury and impaired lipid handling, certain calcium channel blockers such as verapamil may have hepatoprotective, anti-steatotic effects. The main findings regarding effects exerted by particular antiarrhythmic drugs on hepatic tissue are highlighted in Figure 5.
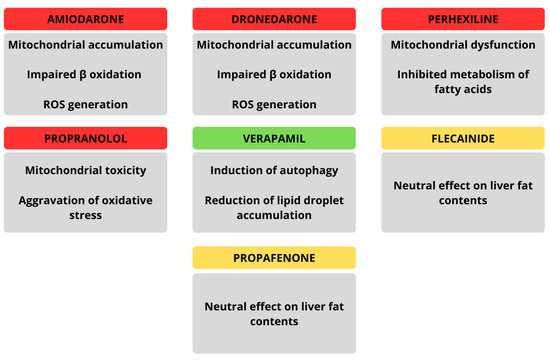
Figure 5.
Summarized effects of particular antiarrhythmic drugs on hepatic tissue.
6. Nutraceuticals’ Role in Prevention and Treatment of MASLD and AF
Nutraceuticals are bioactive compounds that provide health benefits beyond basic nutrition. Although they may originate from animals, microorganisms, or plants, a narrower definition limits this class to secondary metabolites obtained or enriched from plants [128,129]. Due to their structural diversity, nutraceuticals exert a wide range of biological activities that can beneficially modulate the course of numerous diseases. Compounds with antioxidant, anti-inflammatory, and metabolism-regulating properties are particularly promising for improving preventive and therapeutic strategies in MASLD and AF. Preclinical studies indicate that such compounds can inhibit disease progression and attenuate fibrosis in both the liver and heart [130,131,132]. However, the data from clinical trials are limited, with a vast majority of them regarding combined interventions with several nutraceuticals. Such an approach limits information on individual compounds’ effects for MASLD or AF. Moreover, the fact that most nutraceuticals exist in various, closely related forms or were administered in different dosage regimens makes comparing particular trials more complex and further limits their significance. It should be noted that none of the compounds discussed below are approved for general use in guideline-directed cardiometabolic therapy [132,133].
6.1. Omega-3 Polyunsaturated Fatty Acids
Omega-3 polyunsaturated fatty acids (Ω-3 PUFAs), primarily derived from fish and seafood, exert favorable effects on lipid metabolism by modulating PPARs, sterol regulatory element-binding protein-1c (SREBP-1c), and carbohydrate response element-binding protein (ChREBP) activity, thereby alleviating dyslipidemia. In MASLD patients, 12 months of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation (3.6 g/day) significantly decreased gamma-glutamyl transferase (GGT) levels [134,135]. Nogueira et al. showed that combination of EPA and DHA with alpha-linolenic acid (ALA) was capable of reducing triglycerides levels in MASH-affected patients. Moreover, individuals who displayed elevations in specific Ω-3 PUFAs in their blood plasma presented also mitigation of liver lobular inflammation, steatosis, and ballooning—histological exponents of progressive disease process in MASLD and MASH [136]. Despite strong cardioprotective and anti-inflammatory properties, several clinical trials using 1–4 g/day of Ω-3 PUFAs found no significant effects on inflammatory cytokines (IL-6, IL-8, IL-10, TNF-α, MCP-1) or recurrence rates of paroxysmal or persistent AF [137,138]. Similar null results were reported in subjects suffering from heart failure [139]. However, perioperative Ω-3 PUFA administration significantly reduced postoperative AF incidence in patients undergoing coronary artery bypass graft (CABG), including those with a prior history of myocardial infarction [140,141].
6.2. Vitamin E
The term vitamin E refers to a group of tocochromanols: α-, β-, γ- and δ-tocopherols with their corresponding tocotrienols. Vitamin E’s beneficial role for human health is predominantly associated with its potential to act as a lipophilic radical-scavenging antioxidant [142,143]. In non-diabetic patients suffering from MASH, a 96-week long administration of vitamin E at dose of 300 mg/day was shown to positively affect liver histological features, alleviating lobular inflammation, steatosis, and fibrosis score. Subjects treated with this antioxidant agent also displayed significantly decreased ALT and AST levels in blood plasma as well as diminished activity of IL-6 [144]. Similar results were acquired by Al-Baiaty et al. in an examination of tocotrienol-rich fraction vitamin E in a pediatric population. Supplementation with 50 mg of this nutraceutical, for 6 months, led to reductions in apolipoprotein A1, AST, and parameters resembling damage of DNA among children participating in the trial. Moreover, ultrasonographic assessment revealed alleviation of hepatic steatosis [145]. As in case of other nutraceuticals referenced in this review, the data regarding their role in preventing development of AF are limited to findings from patients undergoing cardiac surgeries and concern postoperative AF. Moreover, available studies were conducted using combination of vitamin E and other antioxidant agents or nutraceuticals, which makes it impossible to determine how much vitamin E contributed to the trials’ outcomes. Combined preparations of Ω-3 PUFAs, vitamin C, and vitamin E showed efficacy in preventing incidence of postoperative AF and improving the activity of antioxidant enzymes (CAT, SOD, and GPX) [146]. This beneficial potential was shown to be more pronounced among elderly populations, especially subjects older than 60 years [147].
6.3. Polyphenols
Polyphenols, a diverse class of plant-derived secondary metabolites, have garnered attention for their broad antioxidant and anti-inflammatory potential in disease prevention and therapy. In MASLD patients with coexisting T2DM, curcumin (1500 mg/day) significantly improved antioxidant enzyme activity (GPX, SOD) and reduced inflammatory cytokines (IL-1, IL-6, TNF-α), resulting in decreased body fat, BMI, hepatic steatosis, and liver stiffness [148]. However, in a trial involving CABG patients, curcumin combined with piperine did not prevent AF, although it reduced CRP levels and enhanced total antioxidant capacity [149]. In contrast, quercetin (1000 mg/day) administered two days preoperatively and for seven days post-CABG significantly lowered AF incidence. The effect was attributed to improved endothelial sensitivity to acetylcholine and attenuation of cellular senescence and inflammaging pathways [150]. Among MASLD patients, quercetin (500 mg/day for 12 weeks) modestly reduced body weight, fat mass, BMI, and intrahepatic lipid content (by approximately 2%), with the strongest effect observed in individuals with lower baseline hepatic lipid levels [151]. Although resveratrol is a well-established polyphenol with general health benefits, clinical trials in MASLD populations have shown its capability to induce only modest improvements in body weight, waist circumference, and liver fat, without significant changes in serum lipid profiles, liver enzymes, or cardiovascular risk markers, including atherogenic indices and blood pressure [152,153,154].
7. Conclusions
The growing prevalence of MASLD and AF underscores the urgent need to recognize their close interconnection. Both conditions share a constellation of cardiometabolic risk factors—including obesity, T2DM, dyslipidemia, and HA—and are increasingly understood as two manifestations of systemic metabolic dysfunction. Current evidence highlights that MASLD not only predisposes individuals to the development of AF but also worsens its recurrence and complications, while AF itself may accelerate hepatic injury through chronic inflammation, oxidative stress, and impaired hemodynamics.
The clinical significance of this bidirectional relationship is profound. Individuals with coexisting MASLD and AF face increased risks of unfavorable cardiovascular events, including stroke and HF. They are also at greater risk of liver fibrosis progression and present higher overall mortality. Importantly, this calls for an integrated diagnostic and therapeutic strategy. The use of non-invasive biomarkers and imaging techniques facilitates early detection of advanced fibrosis and arrhythmic risk, while structured risk stratification should guide multidisciplinary care. Lifestyle interventions, particularly sustained weight loss and metabolic optimization, remain the cornerstone of management with proven benefits in both liver and cardiac outcomes.
Pharmacological strategies such as utilization of GLP-1 receptor agonists, SGLT-2 inhibitors, statins, and thiazolidinediones have shown promise in simultaneously addressing hepatic steatosis and reducing AF burden. Furthermore, appropriate anticoagulation and rhythm-control strategies must be carefully tailored to MASLD patients to balance thromboembolic risk with hepatic safety. Nevertheless, most current evidence is derived from observational studies, and large-scale randomized trials are required to establish causality and refine therapeutic recommendations.
Future research should focus on clarifying the molecular mediators of the liver–heart axis, validating novel biomarkers for risk prediction, and developing multidisciplinary treatment models that bridge hepatology, cardiology, endocrinology, and primary care. Ultimately, recognizing MASLD as a systemic disease with significant cardiovascular consequences represents a critical step toward improving outcomes, reducing mortality, and lessening the global healthcare burden associated with both MASLD and AF.
Author Contributions
Conceptualization, M.R-K.; methodology, M.K.; validation, M.K. and M.R.-K.; formal analysis, A.M.; investigation, A.M.; resources, A.M. and M.K.; data curation, A.M. and M.K.; writing—original draft preparation, A.M.; writing—review and editing, M.G. and M.R-K.; visualization, M.K. and R.F.; supervision, M.K.; project administration, M.K.; funding acquisition, M.R-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- EASL; EASD; EASO. EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes. Facts 2024, 17, 374–444. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef]
- Frankowski, R.; Kobierecki, M.; Wittczak, A.; Różycka-Kosmalska, M.; Pietras, T.; Sipowicz, K.; Kosmalski, M. Type 2 Diabetes Mellitus, Non-Alcoholic Fatty Liver Disease, and Metabolic Repercussions: The Vicious Cycle and Its Interplay with Inflammation. Int. J. Mol. Sci. 2023, 24, 9677. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Syed-Abdul, M.M. Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD). Metabolites 2023, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Shang, Y.; Hegmar, H.; Nasr, P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol. Hepatol. 2024, 9, 944–956. [Google Scholar] [CrossRef]
- Lekakis, V.; Papatheodoridis, G.V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Lee, H.A.; Kim, E.-J.; Kim, H.Y.; Kim, H.C.; Ahn, S.H.; Lee, H.; Kim, S.U. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut 2024, 73, 533–540. [Google Scholar] [CrossRef]
- Bharaj, I.S.; Brar, A.S.; Kahlon, J.; Singh, A.; Hotwani, P.; Kumar, V.; Sohal, A.; Batta, A. Metabolic-dysfunction associated steatotic liver disease and atrial fibrillation: A review of pathogenesis. World J. Cardiol. 2025, 17, 106147. [Google Scholar] [CrossRef] [PubMed]
- Mellemkjær, A.; Kjær, M.B.; Haldrup, D.; Grønbæk, H.; Thomsen, K.L. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 28–34. [Google Scholar] [CrossRef]
- Kwak, M.; Kim, H.; Jiang, Z.G.; Yeo, Y.H.; Trivedi, H.D.; Noureddin, M.; Yang, J.D. MASLD/MetALD and mortality in individuals with any cardio-metabolic risk factor: A population-based study with 26.7 years of follow-up. Hepatology 2025, 81, 228–237. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Wang, T.J.; Leip, E.P.; Larson, M.G.; Levy, D.; Vasan, R.S.; D’Agostino, R.B.; Massaro, J.M.; Beiser, A.; Wolf, P.A.; et al. Lifetime Risk for Development of Atrial Fibrillation. Circulation 2004, 110, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation. J. Am. Coll. Cardiol. 2024, 83, 109–279. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Kang, M.K.; Park, J.G.; Kim, M.C. Association between Atrial Fibrillation and Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Yonsei Med. J. 2020, 61, 860. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef]
- Tang, Y.; Fan, J.; Hou, X.; Wu, H.; Zhang, J.; Wu, J.; Wang, Y.; Zhang, Z.; Lu, B.; Zheng, J. Metabolic dysfunction-associated steatotic liver disease and increased risk of atrial fibrillation in the elderly: A longitudinal cohort study. IJC Heart Vasc. 2025, 58, 101676. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, S.-R.; Choi, E.-K.; Go, Y.-H.; Lee, K.-Y.; Choi, J.; Han, S.; Kim, J.-H.; Ahn, H.-J.; Kwon, S.; et al. Impact of steatotic liver diseases on diabetes mellitus risk in patients with atrial fibrillation: A nationwide population study. Cardiovasc. Diabetol. 2025, 24, 242. [Google Scholar] [CrossRef]
- Elliott, A.D.; Middeldorp, M.E.; Van Gelder, I.C.; Albert, C.M.; Sanders, P. Epidemiology and modifiable risk factors for atrial fibrillation. Nat. Rev. Cardiol. 2023, 20, 404–417. [Google Scholar] [CrossRef]
- Pastori, D.; Sciacqua, A.; Marcucci, R.; Farcomeni, A.; Perticone, F.; Del Ben, M.; Angelico, F.; Baratta, F.; Pignatelli, P.; Violi, F.; et al. Prevalence and Impact of Nonalcoholic Fatty Liver Disease in Atrial Fibrillation. Mayo Clin. Proc. 2020, 95, 513–520. [Google Scholar] [CrossRef]
- Riley, D.R.; Hydes, T.; Hernadez, G.; Zhao, S.S.; Alam, U.; Cuthbertson, D.J. The synergistic impact of type 2 diabetes and MASLD on cardiovascular, liver, diabetes-related and cancer outcomes. Liver Int. 2024, 44, 2538–2550. [Google Scholar] [CrossRef]
- Simon, T.G.; Ebrahimi, F.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Incident cardiac arrhythmias associated with metabolic dysfunction-associated steatotic liver disease: A nationwide histology cohort study. Cardiovasc. Diabetol. 2023, 22, 343. [Google Scholar] [CrossRef]
- Brunner, K.T.; Henneberg, C.J.; Wilechansky, R.M.; Long, M.T. Nonalcoholic Fatty Liver Disease and Obesity Treatment. Curr. Obes. Rep. 2019, 8, 220–228. [Google Scholar] [CrossRef]
- Kim, M.; Son, M.; Moon, S.Y.; Baek, Y.H. Metabolic Dysfunction–Associated Steatotic Liver Disease, Alcohol Consumption, and the Risk of Atrial Fibrillation: A Nationwide Population-Based Study. J. Am. Heart Assoc. 2025, 14, e042003. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, G.; Lee, K.; Oh, R.; Kim, J.Y.; Jang, M.; Lee, Y.-B.; Jin, S.-M.; Hur, K.Y.; Han, K.; et al. Impact of steatotic liver disease categories on atrial fibrillation in type 2 diabetes: A nationwide study. Sci. Rep. 2025, 15, 11430. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Chonchol, M.; Pichiri, I.; Zoppini, G. Risk of cardiovascular disease and chronic kidney disease in diabetic patients with non-alcoholic fatty liver disease: Just a coincidence? J. Endocrinol. Investig. 2011, 34, 544–551. [Google Scholar]
- Jaiswal, V.; Ang, S.P.; Huang, H.; Momi, N.K.; Hameed, M.; Naz, S.; Batra, N.; Ishak, A.; Doshi, N.; Gera, A.; et al. Association between nonalcoholic fatty liver disease and atrial fibrillation and other clinical outcomes: A meta-analysis. J. Investig. Med. 2023, 71, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Morandin, R.; Sani, E.; Fiorio, V.; Shtembari, E.; Bonapace, S.; Petta, S.; Polyzos, S.A.; Byrne, C.D.; Targher, G. MASLD Is Associated With an Increased Long-Term Risk of Atrial Fibrillation: An Updated Systematic Review and Meta-Analysis. Liver Int. 2025, 45, e16218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Wei, B.; Zhang, R.; Zhang, X.; Xu, W. Metabolic dysfunction associated steatotic liver disease is associated with atrial fibrillation recurrence following cryoballoon ablation. Sci. Rep. 2025, 15, 6287. [Google Scholar] [CrossRef]
- Moon, J.H.; Jeong, S.; Jang, H.; Koo, B.K.; Kim, W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: A nationwide cohort study. eClinicalMedicine 2023, 65, 102292. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Mantovani, A.; Tilg, H.; Targher, G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 425–439. [Google Scholar] [CrossRef]
- Miller, J.D.; Aronis, K.N.; Chrispin, J.; Patil, K.D.; Marine, J.E.; Martin, S.S.; Blaha, M.J.; Blumenthal, R.S.; Calkins, H. Obesity, Exercise, Obstructive Sleep Apnea, and Modifiable Atherosclerotic Cardiovascular Disease Risk Factors in Atrial Fibrillation. J. Am. Coll. Cardiol. 2015, 66, 2899–2906. [Google Scholar] [CrossRef]
- Knezović, E.; Hefer, M.; Blažanović, S.; Petrović, A.; Tomičić, V.; Srb, N.; Kirner, D.; Smolić, R.; Smolić, M. Drug Pipeline for MASLD: What Can Be Learned from the Successful Story of Resmetirom. Curr. Issues Mol. Biol. 2025, 47, 154. [Google Scholar] [CrossRef]
- Cheung, J.; Cheung, B.M.-Y.; Yiu, K.-H.; Tse, H.-F.; Chan, Y.-H. Role of metabolic dysfunction-associated fatty liver disease in atrial fibrillation and heart failure: Molecular and clinical aspects. Front. Cardiovasc. Med. 2025, 12, 1573841. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Diaconu, C.C. Metabolic Dysfunction-Associated Steatotic Liver Disease: Pathogenetic Links to Cardiovascular Risk. Biomolecules 2025, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dauriz, M.; Sandri, D.; Bonapace, S.; Zoppini, G.; Tilg, H.; Byrne, C.D.; Targher, G. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int. 2019, 39, 758–769. [Google Scholar] [CrossRef]
- Svobodová, G.; Horní, M.; Velecká, E.; Boušová, I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: A review. Arch. Toxicol. 2025, 99, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, J.; Xie, K.; Tang, C.; Gan, C.; Gao, J. MASLD development: From molecular pathogenesis toward therapeutic strategies. Chin. Med. J. 2025, 138, 1807–1824. [Google Scholar] [CrossRef]
- Lv, X.; Nie, C.; Shi, Y.; Qiao, Q.; Gao, J.; Zou, Y.; Yang, J.; Chen, L.; Hou, X. Ergothioneine ameliorates metabolic dysfunction-Associated Steatotic Liver Disease (MASLD) by enhancing autophagy, inhibiting oxidative damage and inflammation. Lipids Health Dis. 2024, 23, 395. [Google Scholar] [CrossRef]
- Qiu, X.; Shen, S.; Jiang, N.; Feng, Y.; Yang, G.; Lu, D. Associations between systemic inflammatory biomarkers and metabolic dysfunction associated steatotic liver disease: A cross-sectional study of NHANES 2017–2020. BMC Gastroenterol. 2025, 25, 42. [Google Scholar] [CrossRef]
- Cho, E.J.; Chung, G.E.; Yoo, J.-J.; Cho, Y.; Lee, K.N.; Shin, D.W.; Kim, Y.J.; Yoon, J.-H.; Han, K.; Yu, S.J. Association of nonalcoholic fatty liver disease with new-onset atrial fibrillation stratified by age groups. Cardiovasc. Diabetol. 2024, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Pfenniger, A.; Yoo, S.; Arora, R. Oxidative stress and atrial fibrillation. J. Mol. Cell. Cardiol. 2024, 196, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 10. [Google Scholar] [CrossRef]
- Xing, Y.; Yan, L.; Li, X.; Xu, Z.; Wu, X.; Gao, H.; Chen, Y.; Ma, X.; Liu, J.; Zhang, J. The relationship between atrial fibrillation and NLRP3 inflammasome: A gut microbiota perspective. Front. Immunol. 2023, 14, 1273524. [Google Scholar] [CrossRef] [PubMed]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Duan, F.; Wu, J.; Chang, J.; Peng, H.; Liu, Z.; Liu, P.; Han, X.; Sun, T.; Shang, D.; Yang, Y.; et al. Deciphering endocrine function of adipose tissue and its significant influences in obesity-related diseases caused by its dysfunction. Differentiation 2025, 141, 100832. [Google Scholar] [CrossRef]
- Hemat Jouy, S.; Mohan, S.; Scichilone, G.; Mostafa, A.; Mahmoud, A.M. Adipokines in the Crosstalk between Adipose Tissues and Other Organs: Implications in Cardiometabolic Diseases. Biomedicines 2024, 12, 2129. [Google Scholar] [CrossRef]
- Francisco, V.; Sanz, M.J.; Real, J.T.; Marques, P.; Capuozzo, M.; Ait Eldjoudi, D.; Gualillo, O. Adipokines in Non-Alcoholic Fatty Liver Disease: Are We on the Road toward New Biomarkers and Therapeutic Targets? Biology 2022, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, H.; Hu, J.; Liu, Z.; Hu, F. The role of novel adipokines and adipose-derived extracellular vesicles (ADEVs): Connections and interactions in liver diseases. Biochem. Pharmacol. 2024, 222, 116104. [Google Scholar] [CrossRef]
- Driessen, S.; Francque, S.M.; Anker, S.D.; Castro Cabezas, M.; Grobbee, D.E.; Tushuizen, M.E.; Holleboom, A.G. Metabolic dysfunction-associated steatotic liver disease and the heart. Hepatology 2025, 82, 487–503. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Valério, C.M.; Júnior, W.S.S.; de Araujo-Neto, J.M.; Sposito, A.C.; Suassuna, J.H.R. CARDIAL-MS (CArdio-Renal-DIAbetes-Liver-Metabolic Syndrome): A new proposition for an integrated multisystem metabolic disease. Diabetol. Metab. Syndr. 2025, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, E.R.; Janssen-Telders, C.; Hulsman, E.L.; Lobe, N.; Zappala, P.; Terpstra, M.M.; Wesselink, R.; de Vries, T.A.C.; Al-Shama, R.F.; van Veen, R.N.; et al. The change of epicardial adipose tissue characteristics and vulnerability for atrial fibrillation upon drastic weight loss. Adipocyte 2024, 13, 2395565. [Google Scholar] [CrossRef]
- Teixeira, B.L.; Cunha, P.S.; Jacinto, A.S.; Portugal, G.; Laranjo, S.; Valente, B.; Lousinha, A.; Cruz, M.C.; Delgado, A.S.; Brás, M.; et al. Epicardial adipose tissue volume assessed by cardiac CT as a predictor of atrial fibrillation recurrence following catheter ablation. Clin. Imaging 2024, 110, 110170. [Google Scholar] [CrossRef]
- Patel, K.H.K.; Hwang, T.; Se Liebers, C.; Ng, F.S. Epicardial adipose tissue as a mediator of cardiac arrhythmias. Am. J. Physiol.-Heart Circ. Physiol. 2022, 322, H129–H144. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, M.; Bai, L.; Zhao, J.; Gao, H.; Bai, M. Recent Advances in Inflammation-Associated Epicardial Adipose Tissue for Atrial Fibrillation Patients. Rev. Cardiovasc. Med. 2025, 26, 36598. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Leeming, D.J.; Henriksen, K.; Mortensen, J.H.; Nielsen, S.H.; Anstee, Q.M.; Sanyal, A.J.; Karsdal, M.A.; Schuppan, D. ECM formation and degradation during fibrosis, repair, and regeneration. npj Metab. Health Dis. 2025, 3, 25. [Google Scholar] [CrossRef]
- van Riet, S.; Julien, A.; Atanasov, A.; Nordling, Å.; Ingelman-Sundberg, M. The role of sinusoidal endothelial cells and TIMP1 in the regulation of fibrosis in a novel human liver 3D NASH model. Hepatol. Commun. 2024, 8, e0374. [Google Scholar] [CrossRef]
- Lai, J.C.-T.; Liang, L.Y.; Wong, G.L.-H. Noninvasive tests for liver fibrosis in 2024: Are there different scales for different diseases? Gastroenterol. Rep. 2023, 12, goae024. [Google Scholar] [CrossRef]
- Kram, M. Galectin-3 inhibition as a potential therapeutic target in non-alcoholic steatohepatitis liver fibrosis. World J. Hepatol. 2023, 15, 201–207. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef] [PubMed]
- Boeckmans, J.; Michel, M.; Gieswinkel, A.; Tüscher, O.; Konstantinides, S.V.; König, J.; Münzel, T.; Lackner, K.J.; Kerahrodi, J.G.; Schuster, A.K.; et al. Inflammation in liver fibrosis and atrial fibrillation: A prospective population-based proteomic study. JHEP Rep. 2024, 6, 101171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, H.; Liu, J.; Huang, Z.; Shi, X.; Zhu, Y. The association between inflammatory, metabolic, and hepatic fibrosis-related biomarkers and atrial fibrillation in older patients with metabolic dysfunction-associated steatotic liver disease. BMC Geriatr. 2025, 25, 459. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsson, G.; Ulfarsson, M.O.; Thorolfsdottir, R.B.; Jonsson, B.A.; Einarsson, E.; Gunnlaugsson, G.; Rognvaldsson, S.; Arnar, D.O.; Baldvinsson, M.; Bjarnason, R.G.; et al. Multiomics study of nonalcoholic fatty liver disease. Nat. Genet. 2022, 54, 1652–1663. [Google Scholar] [CrossRef]
- Amangurbanova, M.; Huang, D.Q.; Loomba, R. Review article: The role of HSD17B13 on global epidemiology, natural history, pathogenesis and treatment of NAFLD. Aliment. Pharmacol. Ther. 2023, 57, 37–51. [Google Scholar] [CrossRef]
- Ajmera, V.; Loomba, R. Advances in the genetics of nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 2023, 39, 150–155. [Google Scholar] [CrossRef]
- Sun, L.; Yue, Z.; Wang, L. Research on the function of epigenetic regulation in the inflammation of non-alcoholic fatty liver disease. Life Med. 2024, 3, lnae030. [Google Scholar] [CrossRef]
- Aghaei, S.M.; Hosseini, S.M. Inflammation-related miRNAs in obesity, CVD, and NAFLD. Cytokine 2024, 182, 156724. [Google Scholar] [CrossRef] [PubMed]
- Vad, O.B.; Monfort, L.M.; Paludan-Müller, C.; Kahnert, K.; Diederichsen, S.Z.; Andreasen, L.; Lotta, L.A.; Nielsen, J.B.; Lundby, A.; Svendsen, J.H.; et al. Rare and Common Genetic Variation Underlying Atrial Fibrillation Risk. JAMA Cardiol. 2024, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Su, X.; He, J.; Gu, Y.; Chen, M.; Wei, Y.; Yi, Y.; Chen, P.; Lin, X.; Li, T.; et al. A Mendelian randomization study reveals a causal association between NASH and the risk of atrial fibrillation. Front. Endocrinol. 2025, 16, 1390259. [Google Scholar] [CrossRef] [PubMed]
- Clayton-Chubb, D.; Roberts, S.K.; Majeed, A.; Woods, R.L.; Tonkin, A.M.; Nelson, M.R.; Chan, A.T.; Ryan, J.; Tran, C.; Hodge, A.; et al. Associations between MASLD, atrial fibrillation, cardiovascular events, mortality and aspirin use in older adults. GeroScience 2024, 47, 1303–1318. [Google Scholar] [CrossRef]
- Attenberger, U.I.; Morelli, J.; Budjan, J.; Henzler, T.; Sourbron, S.; Bock, M.; Riffel, P.; Hernando, D.; Ong, M.M.; Schoenberg, S.O. Fifty Years of Technological Innovation. Investig. Radiol. 2015, 50, 584–593. [Google Scholar] [CrossRef]
- Kosmalski, M.; Mokros, Ł. Non-Alcoholic Fatty Liver Disease in Everyday Clinical Practice: From Diagnosis to Therapy. Life 2025, 15, 363. [Google Scholar] [CrossRef]
- Dawod, S.; Brown, K. Non-invasive testing in metabolic dysfunction-associated steatotic liver disease. Front. Med. 2024, 11, 1499013. [Google Scholar] [CrossRef]
- Long, L.; Wu, Y.; Tang, H.; Xiao, Y.; Wang, M.; Shen, L.; Shi, Y.; Feng, S.; Li, C.; Lin, J.; et al. Development and validation of a scoring system to predict MASLD patients with significant hepatic fibrosis. Sci. Rep. 2025, 15, 9639. [Google Scholar] [CrossRef]
- Cao, K.; Miao, X.; Chen, X. Association of inflammation and nutrition-based indicators with chronic obstructive pulmonary disease and mortality. J. Health Popul. Nutr. 2024, 43, 209. [Google Scholar] [CrossRef]
- Sun, H.; Liu, H.; Li, J.; Kou, J.; Yang, C. Analysis of the Clinical Predictive Value of the Novel Inflammatory Indices SII, SIRI, MHR and NHR in Patients with Acute Myocardial Infarction and Their Extent of Coronary Artery Disease. J. Inflamm. Res. 2024, 17, 7325–7338. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, S.; Dong, Y.; Liu, C.; Zhu, W. Comparison of liver fibrosis scores for predicting mortality and morbidity in heart failure with preserved ejection fraction. ESC Heart Fail. 2023, 10, 1771–1780. [Google Scholar] [CrossRef]
- Raposeiras-Roubín, S.; Parada Barcia, J.A.; Lizancos Castro, A.; Noriega Caro, V.; Ledo Piñeiro, A.; González Bermúdez, I.; González Ferreiro, R.; Íñiguez-Romo, A.; Abu-Assi, E. Liver fibrosis and outcomes of atrial fibrillation: The FIB-4 index. Clin. Res. Cardiol. 2024, 113, 313–323. [Google Scholar] [CrossRef]
- Clayton-Chubb, D.; Commins, I.; Roberts, S.K.; Majeed, A.; Woods, R.L.; Ryan, J.; Schneider, H.G.; Lubel, J.S.; Hodge, A.D.; McNeil, J.J.; et al. Scores to predict steatotic liver disease—Correlates and outcomes in older adults. npj Gut Liver 2025, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Dimala, C.A.; Nso, N.; Wasserlauf, J.; Njei, B. Electrocardiographic abnormalities in patients with metabolic dysfunction-associated steatotic liver disease: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102580. [Google Scholar] [CrossRef] [PubMed]
- Awashra, A.; Nouri, A.; Hamdan, A.; Said, H.; Rajab, I.; Hussein, A.; Abu Rmilah, A. Metabolic Dysfunction-Associated Steatotic Liver Disease as a Cardiovascular Risk Factor: Focus on Atrial Fibrillation. Cardiol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Koutoukidis, D.A.; Astbury, N.M.; Tudor, K.E.; Morris, E.; Henry, J.A.; Noreik, M.; Jebb, S.A.; Aveyard, P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease. JAMA Intern. Med. 2019, 179, 1262. [Google Scholar] [CrossRef]
- Pathak, R.K.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Wong, C.X.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort. J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef]
- Haghbin, H. Nonalcoholic fatty liver disease and atrial fibrillation: Possible pathophysiological links and therapeutic interventions. Ann. Gastroenterol. 2020, 33, 1–12. [Google Scholar] [CrossRef]
- Askarpour, M.; Khani, D.; Sheikhi, A.; Ghaedi, E.; Alizadeh, S. Effect of Bariatric Surgery on Serum Inflammatory Factors of Obese Patients: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 2631–2647. [Google Scholar] [CrossRef]
- Ratziu, V.; Francque, S.; Sanyal, A. Breakthroughs in therapies for NASH and remaining challenges. J. Hepatol. 2022, 76, 1263–1278. [Google Scholar] [CrossRef]
- Tacke, F.; Puengel, T.; Loomba, R.; Friedman, S.L. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J. Hepatol. 2023, 79, 552–566. [Google Scholar] [CrossRef]
- Newsome, P.N.; Ambery, P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J. Hepatol. 2023, 79, 1557–1565. [Google Scholar] [CrossRef]
- Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1703. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, H.; Liu, Q.; Zhou, S.; Liu, Z.; Xiao, Y. GLP-1 receptor agonists and myocardial metabolism in atrial fibrillation. J. Pharm. Anal. 2024, 14, 100917. [Google Scholar] [CrossRef]
- Saglietto, A.; Falasconi, G.; Penela, D.; Francia, P.; Sau, A.; Ng, F.S.; Dusi, V.; Castagno, D.; Gaita, F.; Berruezo, A.; et al. Glucagon-like peptide-1 receptor agonist semaglutide reduces atrial fibrillation incidence: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2024, 54, e14292. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Boyle, T.A.; Lyu, B.; Ballew, S.H.; Selvin, E.; Chang, A.R.; Inker, L.A.; Grams, M.E.; Shin, J.-I. Glucagon-like peptide-1 receptor agonists and the risk of atrial fibrillation in adults with diabetes: A real-world study. J. Gen. Intern. Med. 2024, 39, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A Placebo-Controlled Trial of Pioglitazone in Subjects with Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, E.; Csako, G.; Pucino, F.; Wesley, R.; Loomba, R. Meta-analysis: Pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2012, 35, 66–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Korantzopoulos, P.; Letsas, K.P.; Tse, G.; Gong, M.; Meng, L.; Li, G.; Liu, T. Thiazolidinedione use and atrial fibrillation in diabetic patients: A meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 96. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Wang, X.; Shi, H.; Tan, H.; Zhou, L.; Gu, J.; Jiang, W.; Wang, Y. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. Europace 2011, 13, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Sun, H.; Yang, N.; Wang, Q.; Zhang, L.; Song, J.; Taiwaikuli, D.; Shang, L.; Zhou, X.H. Pioglitazone Alleviates β1-adrenergic Receptor Antibody-induced Atrial Fibrillation Susceptivity via Mitigation of PPAR-γ-mediated Metabolic Inflexibility. Curr. Med. Chem. 2025, 32, 5861–5878. [Google Scholar] [CrossRef]
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus. Ann. Intern. Med. 2016, 165, 305–315. [Google Scholar] [CrossRef]
- O’Malley, P.G.; Arnold, M.J.; Kelley, C.; Spacek, L.; Buelt, A.; Natarajan, S.; Donahue, M.P.; Vagichev, E.; Ballard-Hernandez, J.; Logan, A.; et al. Management of Dyslipidemia for Cardiovascular Disease Risk Reduction: Synopsis of the 2020 Updated, U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann. Intern. Med. 2020, 173, 822–829. [Google Scholar] [CrossRef]
- Schreiner, A.D.; Zhang, J.; Petz, C.A.; Moran, W.P.; Koch, D.G.; Marsden, J.; Bays, C.; Mauldin, P.D.; Gebregziabher, M. Statin prescriptions and progression of advanced fibrosis risk in primary care patients with MASLD. BMJ Open Gastroenterol. 2024, 11, e001404. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Kim, S.U.; Yip, T.C.-F.; Petta, S.; Nakajima, A.; Tsochatzis, E.; Boursier, J.; Bugianesi, E.; Hagström, H.; Chan, W.K.; et al. Long-term liver-related outcomes and liver stiffness progression of statin usage in steatotic liver disease. Gut 2024, 73, 1883–1892. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.-R.; Zhang, M.-J.; Li, Y. Effects of Atorvastatin on Serum High-Sensitive C-Reactive Protein and Total Cholesterol Levels in Asian Patients With Atrial Fibrillation. Am. J. Ther. 2017, 24, e20–e29. [Google Scholar] [CrossRef]
- Engström, A.; Wintzell, V.; Melbye, M.; Hviid, A.; Eliasson, B.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Svanström, H.; Pasternak, B.; et al. Sodium–Glucose Cotransporter 2 Inhibitor Treatment and Risk of Atrial Fibrillation: Scandinavian Cohort Study. Diabetes Care 2023, 46, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Nespoux, J.; Vallon, V. Renal effects of SGLT2 inhibitors. Curr. Opin. Nephrol. Hypertens. 2020, 29, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Harrison, S.A.; Manghi, F.P.; Smith, W.B.; Alpenidze, D.; Aizenberg, D.; Klarenbeek, N.; Chen, C.-Y.; Zuckerman, E.; Ravussin, E.; Charatcharoenwitthaya, P.; et al. Licogliflozin for nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a study. Nat. Med. 2022, 28, 1432–1438. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.M.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus. Circulation 2020, 141, 1227–1234. [Google Scholar] [CrossRef]
- Li, P.; Chen, W.; Chen, R.; Zhang, H.; Yu, Z.; Yan, H.; Du, B.; Yang, P. Long-term effects of SGLT2 inhibitors on arrhythmias: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1558367. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, C.; He, L.; Zheng, S.; Wang, Y.; Gao, M.; Lai, Y.; Zhang, J.; Li, M.; Dai, W.; et al. Impact of Sodium-Glucose Cotransporter 2 Inhibitor on Recurrence After Catheter Ablation for Atrial Fibrillation in Patients With Diabetes: A Propensity-Score Matching Study and Meta-Analysis. J. Am. Heart Assoc. 2023, 12, e031269. [Google Scholar] [CrossRef]
- Zachou, M.; Flevari, P.; Nasiri-Ansari, N.; Varytimiadis, C.; Kalaitzakis, E.; Kassi, E.; Androutsakos, T. The role of anti-diabetic drugs in NAFLD. Have we found the Holy Grail? A narrative review. Eur. J. Clin. Pharmacol. 2024, 80, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Deaton, J.G.; Nappe, T.M. Warfarin Toxicity; StatPearls Publishing: Petersburg, FL, USA, 2025. [Google Scholar]
- Margetić, S.; Goreta, S.Š.; Ćelap, I.; Razum, M. Direct oral anticoagulants (DOACs): From the laboratory point of view. Acta Pharm. 2022, 72, 459–482. [Google Scholar] [CrossRef] [PubMed]
- Bucci, T.; Nabrdalik, K.; Baratta, F.; Pastori, D.; Pignatelli, P.; Hydes, T.; Alam, U.; Violi, F.; Lip, G.Y.H. Risk of Adverse Events in Anticoagulated Patients With Atrial Fibrillation and Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2024, 110, 208–217. [Google Scholar] [CrossRef]
- Costache, R.S.; Dragomirică, A.S.; Gheorghe, B.E.; Balaban, V.D.; Stanciu, S.M.; Jinga, M.; Costache, D.O. Oral Anticoagulation in Patients with Chronic Liver Disease. Medicina 2023, 59, 346. [Google Scholar] [CrossRef]
- Qamar, A.; Antman, E.M.; Ruff, C.T.; Nordio, F.; Murphy, S.A.; Grip, L.T.; Greenberger, N.J.; Yin, O.Q.P.; Choi, Y.; Lanz, H.J.; et al. Edoxaban Versus Warfarin in Patients With Atrial Fibrillation and History of Liver Disease. J. Am. Coll. Cardiol. 2019, 74, 179–189. [Google Scholar] [CrossRef]
- Menichelli, D.; Ronca, V.; Di Rocco, A.; Pignatelli, P.; Marco Podda, G. Direct oral anticoagulants and advanced liver disease: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13397. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, W.; Wang, L.; Chai, L.; Ageno, W.; Romeiro, F.G.; Li, H.; Qi, X. Risk of Bleeding in Liver Cirrhosis Receiving Direct Oral Anticoagulants: A Systematic Review and Meta-analysis. Thromb. Haemost. 2023, 123, 1072–1088. [Google Scholar] [CrossRef] [PubMed]
- Puengel, T.; Tacke, F. Pharmacotherapeutic options for metabolic dysfunction-associated steatotic liver disease: Where are we today? Expert Opin. Pharmacother. 2024, 25, 1249–1263. [Google Scholar] [CrossRef]
- Miele, L.; Liguori, A.; Marrone, G.; Biolato, M.; Araneo, C.; Vaccaro, F.G.; Gasbarrini, A.; Grieco, A. Fatty liver and drugs: The two sides of the same coin. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 86–94. [Google Scholar]
- López-Pascual, E.; Rienda, I.; Perez-Rojas, J.; Rapisarda, A.; Garcia-Llorens, G.; Jover, R.; Castell, J.V. Drug-Induced Fatty Liver Disease (DIFLD): A Comprehensive Analysis of Clinical, Biochemical, and Histopathological Data for Mechanisms Identification and Consistency with Current Adverse Outcome Pathways. Int. J. Mol. Sci. 2024, 25, 5203. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-L.; Lian, Y.-E.; Wu, J.-Y.; Wang, Y.-D.; Bai, Y.-N. Verapamil induces autophagy to improve liver regeneration in non-alcoholic fatty liver mice. Adipocyte 2021, 10, 532–545. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Kumar, P.; Kim, B.; Coutinho, H.D.M. Nutraceuticals: Pharmacologically Active Potent Dietary Supplements. BioMed Res. Int. 2022, 2022, 2051017. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, X.; Liang, W.; Qin, X.; Bian, L.; He, K.; Wu, Z. Curcumin, novel application in reversing myocardial fibrosis in the treatment for atrial fibrillation from the perspective of transcriptomics in rat model. Biomed. Pharmacother. 2022, 146, 112522. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Cui, X.; Kuang, W.; Li, D.; Wang, J. Quercetin prevents isoprenaline-induced myocardial fibrosis by promoting autophagy via regulating miR-223-3p/FOXO. Cell Cycle 2021, 20, 1253–1269. [Google Scholar] [CrossRef] [PubMed]
- Sokal-Dembowska, A.; Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Can Nutraceuticals Support the Treatment of MASLD/MASH, and thus Affect the Process of Liver Fibrosis? Int. J. Mol. Sci. 2024, 25, 5238. [Google Scholar] [CrossRef] [PubMed]
- Vrentzos, E.; Pavlidis, G.; Korakas, E.; Kountouri, A.; Pliouta, L.; Dimitriadis, G.D.; Lambadiari, V. Nutraceutical Strategies for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Path to Liver Health. Nutrients 2025, 17, 1657. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.A.; Oliveira, C.P.; Ferreira Alves, V.A.; Stefano, J.T.; dos Reis Rodrigues, L.S.; Torrinhas, R.S.; Cogliati, B.; Barbeiro, H.; Carrilho, F.J.; Waitzberg, D.L. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2016, 35, 578–586. [Google Scholar] [CrossRef]
- Vanderbilt, C.; Free, M.; Li, J.; Gebretsadik, T.; Bian, A.; Shintani, A.; McBride, B.F.; Solus, J.; Milne, G.; Crossley, G.H.; et al. Effect of Omega-Three Polyunsaturated Fatty Acids on Inflammation, Oxidative Stress, and Recurrence of Atrial Fibrillation. Am. J. Cardiol. 2015, 115, 196–201. [Google Scholar] [CrossRef]
- Macchia, A.; Grancelli, H.; Varini, S.; Nul, D.; Laffaye, N.; Mariani, J.; Ferrante, D.; Badra, R.; Figal, J.; Ramos, S.; et al. Omega-3 Fatty Acids for the Prevention of Recurrent Symptomatic Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 61, 463–468. [Google Scholar] [CrossRef]
- Aleksova, A.; Masson, S.; Maggioni, A.P.; Lucci, D.; Fabbri, G.; Beretta, L.; Mos, L.; Paino, A.M.; Nicolosi, G.L.; Marchioli, R.; et al. n -3 polyunsaturated fatty acids and atrial fibrillation in patients with chronic heart failure: The GISSI-HF trial. Eur. J. Heart Fail. 2013, 15, 1289–1295. [Google Scholar] [CrossRef]
- Sorice, M.; Tritto, F.P.; Sordelli, C.; Gregorio, R.; Piazza, L. N-3 polyunsaturated fatty acids reduces post-operative atrial fibrillation incidence in patients undergoing “on-pump” coronary artery bypass graft surgery. Monaldi Arch. Chest Dis. 2015, 76, 93–98. [Google Scholar] [CrossRef]
- Wilbring, M.; Ploetze, K.; Bormann, S.; Waldow, T.; Matschke, K. Omega-3 Polyunsaturated Fatty Acids Reduce the Incidence of Postoperative Atrial Fibrillation in Patients with History of Prior Myocardial Infarction Undergoing Isolated Coronary Artery Bypass Grafting. Thorac. Cardiovasc. Surg. 2014, 62, 569–574. [Google Scholar] [CrossRef]
- Niki, E. Evidence for beneficial effects of vitamin E. Korean J. Intern. Med. 2015, 30, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, N.; Niki, E. Vitamin E nomenclature. Is RRR-α-tocopherol the only vitamin E? Free Radic. Biol. Med. 2024, 221, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ni, W.; Zheng, M.; Sheng, H.; Wang, J.; Xie, S.; Yang, Y.; Chi, X.; Chen, J.; He, F.; et al. Vitamin E (300 mg) in the treatment of MASH: A multi-center, randomized, double-blind, placebo-controlled study. Cell Rep. Med. 2025, 6, 101939. [Google Scholar] [CrossRef]
- Al-Baiaty, F.D.R.; Ishak, S.; Mohd Zaki, F.; Masra, F.; Abdul Aziz, D.A.; Wan Md Zin, W.N.; Yee Hing, E.; Kuthubul Zaman, A.S.; Abdul Wahab, N.; Muhammad Nawawi, K.N.; et al. Assessing the efficacy of tocotrienol-rich fraction vitamin E in obese children with non-alcoholic fatty liver disease: A single-blind, randomized clinical trial. BMC Pediatr. 2024, 24, 529. [Google Scholar] [CrossRef]
- Rodrigo, R.; Korantzopoulos, P.; Cereceda, M.; Asenjo, R.; Zamorano, J.; Villalabeitia, E.; Baeza, C.; Aguayo, R.; Castillo, R.; Carrasco, R.; et al. A Randomized Controlled Trial to Prevent Post-Operative Atrial Fibrillation by Antioxidant Reinforcement. J. Am. Coll. Cardiol. 2013, 62, 1457–1465. [Google Scholar] [CrossRef]
- Rodrigo, R.; Gutierrez, R.; Fernandez, R.; Guzman, P. Ageing improves the antioxidant response against postoperative atrial fibrillation: A randomized controlled trial. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin for Inflammation Control in Individuals with Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomized Controlled Trial. Nutrients 2025, 17, 1972. [Google Scholar] [CrossRef]
- Tehrani, S.D.; Hosseini, A.; Shahzamani, M.; Heidari, Z.; Askari, G.; Majeed, M.; Sahebkar, A.; Bagherniya, M. Evaluation of the effectiveness of curcumin and piperine co-supplementation on inflammatory factors, cardiac biomarkers, atrial fibrillation, and clinical outcomes after coronary artery bypass graft surgery. Clin. Nutr. ESPEN 2024, 62, 57–65. [Google Scholar] [CrossRef]
- Mury, P.; Dagher, O.; Fortier, A.; Diaz, A.; Lamarche, Y.; Noly, P.; Ibrahim, M.; Pagé, P.; Demers, P.; Bouchard, D.; et al. Quercetin Reduces Vascular Senescence and Inflammation in Symptomatic Male but Not Female Coronary Artery Disease Patients. Aging Cell 2025, 24, e70108. [Google Scholar] [CrossRef]
- Li, N.; Cui, C.; Xu, J.; Mi, M.; Wang, J.; Qin, Y. Quercetin intervention reduced hepatic fat deposition in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled crossover clinical trial. Am. J. Clin. Nutr. 2024, 120, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ali Sangouni, A.; Abdollahi, S.; Mozaffari-Khosravi, H. Effect of resveratrol supplementation on hepatic steatosis and cardiovascular indices in overweight subjects with type 2 diabetes: A double-blind, randomized controlled trial. BMC Cardiovasc. Disord. 2022, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Asghari, S.; Rafraf, M.; Asghari-Jafarabadi, M.; Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2020, 90, 279–289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).