Protective Anti-Inflammatory and Antioxidant Mechanisms of Ohwia caudata Leaf Hydroethanolic Extract in a Dermatitis Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ohwia caudata Leaf Hydroethanolic Extract
2.3. Animal Experiment Design
2.4. Frozen Section
2.5. Immunofluorescence Staining
2.6. Immunoblot Assay
2.7. Statistical Analysis
3. Results
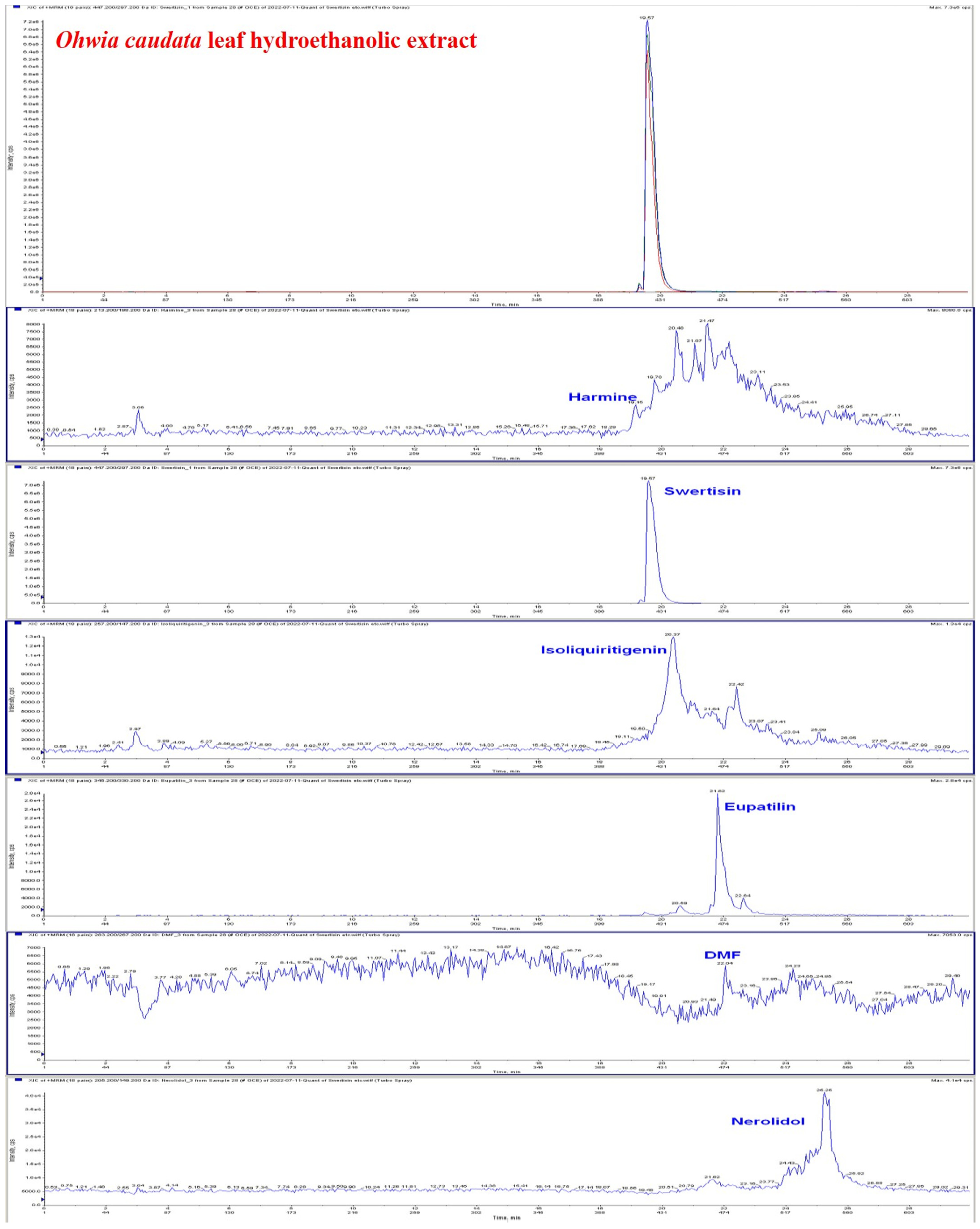
3.1. Identification of Major Bioactive Compounds in the Hydroethanolic Extract of Ohwia caudata Leaves
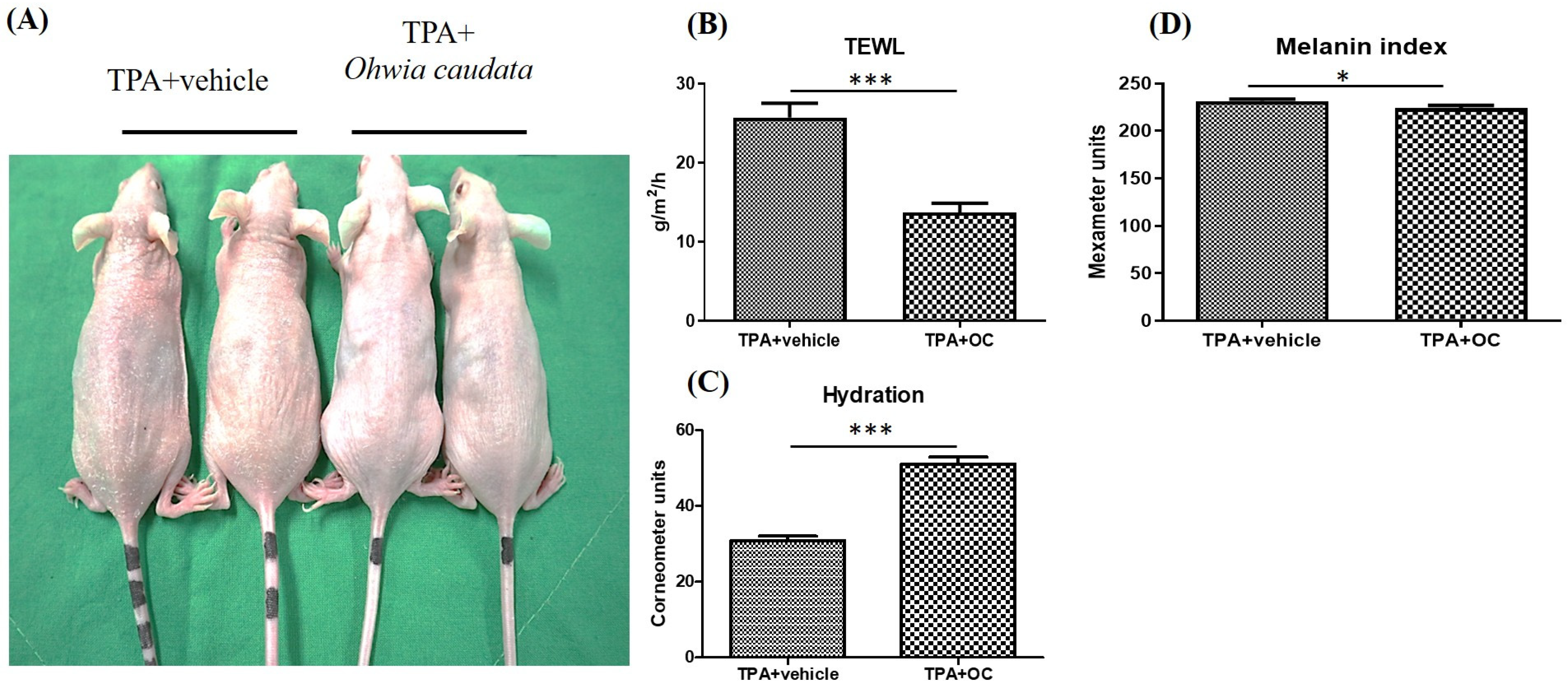
3.2. The Adverse Effects of TPA on the Skin Were Mitigated by the Ohwia caudata Leaf Hydroethanolic Extract
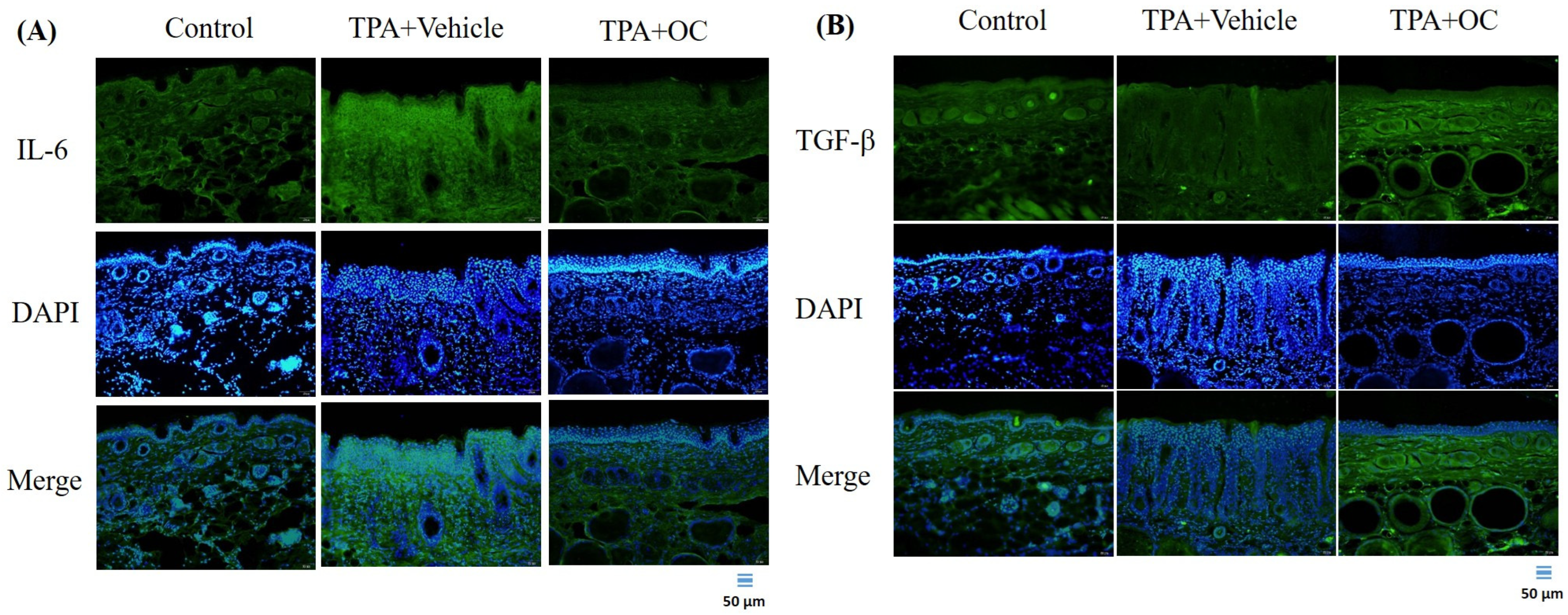
3.3. Ohwia Caudata Leaf Hydroethanolic Extract Attenuated TPA-Induced Skin Inflammation
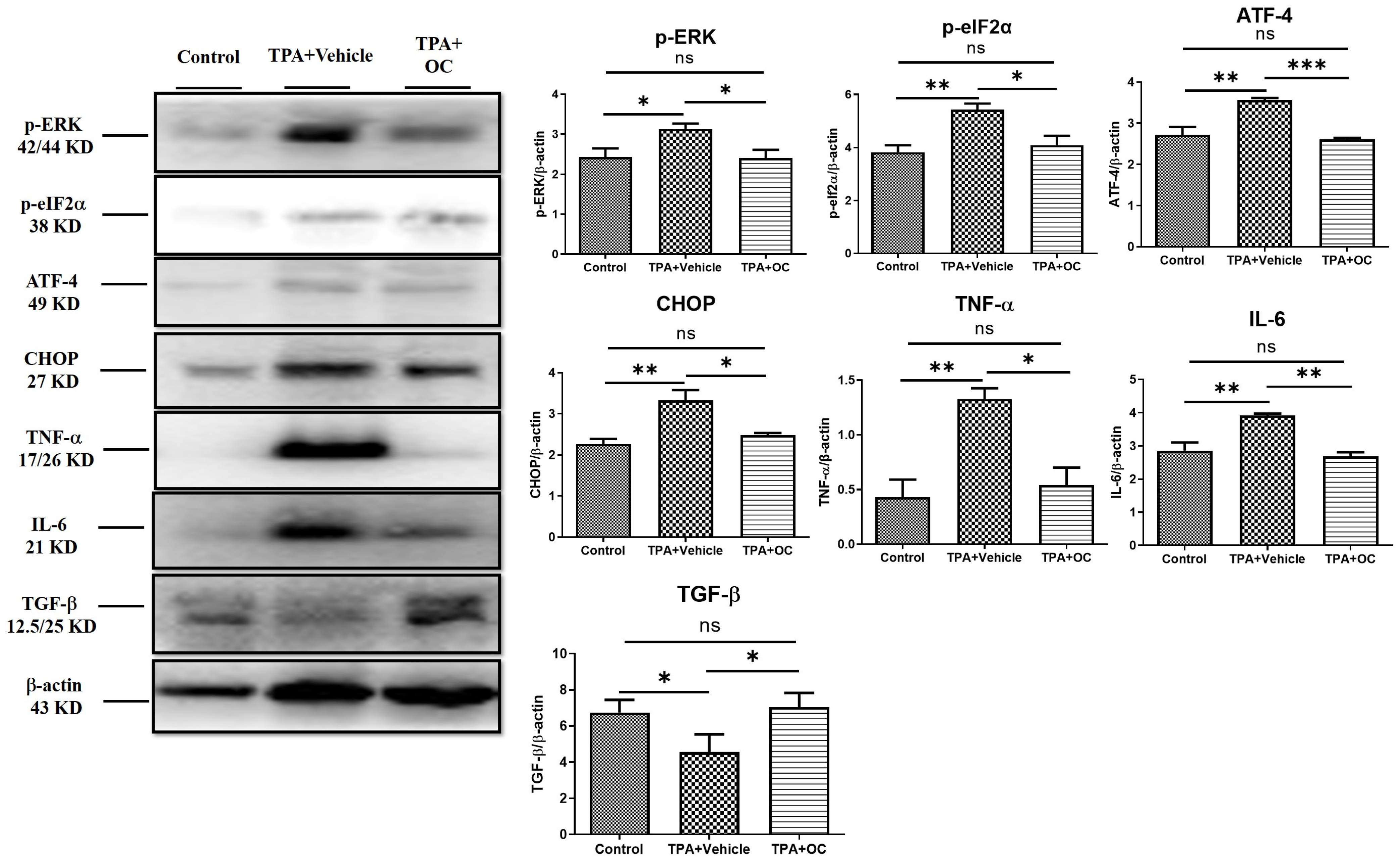
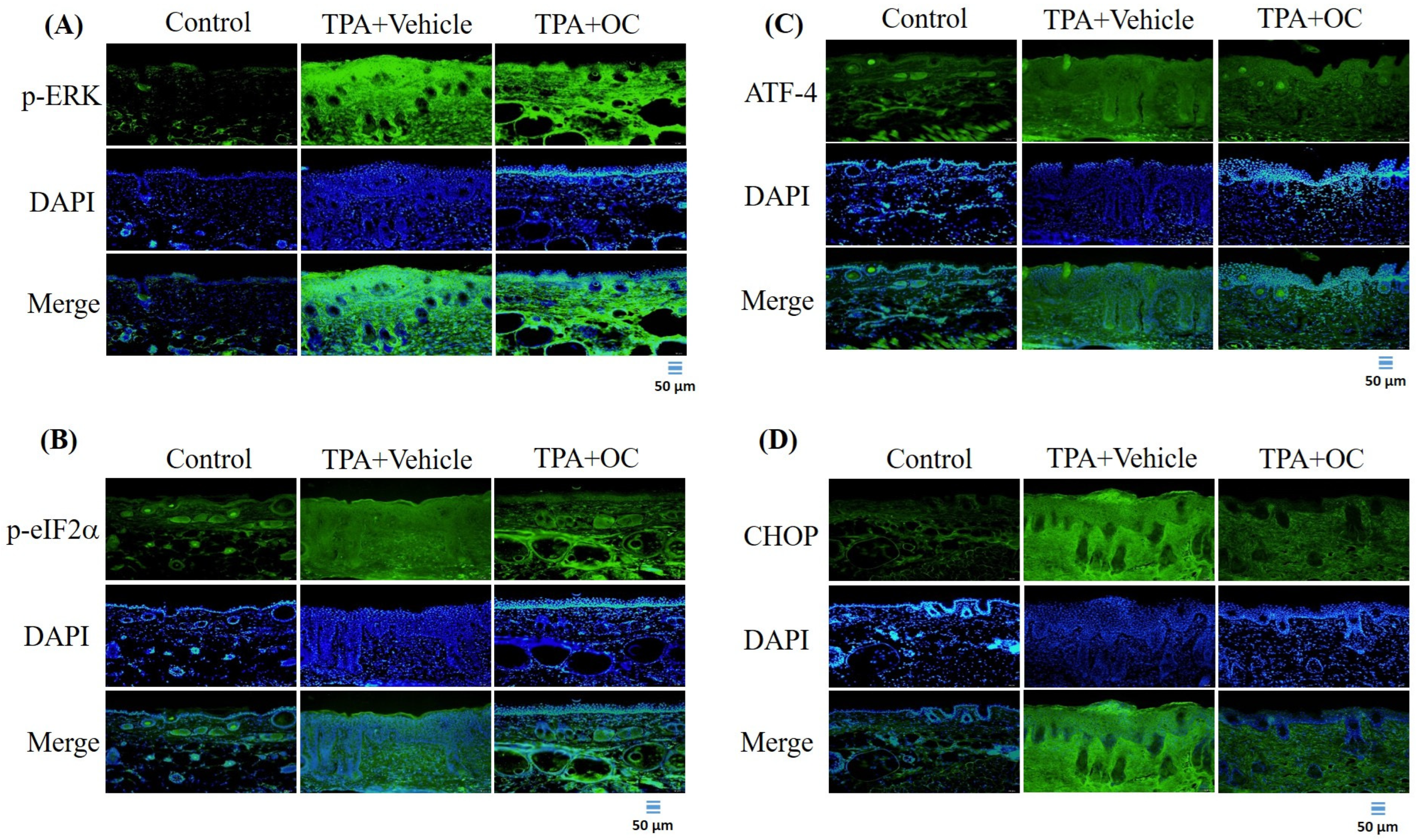
3.4. The ERK Signaling Pathway Was Attenuated by Ohwia caudata Leaf Hydroethanolic Extract
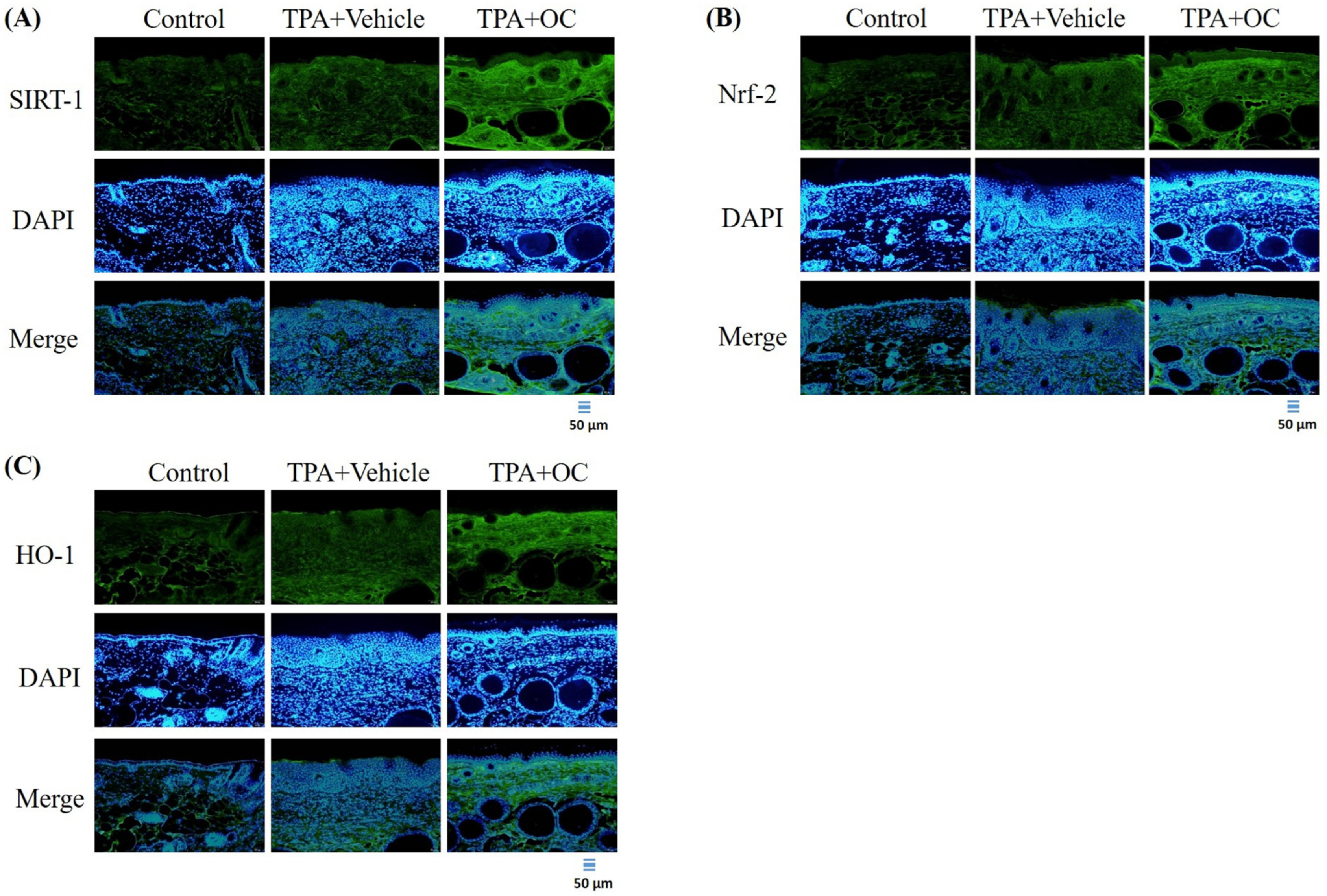
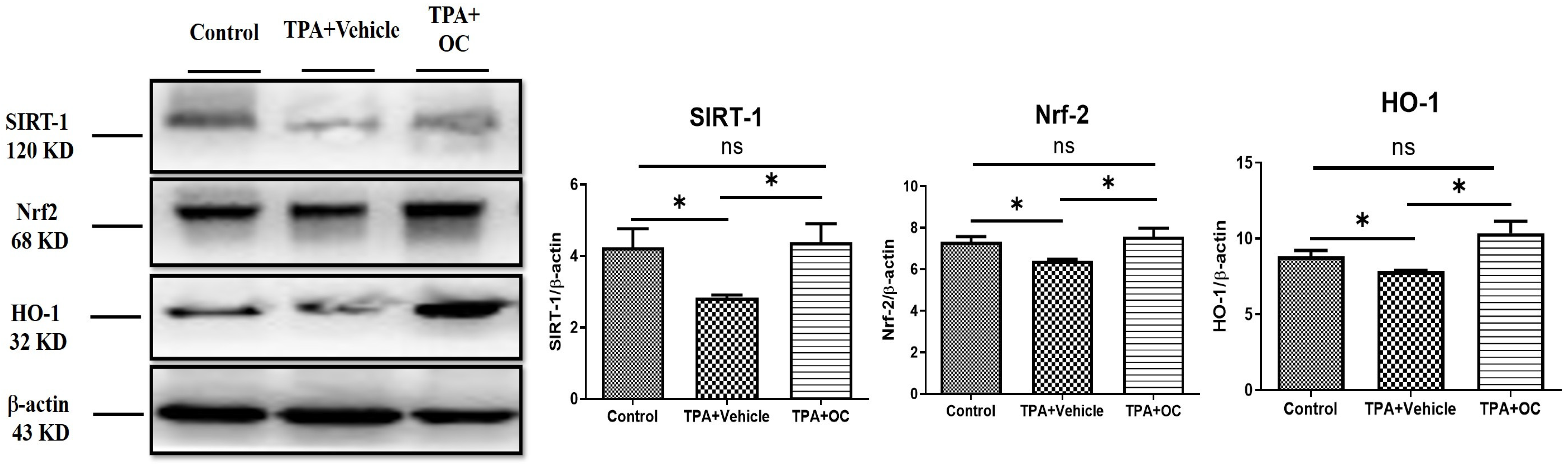
3.5. Ohwia caudata Leaf Hydroethanolic Extract Enhanced SIRT1/Nrf2/HO-1 Antioxidant Signaling in TPA-Stimulated Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATF-4 | activating transcription factor 4 |
| CHOP | C/EBP homologous protein |
| p-eIF2α | phosphorylated eukaryotic initiation factor 2 |
| p-ERK | phosphorylated extracellular signal-regulated kinase |
| HO-1 | heme oxygenase-1 |
| HPLC–MS | high-performance liquid chromatography coupled with tandem mass spectrometry |
| IL-6 | interleukin-6 |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| SIRT1 | Sirtuin 1 |
| TEWL | Transepidermal water loss |
| TGF-β | transforming growth factor beta |
| TNF-α | tumor necrosis factor-alpha |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
References
- Lopez-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A.M. Anatomy, skin (integument). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464/ (accessed on 13 July 2025).
- Frisoli, M.L.; Ko, W.C.; Martinez, N.; Afshari, K.; Wang, Y.; Garber, M.; Harris, J.E. Single-Cell RNA Sequencing Reveals Molecular Signatures that Distinguish Allergic from Irritant Contact Dermatitis. J. Investig. Dermatol. 2025, 145, 1117–1126.e14. [Google Scholar] [CrossRef]
- Aristizabal, M.A.; Bruce, C.J.; Caruso, M.A.; Wieczorek, M.A.; Pacheco-Spann, L.M.; Carter, R.E.; Bruce, A.J.; Hall, M.R. Allergic contact dermatitis revisited: A comprehensive review. JAAD Rev. 2025, 4, 92–103. [Google Scholar] [CrossRef]
- Patel, K.; Nixon, R. Irritant Contact Dermatitis—A Review. Curr. Dermatol. Rep. 2022, 11, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wi, K.; Hwang, S.Y.; Kim, Y.G.; Lee, S.I.; Lee, C.J.; Bang, G.; Lee, J.H.; Lee, M.H. Costunolide inhibits the progression of TPA-induced cell transformation and DMBA/TPA-induced skin carcinogenesis by regulation of AKT-mediated signaling. Cancer Cell Int. 2025, 25, 106. [Google Scholar] [CrossRef] [PubMed]
- Vähätupa, M.; Pemmari, T.; Junttila, I.; Pesu, M.; Järvinen, T.A.H. Chemical-Induced Skin Carcinogenesis Model Using Dimethylbenz[a]Anthracene and 12-O-Tetradecanoyl Phorbol-13-Acetate (DMBA-TPA). J. Vis. Exp. 2019, 154, e60445. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Du, Z.; Yuan, B.; Gao, Z.; Song, M.; Pan, C.; Han, Y.; Wu, X.; Tang, Z.; Zhang, G.; et al. Inhibitory effects of 7,7′-bromo-curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation. Eur. J. Pharmacol. 2019, 858, 172479. [Google Scholar] [CrossRef]
- Wei, W.C.; Lin, S.Y.; Chen, Y.J.; Wen, C.C.; Huang, C.Y.; Palanisamy, A.; Yang, N.S.; Sheu, J.H. Topical application of marine briarane-type diterpenes effectively inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and dermatitis in murine skin. J. Biomed. Sci. 2011, 18, 94. [Google Scholar] [CrossRef]
- Choi, S.Y.; Heo, M.J.; Lee, C.; Choi, Y.M.; An, I.S.; Bae, S.; An, S.; Jung, J.H. 2-deoxy-d-glucose Ameliorates Animal Models of Dermatitis. Biomedicines 2020, 8, 20. [Google Scholar] [CrossRef]
- Ho, T.J.; Ahmed, T.; Shibu, M.A.; Lin, Y.J.; Shih, C.Y.; Lin, P.Y.; Ling, S.Z.; Chiang, C.Y.; Kuo, W.W.; Huang, C.Y. A prospective review of the health-promoting potential of Jing Si Herbal Tea. Tzu. Chi. Med. J. 2024, 36, 1–22. [Google Scholar] [CrossRef]
- Chang, W.S.; Tsai, C.W.; Yang, J.S.; Hsu, Y.M.; Shih, L.C.; Chiu, H.Y.; Bau, D.T.; Tsai, F.J. Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Velmurugan, B.K.; Kuo, W.-W.; Chen, Y.-S.; Ho, T.-J.; Tsai, C.-T.; Ye, C.-X.; Tsai, C.-H.; Tsai, F.-J.; Huang, C.-Y. Inhibitory effect of alpinate Oxyphyllae fructus extracts on Ang II-induced cardiac pathological remodeling-related pathways in H9c2 cardiomyoblast cells. BioMedicine 2013, 3, 148–152. [Google Scholar] [CrossRef]
- Lee, H.-P.; Wang, S.-W.; Wu, Y.-C.; Tsai, C.-H.; Tsai, F.-J.; Chung, J.-G.; Huang, C.-Y.; Yang, J.-S.; Hsu, Y.-M.; Yin, M.-C.; et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric. Immunol. 2019, 30, 1033–1045. [Google Scholar] [CrossRef]
- Liu, S.P.; Shibu, M.A.; Tsai, F.J.; Hsu, Y.M.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Wang, S.; Li, Q.; et al. Tetramethylpyrazine reverses high-glucose induced hypoxic effects by negatively regulating HIF-1α induced BNIP3 expression to ameliorate H9c2 cardiomyoblast apoptosis. Nutr. Metab. 2020, 17, 12. [Google Scholar] [CrossRef]
- Hsieh, D.J.-Y.; Tsai, B.C.-K.; Barik, P.; Shibu, M.A.; Kuo, C.-H.; Kuo, W.-W.; Lin, P.-Y.; Shih, C.-Y.; Lin, S.-Z.; Ho, T.-J.; et al. Human adipose-derived stem cells preconditioned with a novel herbal formulation Jing Shi attenuate doxorubicin-induced cardiac damage. Aging 2023, 15, 9167–9181. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Bolatkyzy, N.; Shepilov, D.; Turmanov, R.; Berillo, D.; Vassilina, T.; Ibragimova, N.; Berganayeva, G.; Dyusebaeva, M. Medicinal Plants for Skin Disorders: Phytochemistry and Pharmacological Insights. Molecules 2025, 30, 3281. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-J.; Zhu, Z.-Z.; Yu, C.-H.; Zhang, H.; Liu, J.; Qin, L.-P. Analgesic, anti-inflammatory, and antipyretic activities of the ethanol extract from Desmodium caudatum. Pharm. Biol. 2011, 49, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.B.; Yang, H.J.; Choi, J.-G.; Li, W. Protective Effect of Flavonoids from Ohwia caudata Against Influenza a Virus Infection. Molecules 2020, 25, 4387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Chae, D.; Hyun, J.W.; Kang, H.K.; Koh, Y.-S.; Kim, Y.H. Anti-inflammatory and antioxidant activities of phenolic compounds from Desmodium caudatum leaves and stems. Arch. Pharmacal Res. 2014, 37, 721–727. [Google Scholar] [CrossRef]
- Lu, C.Y.; Kuo, C.H.; Kuo, W.W.; Hsieh, D.J.; Wang, T.F.; Shih, C.Y.; Lin, P.Y.; Lin, S.Z.; Ho, T.J.; Huang, C.Y. Ohwia caudata extract relieves the IL-17A-induced inflammatory response of synoviocytes through modulation of SOCS3 and JAK2/STAT3 activation. Environ. Toxicol. 2023, 38, 1914–1924. [Google Scholar] [CrossRef]
- Sun, Y.-W.; Wang, Y.; Guo, Z.-F.; Du, K.-C.; Meng, D.-L. Systems pharmacological approach to investigate the mechanism of Ohwia caudata for application to alzheimer’s disease. Molecules 2019, 24, 1499. [Google Scholar] [CrossRef]
- Lee, P.Y.; Tsai, B.C.K.; Sitorus, M.A.; Lin, P.Y.; Lin, S.Z.; Shih, C.Y.; Lu, S.Y.; Lin, Y.M.; Ho, T.J.; Huang, C.Y. Ohwia caudata aqueous extract attenuates doxorubicin-induced mitochondrial dysfunction in Wharton’s jelly-derived mesenchymal stem cells. Environ. Toxicol. 2023, 38, 2450–2461. [Google Scholar] [CrossRef]
- Ho, T.J.; Tsai, B.C.K.; Debakshee, G.; Shibu, M.A.; Kuo, C.H.; Lin, C.H.; Lin, P.Y.; Lin, S.Z.; Kuo, W.W.; Huang, C.Y. Ohwia caudata aqueous extract attenuates senescence in aging adipose-derived mesenchymal stem cells. Heliyon 2024, 10, e29729. [Google Scholar] [CrossRef]
- Yuan Hsieh, D.J.; Islam, M.N.; Kuo, W.W.; Shibu, M.A.; Lai, C.H.; Lin, P.Y.; Lin, S.Z.; Chen, M.Y.; Huang, C.Y. A combination of isoliquiritigenin with Artemisia argyi and Ohwia caudata water extracts attenuates oxidative stress, inflammation, and apoptosis by modulating Nrf2/Ho-1 signaling pathways in SD rats with doxorubicin-induced acute cardiotoxicity. Environ. Toxicol. 2023, 38, 3026–3042. [Google Scholar] [CrossRef]
- Kan, S.A.; Ali, A.; Kao, S.W.; Tsai, B.C.K.; Lin, Y.M.; Hsieh, D.J.Y.; Kuo, C.H.; Kuo, W.W.; Lin, S.Z.; Huang, C.Y. A Novel Therapeutic Strategy for Ameliorating Hyperglycemia-Induced Liver Injury via Overexpression of the Carboxyl Terminus of HSP70-Interacting Protein in Wharton’s Jelly Mesenchymal Stem Cells. Biotechnol. Appl. Biochem. 2025, e2771. [Google Scholar] [CrossRef]
- Tsai, B.C.-K.; Hsieh, D.J.-Y.; Lin, W.-T.; Tamilselvi, S.; Day, C.H.; Ho, T.-J.; Chang, R.-L.; Viswanadha, V.P.; Kuo, C.-H.; Huang, C.-Y. Functional potato bioactive peptide intensifies Nrf2-dependent antioxidant defense against renal damage in hypertensive rats. Food Res. Int. 2020, 129, 108862. [Google Scholar] [CrossRef] [PubMed]
- Tsai, B.C.-K.; Kuo, W.-W.; Day, C.H.; Hsieh, D.J.-Y.; Kuo, C.-H.; Daddam, J.; Chen, R.-J.; Padma, V.V.; Wang, G.; Huang, C.-Y. The soybean bioactive peptide VHVV alleviates hypertension-induced renal damage in hypertensive rats via the SIRT1-PGC1α/Nrf2 pathway. J. Funct. Foods 2020, 75, 104255. [Google Scholar] [CrossRef]
- Green, M.; Kashetsky, N.; Feschuk, A.; Maibach, H.I. Transepidermal water loss (TEWL): Environment and pollution—A systematic review. Skin. Health Dis. 2022, 2, e104. [Google Scholar] [CrossRef]
- Yoshimura, A.; Wakabayashi, Y.; Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF-β. J. Biochem. 2010, 147, 781–792. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Zeze, N.; Kido-Nakahara, M.; Tsuji, G.; Maehara, E.; Sato, Y.; Sakai, S.; Fujishima, K.; Hashimoto-Hachiya, A.; Furue, M.; Nakahara, T. Role of ERK Pathway in the Pathogenesis of Atopic Dermatitis and Its Potential as a Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3467. [Google Scholar] [CrossRef] [PubMed]
- Radaszkiewicz, K.A.; Beckerová, D.; Woloszczuková, L.; Radaszkiewicz, T.W.; Lesáková, P.; Blanářová, O.V.; Kubala, L.; Humpolíček, P.; Pachernik, J. 12-O-Tetradecanoylphorbol-13-acetate increases cardiomyogenesis through PKC/ERK signaling. Sci. Rep. 2020, 10, 15922. [Google Scholar] [CrossRef]
- Cohen, A.; Brodie, C.; Sarid, R. An essential role of ERK signalling in TPA-induced reactivation of Kaposi’s sarcoma-associated herpesvirus. J. Gen. Virol. 2006, 87, 795–802. [Google Scholar] [CrossRef]
- Thiaville, M.M.; Pan, Y.X.; Gjymishka, A.; Zhong, C.; Kaufman, R.J.; Kilberg, M.S. MEK signaling is required for phosphorylation of eIF2α following amino acid limitation of HepG2 human hepatoma cells. J. Biol. Chem. 2008, 283, 10848–10857. [Google Scholar] [CrossRef]
- Oh, Y.T.; Liu, X.; Yue, P.; Kang, S.; Chen, J.; Taunton, J.; Khuri, F.R.; Sun, S.Y. ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J. Biol. Chem. 2010, 285, 41310–41319. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.; Younis, S.M.; Al Tamimi, M.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting deregulated oxidative stress in skin inflammatory diseases: An update on clinical importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef] [PubMed]
- Wallen-Russell, C. Is there a relationship between transepidermal water loss and microbial biodiversity on the skin? Cosmetics 2019, 6, 18. [Google Scholar] [CrossRef]
- Załęcki, P.; Rogowska, K.; Wąs, P.; Łuczak, K.; Wysocka, M.; Nowicka, D. Impact of Lifestyle on Differences in Skin Hydration of Selected Body Areas in Young Women. Cosmetics 2024, 11, 13. [Google Scholar] [CrossRef]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, J.; Wang, L.; Li, B.; Guo, J.; Guan, X.; Han, Q.; Zhang, H. Caffeic acid reduces cutaneous tumor necrosis factor alpha (TNF-α), IL-6 and IL-1β levels and ameliorates skin edema in acute and chronic model of cutaneous inflammation in mice. Biol. Pharm. Bull. 2014, 37, 347–354. [Google Scholar] [CrossRef]
- Chipurupalli, S.; Samavedam, U.; Robinson, N. Crosstalk Between ER Stress, Autophagy and Inflammation. Front. Med. 2021, 8, 758311. [Google Scholar] [CrossRef]
- Cagnetta, R.; Wong, H.H.; Frese, C.K.; Mallucci, G.R.; Krijgsveld, J.; Holt, C.E. Noncanonical Modulation of the eIF2 Pathway Controls an Increase in Local Translation During Neural Wiring. Mol. Cell 2019, 73, 474–489.e5. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Jalili, C.; Darakhshan, S.; Azimi, M. Harmine mitigates liver injury induced by mercuric chloride via the inhibition of oxidative stress. Res. J. Pharmacogn. 2021, 8, 13–23. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Yao, Q.; Liu, Y.; Yu, J.; Zang, L.; Wang, S.; Zhou, L.; Wen, S.; Luo, Y.; et al. Harmine ameliorates CCl4-induced acute liver injury through suppression of autophagy and inflammation. Int. Immunopharmacol. 2024, 129, 111538. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhang, M.; Wang, Q.; Xie, J. Ultrasonic-assisted extraction of swertisin from sour Jujube seed and comprehensive revelation of its antioxidant activity. J. Food Biochem. 2022, 46, e14433. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Jeon, S.J.; Ryu, B.; Park, S.J.; Ko, S.Y.; Lee, Y.; Kim, E.; Lee, S.; Kim, H.; Jang, D.S.; et al. Swertisin, a C-glucosylflavone, ameliorates scopolamine-induced memory impairment in mice with its adenosine A1 receptor antagonistic property. Behav. Brain Res. 2016, 306, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; El-Shiekh, R.A.; Esmail, M.M.; Hassan, E.; Senna, M.M.; Ebid, N.; Elgindy, A.M. Surveying the Therapeutic Potentials of Isoliquiritigenin (ISL): A Comprehensive Review. Chem. Biodivers. 2025, 22, e202500456. [Google Scholar] [CrossRef]
- Alresheedi, N.H.; Samaha, M.M.; Ibrahim, T.M.; Abu-Elsaad, N.M. Eupatilin Protects Against Isotretinoin Induced Hepatotoxicity Through Immunomodulation of TLR4/MyD88/TRAF6 and NF-κB/NrF2 Pathways. Eur. J. Pharmacol. 2025, 1003, 177920. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, G.; Lee, Y.; Kim, H.; Song, M.J.; Lee, D.H.; Chung, J.H. A natural compound harmine decreases melanin synthesis through regulation of the DYRK1A/NFATC3 pathway. J. Dermatol. Sci. 2021, 103, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Fu, Y.; Cao, Y.; Jiang, S.; Yang, Y.; Song, G.; Yun, C.; Gao, R. Isoliquiritigenin inhibits melanogenesis, melanocyte dendricity and melanosome transport by regulating ERK-mediated MITF degradation. Exp. Dermatol. 2020, 29, 149–157. [Google Scholar] [CrossRef]
- Bai, D.; Cheng, X.; Li, Q.; Zhang, B.; Zhang, Y.; Lu, F.; Sun, T.; Hao, J. Eupatilin inhibits keratinocyte proliferation and ameliorates imiquimod-induced psoriasis-like skin lesions in mice via the p38 MAPK/NF-κB signaling pathway. Immunopharmacol. Immunotoxicol. 2023, 45, 133–139. [Google Scholar] [CrossRef]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical approaches to create murine models of human wound healing. BioMed Res. Int. 2011, 2011, 969618. [Google Scholar] [CrossRef]
- Griffin, M.F.; desJardins-Park, H.E.; Mascharak, S.; Borrelli, M.R.; Longaker, M.T. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis. Models Mech. 2020, 13, dmm044164. [Google Scholar] [CrossRef] [PubMed]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Medetgul-Ernar, K.; Davis, M.M. Standing on the shoulders of mice. Immunity 2022, 55, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-K.; Tsai, B.C.-K.; Kao, S.-W.; Tsai, C.-L.; Kuo, C.-H.; Ho, T.-J.; Hsieh, D.J.-Y.; Lin, S.-Z.; Kuo, W.-W.; Huang, C.-Y. Protective Anti-Inflammatory and Antioxidant Mechanisms of Ohwia caudata Leaf Hydroethanolic Extract in a Dermatitis Mouse Model. Life 2025, 15, 1707. https://doi.org/10.3390/life15111707
Lin T-K, Tsai BC-K, Kao S-W, Tsai C-L, Kuo C-H, Ho T-J, Hsieh DJ-Y, Lin S-Z, Kuo W-W, Huang C-Y. Protective Anti-Inflammatory and Antioxidant Mechanisms of Ohwia caudata Leaf Hydroethanolic Extract in a Dermatitis Mouse Model. Life. 2025; 15(11):1707. https://doi.org/10.3390/life15111707
Chicago/Turabian StyleLin, Tzu-Kai, Bruce Chi-Kang Tsai, Shih-Wen Kao, Chia-Lun Tsai, Chia-Hua Kuo, Tsung-Jung Ho, Dennis Jine-Yuan Hsieh, Shinn-Zong Lin, Wei-Wen Kuo, and Chih-Yang Huang. 2025. "Protective Anti-Inflammatory and Antioxidant Mechanisms of Ohwia caudata Leaf Hydroethanolic Extract in a Dermatitis Mouse Model" Life 15, no. 11: 1707. https://doi.org/10.3390/life15111707
APA StyleLin, T.-K., Tsai, B. C.-K., Kao, S.-W., Tsai, C.-L., Kuo, C.-H., Ho, T.-J., Hsieh, D. J.-Y., Lin, S.-Z., Kuo, W.-W., & Huang, C.-Y. (2025). Protective Anti-Inflammatory and Antioxidant Mechanisms of Ohwia caudata Leaf Hydroethanolic Extract in a Dermatitis Mouse Model. Life, 15(11), 1707. https://doi.org/10.3390/life15111707






