Understanding Drug Permeability in Pseudomonas aeruginosa
Abstract
1. Introduction
1.1. Study Selection Criteria
1.2. Understanding and Quantifying Outer Membrane Permeability in Pseudomonas aeruginosa
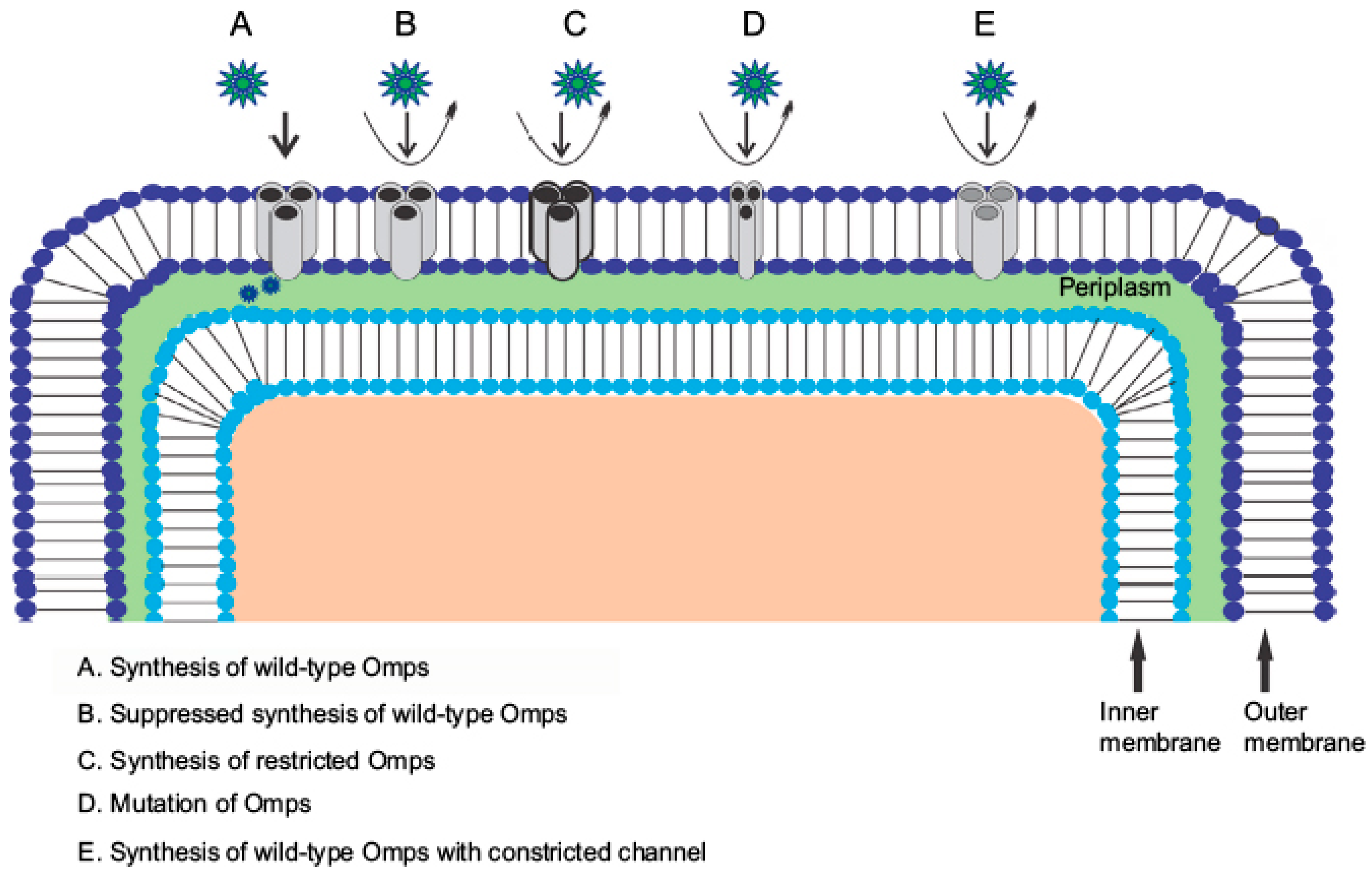
2. Experimental Approaches to Understanding the Role of Outer Membrane Porins in Pseudomonas aeruginosa Towards Antibiotic Influx
2.1. Electrophysiological Measurements
2.2. Flux Measurements
2.3. Integrating Data: A Comprehensive Picture of Antibiotic Influx
3. Discussion: Porin Modifications and Their Role in Antibiotic Resistance
4. Conclusive Remarks: The Path Forward
4.1. Key Implications for Antibiotic Development
4.1.1. Porin-Targeted Drug Design
4.1.2. Dynamic Permeability Modeling
4.1.3. Combination Therapies
4.2. Limitations and Future Perspectives
- Variability in Experimental Approaches
- 2.
- Limited Physiological Relevance of In Vitro Models
- 3.
- Underrepresentation of Efflux–Permeability Interplay
- 4.
- Structural and Dynamic Uncertainty in Porin Models
- 5.
- Lack of Standardized Permeability Databases
- 6.
- Translational Gap between Basic Research and Drug Discovery
4.3. Future Directions
- Develop integrated experimental platforms combining electrophysiology, flux assays, and live-cell uptake measurements under physiologically relevant conditions.
- Create high-throughput screening systems that evaluate permeability across multiple porins simultaneously using microfluidic or biosensor-based technologies.
- Employ machine learning and AI models trained on existing permeability data to predict antibiotic uptake and identify physicochemical features that favor porin passage.
- Explore dynamic regulatory mechanisms of porin expression under clinical stressors such as antibiotic exposure, oxidative stress, and host immune factors.
- Encourage collaborative databases and open-access repositories that standardize permeability reporting across research groups.
- Foster translational collaborations between structural biologists, microbiologists, and medicinal chemists to design next-generation antibiotics optimized for both target affinity and membrane penetration.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghai, I.; Winterhalter, M.; Wagner, R. Probing transport of charged beta-lactamase inhibitors through OmpC, a membrane channel from E. coli. Biochem. Biophys. Res. Commun. 2017, 484, 51–55. [Google Scholar] [CrossRef]
- Modi, N.; Benz, R.; Hancock, R.E.; Kleinekathofer, U. Modeling the Ion Selectivity of the Phosphate Specific Channel OprP. J. Phys. Chem. Lett. 2012, 3, 3639–3645. [Google Scholar] [CrossRef]
- Samanta, S.; D’Agostino, T.; Ghai, I.; Pathania, M.; Acosta Gutierrez, S.; Andrea Scorciapino, M.; Bodrenko, I.; Wagner, R.; van den Berg, B.; Winterhalter, M.; et al. How to Get Large Drugs through Small Pores? Exploiting the Porins Pathway in Pseudomonas Aeruginosa. Biophys. J. 2017, 112 (Suppl. S1), 416a. [Google Scholar] [CrossRef]
- Winterhalter, M.; Ceccarelli, M. Physical methods to quantify small antibiotic molecules uptake into Gram-negative bacteria. Eur. J. Pharm. Biopharm. 2015, 95 Pt A, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Ghai, I.; Hörömpöli, D.; Brötz-Oesterhelt, H.; Winterhalter, M.; Bafna, J.A. Uptake of aminoglycosides through outer membrane porins in Escherichia coli. bioRxiv 2022. [Google Scholar] [CrossRef]
- Masi, M.; Winterhalter, M.; Pages, J.M. Outer Membrane Porins. Subcell. Biochem. 2019, 92, 79–123. [Google Scholar]
- Pages, J.M.; James, C.E.; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008, 6, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Stavenger, R.A.; Winterhalter, M. TRANSLOCATION project: How to get good drugs into bad bugs. Sci. Transl. Med. 2014, 6, 228ed7. [Google Scholar] [CrossRef]
- Winterhalter, M. Antibiotic uptake through porins located in the outer membrane of Gram-negative bacteria. Expert. Opin. Drug Deliv. 2021, 18, 449–457. [Google Scholar] [CrossRef]
- Weingart, H.; Petrescu, M.; Winterhalter, M. Biophysical characterization of in- and efflux in Gram-negative bacteria. Curr. Drug Targets 2008, 9, 789–796. [Google Scholar] [CrossRef]
- Ghai, I. A Barrier to Entry: Examining the Bacterial Outer Membrane and Antibiotic Resistance. Appl. Sci. 2023, 13, 4238. [Google Scholar] [CrossRef]
- Sathe, N.; Beech, P.; Croft, L.; Suphioglu, C.; Kapat, A.; Athan, E. Pseudomonas aeruginosa: Infections and novel approaches to treatment “Knowing the enemy” the threat of Pseudomonas aeruginosa and exploring novel approaches to treatment. Infect. Med. 2023, 2, 178–194. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Mejri, S.; Okwume, C.C.; Lawal, N.A.; Olusegun, O.A.; Sallem, R.B.; Slama, K.B. Global epidemiology of high priority and pandemic Pseudomonas aeruginosa in pets, livestock, wild, and aquatic animals: A systematic review and meta-analysis. Lett. Appl. Microbiol. 2025, 78, ovaf028. [Google Scholar] [CrossRef]
- Avakh, A.; Grant, G.D.; Cheesman, M.J.; Kalkundri, T.; Hall, S. The art of war with Pseudomonas aeruginosa: Targeting Mex efflux pumps directly to strategically enhance antipseudomonal drug efficacy. Antibiotics 2023, 12, 1304. [Google Scholar] [CrossRef]
- Chalhoub, H.; Pletzer, D.; Weingart, H.; Braun, Y.; Tunney, M.M.; Elborn, J.S.; Rodriguez-Villalobos, H.; Plesiat, P.; Kahl, B.C.; Denis, O.; et al. Mechanisms of intrinsic resistance and acquired susceptibility of Pseudomonas aeruginosa isolated from cystic fibrosis patients to temocillin, a revived antibiotic. Sci. Rep. 2017, 7, 40208. [Google Scholar] [CrossRef]
- Elfadadny, A.; Ragab, R.F.; AlHarbi, M.; Badshah, F.; Ibáñez-Arancibia, E.; Farag, A.; Hendawy, A.O.; De los Ríos-Escalante, P.R.; Aboubakr, M.; Zakai, S.A. Antimicrobial resistance of Pseudomonas aeruginosa: Navigating clinical impacts, current resistance trends, and innovations in breaking therapies. Front. Microbiol. 2024, 15, 1374466. [Google Scholar] [CrossRef]
- Nikaido, H. Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol. Ther. 1985, 27, 197–231. [Google Scholar] [CrossRef]
- Warren, J.W. Providencia stuartii: A common cause of antibiotic-resistant bacteriuria in patients with long-term indwelling catheters. Rev. Infect. Dis. 1986, 8, 61–67. [Google Scholar] [CrossRef]
- Richter, M.F.; Drown, B.S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef]
- Agah Terzi, H.; Kulah, C.; Riza Atasoy, A.; Hakki Ciftci, I. Investigation of OprD Porin Protein Levels in Carbapenem-Resistant Pseudomonas aeruginosa Isolates. Jundishapur J. Microbiol. 2015, 8, e25952. [Google Scholar] [CrossRef]
- He, J.; Jia, X.; Yang, S.; Xu, X.; Sun, K.; Li, C.; Yang, T.; Zhang, L. Heteroresistance to carbapenems in invasive pseudomonas aeruginosa infections. Int. J. Antimicrob. Agents 2017, 51, 413–421. [Google Scholar] [CrossRef]
- Humphries, R.M.; Yang, S.; Hemarajata, P.; Ward, K.W.; Hindler, J.A.; Miller, S.A.; Gregson, A.L. First Report of Ceftazidime-Avibactam Resistance in a KPC-3-Expressing Klebsiella pneumoniae Isolate. Antimicrob. Agents Chemother. 2015, 59, 6605–6607. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- AHMAD, N.; SIDDIQUI, A.H.; SHARMA, M.; ARYA, A. Pathogenicity and Antibiotic Resistance of Pseudomonas aeruginosa: A Comprehensive Review. J. Clin. Diagn. Res. 2024, 18, DE6–DE13. [Google Scholar] [CrossRef]
- Alharbi, M.T. Unravelling the Versatile Nature of Pseudomonas aeruginosa: Challenges and Innovations in Infection Management. J. King Abdulaziz Univ. Med. Sci. 2024, 31, 21–30. [Google Scholar]
- Yang, J.; Xu, J.-F.; Liang, S. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and emerging treatment. Crit. Rev. Microbiol. 2025, 51, 841–859. [Google Scholar] [CrossRef]
- Eren, E.; Parkin, J.; Adelanwa, A.; Cheneke, B.; Movileanu, L.; Khalid, S.; van den Berg, B. Toward understanding the outer membrane uptake of small molecules by Pseudomonas aeruginosa. J. Biol. Chem. 2013, 288, 12042–12053. [Google Scholar] [CrossRef]
- Isabella, V.M.; Campbell, A.J.; Manchester, J.; Sylvester, M.; Nayar, A.S.; Ferguson, K.E.; Tommasi, R.; Miller, A.A. Toward the rational design of carbapenem uptake in Pseudomonas aeruginosa. Chem. Biol. 2015, 22, 535–547. [Google Scholar] [CrossRef]
- Iyer, R.; Sylvester, M.A.; Velez-Vega, C.; Tommasi, R.; Durand-Reville, T.F.; Miller, A.A. Whole-Cell-Based Assay To Evaluate Structure Permeation Relationships for Carbapenem Passage through the Pseudomonas aeruginosa Porin OprD. ACS Infect. Dis. 2017, 3, 310–319. [Google Scholar] [CrossRef]
- Jones, R.A. The Role of Membrane Lipid Remodelling in the Antimicrobial Resistance Arsenal of Pseudomonas aeruginosa. Doctoral Dissertation, University of Warwick, Coventry, UK, 2020. [Google Scholar]
- Cama, J.; Henney, A.M.; Winterhalter, M. Breaching the barrier: Quantifying antibiotic permeability across gram-negative bacterial membranes. J. Mol. Biol. 2019, 431, 3531–3546. [Google Scholar] [CrossRef]
- Catel-Ferreira, M.; Nehme, R.; Molle, V.; Aranda, J.; Bouffartigues, E.; Chevalier, S.; Bou, G.; Jouenne, T.; De, E. Deciphering the function of the outer membrane protein OprD homologue of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 3826–3832. [Google Scholar] [CrossRef]
- Cheneke, B.R.; Indic, M.; van den Berg, B.; Movileanu, L. An outer membrane protein undergoes enthalpy- and entropy-driven transitions. Biochemistry 2012, 51, 5348–5358. [Google Scholar] [CrossRef]
- Dogan Guzel, F.; Pletzer, D.; Norouz Dizaji, A.; Al-Nahas, K.; Bajrai, M.; Winterhalter, M. Towards understanding single-channel characteristics of OccK8 purified from Pseudomonas aeruginosa. Eur. Biophys. J. 2021, 50, 87–98. [Google Scholar] [CrossRef]
- Ghai, I. Quantifying the Flux of Charged Molecules through Bacterial Membrane Proteins. PhD Thesis, Jacobs University, Bremen, Germany, 2017. [Google Scholar]
- Ghai, I.; Ghai, S. Exploring bacterial outer membrane barrier to combat bad bugs. Infect. Drug Resist. 2017, 10, 261–273. [Google Scholar] [CrossRef]
- Ghai, I. Mapping Ammonium Flux Across Bacterial Porins: A Novel Electrophysiological Assay with Antimicrobial Relevance. Appl. Sci. 2025, 15, 7677. [Google Scholar] [CrossRef]
- Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. How to enter a bacterium: Bacterial porins and the permeation of antibiotics. Chem. Rev. 2021, 121, 5158–5192. [Google Scholar] [CrossRef]
- Shukla, S. Probing Structure, Function and Dynamics in Bacterial Primary and Secondary Transporter-Associated Binding Proteins. Doctoral Dissertation, University of Tennessee, Knoxville, TN, USA, 2020. [Google Scholar]
- Hall, L.T.; Hill, C.D.; Cole, J.H.; Städler, B.; Caruso, F.; Mulvaney, P.; Wrachtrup, J.; Hollenberg, L.C. Monitoring ion-channel function in real time through quantum decoherence. Proc. Natl. Acad. Sci. USA 2010, 107, 18777–18782. [Google Scholar] [CrossRef]
- Tran, Q.T.; Mahendran, K.R.; Hajjar, E.; Ceccarelli, M.; Davin-Regli, A.; Winterhalter, M.; Weingart, H.; Pages, J.M. Implication of porins in beta-lactam resistance of Providencia stuartii. J. Biol. Chem. 2010, 285, 32273–32281. [Google Scholar] [CrossRef]
- Winterhalter, M.; Hilty, C.; Bezrukov, S.M.; Nardin, C.; Meier, W.; Fournier, D. Controlling membrane permeability with bacterial porins: Application to encapsulated enzymes. Talanta 2001, 55, 965–971. [Google Scholar] [CrossRef]
- Choudhury, D.; Talukdar, A.D.; Choudhury, M.D.; Maurya, A.P.; Chanda, D.D.; Chakravorty, A.; Bhattacharjee, A. Carbapenem nonsusceptibility with modified OprD in clinical isolates of Pseudomonas aeruginosa from India. Indian. J. Med. Microbiol. 2017, 35, 137–139. [Google Scholar] [CrossRef]
- Tamber, S.; Maier, E.; Benz, R.; Hancock, R.E. Characterization of OpdH, a Pseudomonas aeruginosa porin involved in the uptake of tricarboxylates. J. Bacteriol. 2007, 189, 929–939. [Google Scholar] [CrossRef]
- Amisano, F. Assessment of Outer Membrane Permeability in Pseudomonas aeruginosa to β-Lactams: Some Aspects of the Role of OpdP in Carbapenem Resistance. Doctoral Thesis, Université de Liège, Liège, Belgium, 2025. [Google Scholar]
- Mammeri, H.; Sereme, Y.; Toumi, E.; Faury, H.; Skurnik, D. Interplay between porin deficiency, fitness, and virulence in carbapenem-non-susceptible Pseudomonas aeruginosa and Enterobacteriaceae. PLoS Pathog. 2025, 21, e1012902. [Google Scholar] [CrossRef]
- Azeem, K.; Fatima, S.; Ali, A.; Ubaid, A.; Husain, F.M.; Abid, M. Biochemistry of bacterial biofilm: Insights into antibiotic resistance mechanisms and therapeutic intervention. Life 2025, 15, 49. [Google Scholar] [CrossRef]
- Aita, S.E.; Ristori, M.V.; Cristiano, A.; Marfoli, T.; De Cesaris, M.; La Vaccara, V.; Cammarata, R.; Caputo, D.; Spoto, S.; Angeletti, S. Proteomic Insights into Bacterial Responses to Antibiotics: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7255. [Google Scholar] [CrossRef]
- Bajaj, H.; Scorciapino, M.A.; Moynie, L.; Page, M.G.; Naismith, J.H.; Ceccarelli, M.; Winterhalter, M. Molecular Basis of Filtering Carbapenems by Porins from beta-Lactam-resistant Clinical Strains of Escherichia coli. J. Biol. Chem. 2016, 291, 2837–2847. [Google Scholar] [CrossRef]
- Biswas, S.; Mohammad, M.M.; Patel, D.R.; Movileanu, L.; van den Berg, B. Structural insight into OprD substrate specificity. Nat. Struct. Mol. Biol. 2007, 14, 1108–1109. [Google Scholar] [CrossRef]
- Danelon, C.; Suenaga, A.; Winterhalter, M.; Yamato, I. Molecular origin of the cation selectivity in OmpF porin: Single channel conductances vs. free energy calculation. Biophys. Chem. 2003, 104, 591–603. [Google Scholar] [CrossRef]
- Ghai, I.; Ghai, S. Understanding antibiotic resistance via outer membrane permeability. Infect. Drug Resist. 2018, 11, 523–530. [Google Scholar] [CrossRef]
- Lovelle, M.; Mach, T.; Mahendran, K.R.; Weingart, H.; Winterhalter, M.; Gameiro, P. Interaction of cephalosporins with outer membrane channels of Escherichia coli. Revealing binding by fluorescence quenching and ion conductance fluctuations. Phys. Chem. Chem. Phys. 2011, 13, 1521–1530. [Google Scholar] [CrossRef]
- Ude, J.; Tripathi, V.; Buyck, J.M.; Söderholm, S.; Cunrath, O.; Fanous, J.; Claudi, B.; Egli, A.; Schleberger, C.; Hiller, S.; et al. Outer membrane permeability: Antimicrobials and diverse nutrients bypass porins in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2107644118. [Google Scholar] [CrossRef]
- Dixon, D.; Darveau, R. Lipopolysaccharide heterogeneity: Innate host responses to bacterial modification of lipid a structure. J. Dent. Res. 2005, 84, 584–595. [Google Scholar] [CrossRef]
- Kaur, M.; Mozaheb, N.; Paiva, T.; Herent, M.-F.; Goormaghtigh, F.; Paquot, A.; Terrasi, R.; Mignolet, E.; Décout, J.-L.; Lorent, J. Insight into the outer membrane asymmetry of P. aeruginosa and the role of MlaA in modulating the lipidic composition, mechanical, biophysical, and functional membrane properties of the cell envelope. Microbiol. Spectr. 2024, 12, e0148424. [Google Scholar] [CrossRef]
- Roy, H. Tuning the properties of the bacterial membrane with aminoacylated phosphatidylglycerol. IUBMB Life 2009, 61, 940–953. [Google Scholar] [CrossRef]
- Huang, H.; Hancock, R.E. The role of specific surface loop regions in determining the function of the imipenem-specific pore protein OprD of Pseudomonas aeruginosa. J. Bacteriol. 1996, 178, 3085–3090. [Google Scholar] [CrossRef]
- Kos, V.N.; McLaughlin, R.E.; Gardner, H.A. Identification of unique in-frame deletions in OprD among clinical isolates of Pseudomonas aeruginosa. Pathog. Dis. 2016, 74, ftw031. [Google Scholar] [CrossRef]
- Vasan, A.K.; Haloi, N.; Ulrich, R.J.; Metcalf, M.E.; Wen, P.C.; Metcalf, W.W.; Hergenrother, P.J.; Shukla, D.; Tajkhorshid, E. Role of internal loop dynamics in antibiotic permeability of outer membrane porins. Proc. Natl. Acad. Sci. USA 2022, 119, e2117009119. [Google Scholar] [CrossRef]
- Acharya, A.; Ghai, I.; Piselli, C.; Prajapati, J.D.; Benz, R.; Winterhalter, M.; Kleinekathöfer, U. Conformational Dynamics of Loop L3 in OmpF: Implications toward Antibiotic Translocation and Voltage Gating. J. Chem. Inf. Model. 2022, 63, 910–927. [Google Scholar] [CrossRef]
- Bafna, J.A.; Sans-Serramitjana, E.; Acosta-Gutiérrez, S.; Bodrenko, I.V.; Hörömpöli, D.; Berscheid, A.; Brötz-Oesterhelt, H.; Winterhalter, M.; Ceccarelli, M. Kanamycin Uptake into Escherichia coli Is Facilitated by OmpF and OmpC Porin Channels Located in the Outer Membrane. ACS Infect. Dis. 2020, 6, 1855–1865. [Google Scholar] [CrossRef]
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef]
- Hirabayashi, A.; Kato, D.; Tomita, Y.; Iguchi, M.; Yamada, K.; Kouyama, Y.; Morioka, H.; Tetsuka, N.; Yagi, T. Risk factors for and role of OprD protein in increasing minimal inhibitory concentrations of carbapenems in clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 2017, 66, 1562–1572. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.F.; Williams, B.J.; Blackwell, T.S.; Xie, C.M. Structure and function of OprD protein in Pseudomonas aeruginosa: From antibiotic resistance to novel therapies. Int. J. Med. Microbiol. 2012, 302, 63–68. [Google Scholar] [CrossRef]
- Liu, N.; Samartzidou, H.; Lee, K.W.; Briggs, J.M.; Delcour, A.H. Effects of pore mutations and permeant ion concentration on the spontaneous gating activity of OmpC porin. Protein Eng. 2000, 13, 491–500. [Google Scholar] [CrossRef]
- Kao, C.Y.; Chen, S.S.; Hung, K.H.; Wu, H.M.; Hsueh, P.R.; Yan, J.J.; Wu, J.J. Overproduction of active efflux pump and variations of OprD dominate in imipenem-resistant Pseudomonas aeruginosa isolated from patients with bloodstream infections in Taiwan. BMC Microbiol. 2016, 16, 107. [Google Scholar] [CrossRef]
- Bartsch, A.; Ives, C.M.; Kattner, C.; Pein, F.; Diehn, M.; Tanabe, M.; Munk, A.; Zachariae, U.; Steinem, C.; Llabrés, S. An antibiotic-resistance conferring mutation in a neisserial porin: Structure, ion flux, and ampicillin binding. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183601. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Nazari, M.; Ahmadi, H.; Hosseinzadeh, S.; Sahebkar, A.; Khademi, F. Imipenem resistance associated with amino acid alterations of the OprD porin in Pseudomonas aeruginosa clinical isolates. Acta Microbiol. Immunol. Hung. 2023, 70, 206–212. [Google Scholar] [CrossRef]
- Richardot, C.; Plesiat, P.; Fournier, D.; Monlezun, L.; Broutin, I.; Llanes, C. Carbapenem resistance in cystic fibrosis strains of Pseudomonas aeruginosa as a result of amino acid substitutions in porin OprD. Int. J. Antimicrob. Agents 2015, 45, 529–532. [Google Scholar] [CrossRef]
- Shu, J.C.; Kuo, A.J.; Su, L.H.; Liu, T.P.; Lee, M.H.; Su, I.N.; Wu, T.L. Development of carbapenem resistance in Pseudomonas aeruginosa is associated with OprD polymorphisms, particularly the amino acid substitution at codon 170. J. Antimicrob. Chemother. 2017, 72, 2489–2495. [Google Scholar] [CrossRef]
- Soge, O.O.; Harger, D.; Schafer, S.; Toevs, K.; Raisler, K.A.; Venator, K.; Holmes, K.K.; Kirkcaldy, R.D. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011). Sex Transm Dis 2012, 39, 877–879. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T 2015, 40, 277–283. [Google Scholar]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef]
- Yin, C.; Alam, M.Z.; Fallon, J.T.; Huang, W. Advances in development of novel therapeutic strategies against multi-drug resistant Pseudomonas aeruginosa. Antibiotics 2024, 13, 119. [Google Scholar] [CrossRef]
- Bhamidimarri, S.P.; Zahn, M.; Prajapati, J.D.; Schleberger, C.; Söderholm, S.; Hoover, J.; West, J.; Kleinekathöfer, U.; Bumann, D.; Winterhalter, M.; et al. A Multidisciplinary Approach toward Identification of Antibiotic Scaffolds for Acinetobacter baumannii. Structure 2019, 27, 268–280.e266. [Google Scholar] [CrossRef]
- Blay, V.; Tolani, B.; Ho, S.P.; Arkin, M.R. High-Throughput Screening: Today’s biochemical and cell-based approaches. Drug Discov. Today 2020, 25, 1807–1821. [Google Scholar] [CrossRef]
- Citak, F.; Ghai, I.; Rosenkotter, F.; Benier, L.; Winterhalter, M.; Wagner, R. Probing transport of fosfomycin through substrate specific OprO and OprP from Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2018, 495, 1454–1460. [Google Scholar] [CrossRef]
- Samanta, S.; Scorciapino, M.A.; Ceccarelli, M. Molecular basis of substrate translocation through the outer membrane channel OprD of Pseudomonas aeruginosa. Phys. Chem. Chem. Phys. 2015, 17, 23867–23876. [Google Scholar] [CrossRef]
- Samanta, S.; Bodrenko, I.; Acosta-Gutierrez, S.; D’Agostino, T.; Pathania, M.; Ghai, I.; Schleberger, C.; Bumann, D.; Wagner, R.; Winterhalter, M.; et al. Getting Drugs through Small Pores: Exploiting the Porins Pathway in Pseudomonas aeruginosa. ACS Infect. Dis. 2018, 4, 1519–1528. [Google Scholar] [CrossRef]
- Soundararajan, G.; Bhamidimarri, S.P.; Winterhalter, M. Understanding Carbapenem Translocation through OccD3 (OpdP) of Pseudomonas aeruginosa. ACS Chem. Biol. 2017, 12, 1656–1664. [Google Scholar] [CrossRef]
- Ghai, I. Electrophysiological Insights into Antibiotic Translocation and Resistance: The Impact of Outer Membrane Proteins. Membranes 2024, 14, 161. [Google Scholar] [CrossRef]
- Pérez, F.J.; Gimeno, C.; Navarro, D.; García-de-Lomas, J. Meropenem permeation through the outer membrane of Pseudomonas aeruginosa can involve pathways other than the OprD porin channel. Chemotherapy 1996, 42, 210–214. [Google Scholar] [CrossRef]
- Piselli, C.; Benz, R. Fosmidomycin transport through the phosphate-specific porins OprO and OprP of Pseudomonas aeruginosa. Mol. Microbiol. 2021, 116, 97–108. [Google Scholar] [CrossRef]
- Liu, J.; Wolfe, A.J.; Eren, E.; Vijayaraghavan, J.; Indic, M.; van den Berg, B.; Movileanu, L. Cation selectivity is a conserved feature in the OccD subfamily of Pseudomonas aeruginosa. Biochim. Biophys. Acta 2012, 1818, 2908–2916. [Google Scholar] [CrossRef]
- Liu, J.; Eren, E.; Vijayaraghavan, J.; Cheneke, B.R.; Indic, M.; van den Berg, B.; Movileanu, L. OccK channels from Pseudomonas aeruginosa exhibit diverse single-channel electrical signatures but conserved anion selectivity. Biochemistry 2012, 51, 2319–2330. [Google Scholar] [CrossRef]
- Pothula, K.R.; Kleinekathofer, U. Theoretical analysis of ion conductance and gating transitions in the OpdK (OccK1) channel. Analyst 2015, 140, 4855–4864. [Google Scholar] [CrossRef]
- Cheneke, B.R.; van den Berg, B.; Movileanu, L. Analysis of gating transitions among the three major open states of the OpdK channel. Biochemistry 2011, 50, 4987–4997. [Google Scholar] [CrossRef]
- Pothula, K.R.; Dhanasekar, N.N.; Lamichhane, U.; Younas, F.; Pletzer, D.; Benz, R.; Winterhalter, M.; Kleinekathofer, U. Single Residue Acts as Gate in OccK Channels. J. Phys. Chem. B 2017, 121, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Pongprayoon, P.; Beckstein, O.; Wee, C.L.; Sansom, M.S. Simulations of anion transport through OprP reveal the molecular basis for high affinity and selectivity for phosphate. Proc. Natl. Acad. Sci. USA 2009, 106, 21614–21618. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.; Barcena-Uribarri, I.; Bains, M.; Benz, R.; Hancock, R.E.; Kleinekathofer, U. Tuning the affinity of anion binding sites in porin channels with negatively charged residues: Molecular details for OprP. ACS Chem. Biol. 2015, 10, 441–451. [Google Scholar] [CrossRef]
- Modi, N.; Barcena-Uribarri, I.; Bains, M.; Benz, R.; Hancock, R.E.; Kleinekathofer, U. Role of the central arginine R133 toward the ion selectivity of the phosphate specific channel OprP: Effects of charge and solvation. Biochemistry 2013, 52, 5522–5532. [Google Scholar] [CrossRef]
- Ganguly, S.; Kesireddy, A.; Barcena-Uribarri, I.; Kleinekathofer, U.; Benz, R. Conversion of OprO into an OprP-like Channel by Exchanging Key Residues in the Channel Constriction. Biophys. J. 2017, 113, 829–834. [Google Scholar] [CrossRef]
- Pragasam, A.K.; Raghanivedha, M.; Anandan, S.; Veeraraghavan, B. Characterization of Pseudomonas aeruginosa with discrepant carbapenem susceptibility profile. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 12. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Shields, R.K.; Chen, L.; Hao, B.; Press, E.G.; Alkrouk, A.; Potoski, B.A.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Evaluation of the In Vitro Activity of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Meropenem-Resistant Pseudomonas aeruginosa Isolates. Antimicrob. Agents Chemother. 2016, 60, 3227–3231. [Google Scholar] [CrossRef]
- Naenna, P.; Noisumdaeng, P.; Pongpech, P.; Tribuddharat, C. Detection of outer membrane porin protein, an imipenem influx channel, in Pseudomonas aeruginosa clinical isolates. Southeast. Asian J. Trop. Med. Public. Health 2010, 41, 614–624. [Google Scholar]
- Modi, N.; Ganguly, S.; Barcena-Uribarri, I.; Benz, R.; van den Berg, B.; Kleinekathofer, U. Structure, Dynamics, and Substrate Specificity of the OprO Porin from Pseudomonas aeruginosa. Biophys. J. 2015, 109, 1429–1438. [Google Scholar] [CrossRef]
- Golla, V.K.; Piselli, C.; Kleinekathöfer, U.; Benz, R. Permeation of Fosfomycin through the Phosphate-Specific Channels OprP and OprO of Pseudomonas aeruginosa. J. Phys. Chem. B 2022, 126, 1388–1403. [Google Scholar] [CrossRef]
- Ghai, I.; Pira, A.; Scorciapino, M.A.; Bodrenko, I.; Benier, L.; Ceccarelli, M.; Winterhalter, M.; Wagner, R. General Method to Determine the Flux of Charged Molecules through Nanopores Applied to beta-Lactamase Inhibitors and OmpF. J. Phys. Chem. Lett. 2017, 8, 1295–1301. [Google Scholar] [CrossRef]
- Yi, M.Y.; Wang, P.Y.; Huang, H.J.; Liu, Y.C. [The roles of active efflux system overexpression and outer membrane protein OprD deficiency or loss in carbapenem resistance of Pseudomonas aeruginosa]. Zhonghua Yi Xue Za Zhi 2006, 86, 457–462. [Google Scholar]
- Eren, E.; Vijayaraghavan, J.; Liu, J.; Cheneke, B.R.; Touw, D.S.; Lepore, B.W.; Indic, M.; Movileanu, L.; van den Berg, B. Substrate specificity within a family of outer membrane carboxylate channels. PLoS Biol. 2012, 10, e1001242. [Google Scholar] [CrossRef]
- Song, W.; Bajaj, H.; Nasrallah, C.; Jiang, H.; Winterhalter, M.; Colletier, J.-P.; Xu, Y. Understanding Voltage Gating of Providencia stuartii Porins at Atomic Level. PLoS Comput. Biol. 2015, 11, e1004255. [Google Scholar] [CrossRef]
- Ghai, I.; Bajaj, H.; Arun Bafna, J.; El Damrany Hussein, H.A.; Winterhalter, M.; Wagner, R. Ampicillin permeation across OmpF, the major outer-membrane channel in Escherichia coli. J. Biol. Chem. 2018, 293, 7030–7037. [Google Scholar] [CrossRef]
- Phale, P.S.; Schirmer, T.; Prilipov, A.; Lou, K.-L.; Hardmeyer, A.; Rosenbusch, J.P. Voltage gating of Escherichia coli porin channels: Role of the constriction loop. Proc. Natl. Acad. Sci. USA 1997, 94, 6741–6745. [Google Scholar] [CrossRef] [PubMed]
- Phale, P.S.; Philippsen, A.; Widmer, C.; Phale, V.P.; Rosenbusch, J.P.; Schirmer, T. Role of charged residues at the OmpF porin channel constriction probed by mutagenesis and simulation. Biochemistry 2001, 40, 6319–6325. [Google Scholar] [CrossRef]
- Bodrenko, I.V.; Wang, J.; Salis, S.; Winterhalter, M.; Ceccarelli, M. Sensing Single Molecule Penetration into Nanopores: Pushing the Time Resolution to the Diffusion Limit. ACS Sens. 2017, 2, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Bafna, J.A.; Schmid, B.; Klingl, S.; Baier, S.; Hemmis, B.; Wagner, R.; Winterhalter, M.; Voll, L.M. Manipulation of charge distribution in the arginine and glutamate clusters of the OmpG pore alters sugar specificity and ion selectivity. Biochim. Biophys. Acta Biomembr. 2019, 1861, 183021. [Google Scholar] [CrossRef]
- Pangeni, S.; Prajapati, J.D.; Bafna, J.; Nilam, M.; Nau, W.M.; Kleinekathöfer, U.; Winterhalter, M. Large-Peptide Permeation Through a Membrane Channel: Understanding Protamine Translocation Through CymA from Klebsiella Oxytoca*. Angew. Chem. Int. Ed. Engl. 2021, 60, 8089–8094. [Google Scholar] [CrossRef]
- Nestorovich, E.M.; Danelon, C.; Winterhalter, M.; Bezrukov, S.M. Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl. Acad. Sci. USA 2002, 99, 9789–9794. [Google Scholar] [CrossRef]
- Mahendran, K.R.; Chimerel, C.; Mach, T.; Winterhalter, M. Antibiotic translocation through membrane channels: Temperature-dependent ion current fluctuation for catching the fast events. Eur. Biophys. J. 2009, 38, 1141–1145. [Google Scholar] [CrossRef]
- Mahendran, K.R.; Kreir, M.; Weingart, H.; Fertig, N.; Winterhalter, M. Permeation of antibiotics through Escherichia coli OmpF and OmpC porins: Screening for influx on a single-molecule level. J. Biomol. Screen. 2010, 15, 302–307. [Google Scholar] [CrossRef]
- Liu, Z.; Ghai, I.; Winterhalter, M.; Schwaneberg, U. Engineering Enhanced Pore Sizes Using FhuA Delta1-160 from E. coli Outer Membrane as Template. ACS Sens. 2017, 2, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Terrasse, R.; Bafna, J.A.; Benier, L.; Winterhalter, M. Electrophysiological Characterization of Transport Across Outer-Membrane Channels from Gram-Negative Bacteria in Presence of Lipopolysaccharides. Angew. Chem. Int. Ed. Engl. 2020, 59, 8517–8521. [Google Scholar] [CrossRef]
- Wang, J.; Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. Dynamic interaction of fluoroquinolones with magnesium ions monitored using bacterial outer membrane nanopores. Chem. Sci. 2020, 11, 10344–10353. [Google Scholar] [CrossRef]
- Bodrenko, I.V.; Zewdie, T.A.; Wang, J.; Paul, E.; Witt, S.; Winterhalter, M. TolC-AcrA complex formation monitored by time dependent single-channel electrophysiology. Biochimie 2023, 205, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, J.D.; Fernández Solano, C.J.; Winterhalter, M.; Kleinekathöfer, U. Characterization of Ciprofloxacin Permeation Pathways across the Porin OmpC Using Metadynamics and a String Method. J. Chem. Theory Comput. 2017, 13, 4553–4566. [Google Scholar] [CrossRef]
- Alcaraz, A.; López, M.L.; Queralt-Martín, M.; Aguilella, V.M. Ion Transport in Confined Geometries below the Nanoscale: Access Resistance Dominates Protein Channel Conductance in Diluted Solutions. ACS Nano 2017, 11, 10392–10400. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, J.; Hub, J.S. Converging PMF Calculations of Antibiotic Permeation across an Outer Membrane Porin with Subkilocalorie per Mole Accuracy. J. Chem. Inf. Model. 2023, 63, 5319–5330. [Google Scholar] [CrossRef]
- Lou, H.; Chen, M.; Black, S.S.; Bushell, S.R.; Ceccarelli, M.; Mach, T.; Beis, K.; Low, A.S.; Bamford, V.A.; Booth, I.R.; et al. Altered antibiotic transport in OmpC mutants isolated from a series of clinical strains of multi-drug resistant E. coli. PLoS ONE 2011, 6, e25825. [Google Scholar] [CrossRef] [PubMed]
- Golla, V.K.; Sans-Serramitjana, E.; Pothula, K.R.; Benier, L.; Bafna, J.A.; Winterhalter, M.; Kleinekathöfer, U. Fosfomycin Permeation through the Outer Membrane Porin OmpF. Biophys. J. 2019, 116, 258–269. [Google Scholar] [CrossRef]
- Schwarz, G.; Danelon, C.; Winterhalter, M. On translocation through a membrane channel via an internal binding site: Kinetics and voltage dependence. Biophys. J. 2003, 84, 2990–2998. [Google Scholar] [CrossRef]
- Neves, P.; Sousa, I.; Winterhalter, M.; Gameiro, P. Fluorescence quenching as a tool to investigate quinolone antibiotic interactions with bacterial protein OmpF. J. Membr. Biol. 2009, 227, 133–140. [Google Scholar] [CrossRef]
- Masi, M.; Vergalli, J.; Ghai, I.; Barba-Bon, A.; Schembri, T.; Nau, W.M.; Lafitte, D.; Winterhalter, M.; Pages, J.M. Cephalosporin translocation across enterobacterial OmpF and OmpC channels, a filter across the outer membrane. Commun. Biol. 2022, 5, 1059. [Google Scholar] [CrossRef]
- Hajjar, E.; Mahendran, K.R.; Kumar, A.; Bessonov, A.; Petrescu, M.; Weingart, H.; Ruggerone, P.; Winterhalter, M.; Ceccarelli, M. Bridging timescales and length scales: From macroscopic flux to the molecular mechanism of antibiotic diffusion through porins. Biophys. J. 2010, 98, 569–575. [Google Scholar] [CrossRef] [PubMed]
| Structural Insights and Functional Dynamics | Antibiotic Class Investigated | Porin(s) Examined | Principal Findings |
|---|---|---|---|
| Meropenem permeation through the outer membrane [84] | Meropenem | OprD, OprF, OprE | Meropenem uptake in Pseudomonas aeruginosa occurs via alternative porins (OprF/OprE) when OprD is absent, unlike imipenem. |
| Imipenem resistance associated with amino acid alterations porin in Pseudomonas aeruginosa clinical isolates [70] | Imipenem, Meropenem | OprD | This study investigates OprD mutations and expression levels in imipenem-resistant Pseudomonas aeruginosa from Ardabil hospitals, highlighting diverse resistance mechanisms. |
| Fosmidomycin uptake via phosphate-specific channels [85] | Fosmidomycin | OprO, OprP | Elucidated the mechanism of fosmidomycin transport through phosphate-specific porins |
| Characterization of single-channel properties [34] | NA | OccK8 | This study detailed single-channel behavior and conductance of OccK8 and analyzed the effects of Arginine, Glycine, and Glutamic Acid. |
| Analysis of conductance and gating in the OccD series [86] | NA | OccD1—OccD6 | Documented a broad conductance range, multiple gating transitions, and cation selectivity across these channels |
| Investigation of carboxylate interactions within the channel [27] | NA | OccD1—OccD6, OccK1—OccK7 | Identified interactions between carboxylate groups and basic residues (arginine/lysine) that influence uptake |
| Examination of multi-state gating dynamics [87] | NA | OccK1—OccK7 | Revealed distinct one-, two-, and three-open sub-states and established anion selectivity linked to constriction zone residues |
| Impact of internal loop deletion on channel behavior [88] | NA | OccK1 | Demonstrated that removal of the constriction loop significantly alters gating properties |
| Thermodynamic characterization of channel gating [33] | NA | OccK1 | Quantified the activation parameters for loop-deletion-induced transitions in channel activity |
| Influence of ion concentration on gating transitions [89] | NA | OpdK | Determined that variations in ion levels modulate the gating kinetics of the channel |
| Structural insight into substrate specificity [50] | NA | OprD | Revealed an 18-stranded β-barrel architecture with a narrow pore, critical for selective substrate transport |
| Role of surface loops in antibiotic translocation [58] | Imipenem | OprD | Demonstrated that specific extracellular loop regions are crucial for imipenem passage |
| Dynamics of amino acid substrate movement [80] | Imipenem, Meropenem | OccD1 (OprD) | Provided insight into the structural dynamics and natural substrate translocation through the pore |
| Uptake kinetics of carbapenem antibiotics [82] | Imipenem, Meropenem | OccD3 (OpdP) | Documented the transport rates of imipenem and meropenem across the channel |
| Mechanism of tricarboxylate and citrate uptake [44] | NA | OccK5 | Clarified the role of OccK5 in the uptake of key metabolites such as isocitrate and citrate |
| Variability in gating behaviors among porin channels [90] | NA | OccK5 | Identified diverse gating properties and conductance states within OccK5 |
| Role in temocillin permeation [15] | Temocillin | OccK1, OccK2 | Confirmed the contribution of these porins to temocillin entry into the cell |
| Energetics of ion selectivity [2,91] | NA | OprP | Established the energetic profile for the selective transport of phosphate, sulfate, chloride, and potassium ions |
| Critical role of acidic residue in binding [92] | NA | OprP | Highlighted the importance of residue D94 in phosphate binding and selectivity |
| Contribution of a central basic residue [93] | NA | OprP | Demonstrated that arginine R133 is vital for defining ion transport properties |
| Determining key constriction determinants [94] | NA | OprP, OprO | Identified essential constriction residues that affect substrate specificity |
| Reduced porin expression in resistance [21] | Imipenem, Meropenem | OprD | Linked decreased OprD levels to carbapenem heteroresistance |
| Correlation between porin levels and antibiotic MIC [64] | Imipenem, Meropenem | OprD | Showed that diminished porin expression elevates carbapenem MICs in clinical isolates |
| Quantification of OprD in resistant strains [20] | Imipenem, Meropenem | OprD | Documented lower OprD transcript levels in carbapenem-resistant isolates |
| Altered permeability and susceptibility profiles [95] | Imipenem, Meropenem | OprD | Revealed discrepancies in carbapenem susceptibility related to changes in outer membrane permeability |
| In vitro evaluation of novel β-lactam combinations [96] | Ceftazidime, Avibactam Ceftolozane, Tazobactam | OprD | Demonstrated activity of ceftazidime-avibactam and ceftolozane-tazobactam against meropenem-resistant isolates |
| Identification of in-frame deletions in clinical isolates [59] | NA | OprD | Detected unique in-frame deletions in OprD among clinical isolates |
| Variability of membrane protein dominance in resistance [67] | Imipenem, Meropenem | OprD | Observed shifts in membrane protein profiles in imipenem-resistant isolates |
| Whole-cell assay for permeability relationships [29] | Imipenem, Meropenem | OprD | Characterized structure–permeation correlations for novel carbapenem analogues |
| Effects of specific amino acid substitutions [72] | NA | OprD | Determined that substitution at codon 170 correlates with increased resistance |
| Impact of single residue changes on resistance [71] | Imipenem, Meropenem | OprD | Demonstrated that individual amino acid alterations significantly affect carbapenem susceptibility |
| Consequences of incapacitating mutations [43] | Imipenem, Meropenem | OprD | Showed that severe mutations and decreased expression contribute to high-level resistance |
| Survey of porin presence in resistant isolates [97] | Imipenem, Meropenem | OprD | Confirmed widespread occurrence of altered OprD in 70 carbapenem-resistant isolates |
| Double mutations altering ion specificity [98] | NA | OprP, OprO | Demonstrated that double mutations can invert specificity between phosphate and diphosphate transport |
| Structural features underpinning amino acid transport [3,81] | Ceftazidime Hydrate, Cefotaxime, Carbenicillin | OccK8 | Defined substrate-specific transport mechanisms for amino acids via OccK8 |
| Permeation of Fosfomycin through the Phosphate-Specific Channels OprP and OprO of Pseudomonas aeruginosa [99] | Fosfomycin, Fosmidomycin | OprP and OprO | Fosfomycin uses specific channels (OprP and OprO) to enter resistant Pseudomonas aeruginosa more effectively than fosmidomycin. Exploiting channel selectivity could improve antibiotic uptake in Gram-negative bacteria. |
| Probing transport of fosfomycin through substrate specific membrane proteins [79] | Fosfomycin | OprO, OprP | Fosfomycin exhibits high permeability through OprO and OprP porins, making it a promising alternative for treating Pseudomonas aeruginosa infections. |
| Major Topic | Antibiotic Class/Molecule | Porins Examined | Principal Findings | |
|---|---|---|---|---|
| Structural Insights | NA | OccK8, OccD1-D6, OprP, OprO, OprD | Characterization of β-barrel architectures, pore diameters, constriction zones, and surface loops critical for substrate specificity. Multi-state gating and charge-selective filters. | [2,27,33,34,50,86,87,88,89,91,92,93,94,98] |
| Functional Dynamics | NA | OccK1–K7, OccD1–D6, OpdK | Detailed conductance ranges, gating transitions, anion/cation selectivity, and effects of loop deletions or point mutations on permeability. | [27,33,86,87,88,89] |
| Porin Modifications and Resistance Mechanisms | Imipenem, Meropenem | OprD | Diverse OprD mutations, in-frame deletions, and reduced expression linked to elevated carbapenem MICs and heteroresistance in clinical isolates. | [20,21,29,43,50,58,59,64,67,70,71,72,80,95,96,97] |
| Transport Mechanisms of Nutrients/Ions | NA | OprP, OprO, OccK5 | Mechanistic insights into phosphate, sulfate, chloride, potassium, isocitrate, and citrate uptake; crucial role of constriction residues and charged residues. | [2,44,79,91,92,93,94,98,99] |
| Antibiotic Uptake Studies | Imipenem, Meropenem, Temocillin | OprD, OccK1, OccK2, OprF, OprE | Carbapenem transport is mainly via OprD; alternative uptake via OprF/OprE when OprD is absent. OccK porins contribute to temocillin entry. | [3,15,20,21,29,43,58,64,67,70,71,80,81,82,84,95,96,97] |
| Fosfomycin/Fosmidomycin Studies | Fosfomycin, Fosmidomycin | OprP, OprO | High permeability of fosfomycin via OprO (~280 molecules/s) compared to OprP (~2.2 molecules/s), exploiting phosphate-specific channels. | [79,85,99] |
| Whole-Cell Permeability Correlations | Imipenem, Meropenem | OprD | Reduced OprD levels directly correlate with increased MICs. Structure–permeability relationships established for novel carbapenems. | [29,64,96] |
| Novel β-lactam Combinations | Ceftazidime-Avibactam, Ceftolozane-Tazobactam | OprD | Showed activity against meropenem-resistant isolates with altered OprD profiles. | [96] |
| Molecules | Omps | Bug | Recalculated Flux at 1 μM Molecules/s | Reported Flux Rate Molecules/s at the Specific Mentioned Gradient |
|---|---|---|---|---|
| Fosfomycin | OprO | Pseudomonas aeruginosa | 28 | ≈280 at gradient 10 μM [79] |
| OprP | Pseudomonas aeruginosa | ≤1 | ≈2.2 at gradient 10 μM [79] | |
| Ceftazidime | OprE | Pseudomonas aeruginosa | ≤1 | ≈0.4 a t gradient 10 μM [81] |
| Cefotaxime | OprE | Pseudomonas aeruginosa | ≤1 | ≈0.1 at gradient 10 μM [81] |
| Carbenicillin | OprE | Pseudomonas aeruginosa | ≤1 | 0.04 at gradient 10 μM [81] |
| Sodium Glutamate | OprE | Pseudomonas aeruginosa | ≤1 | ≈0.6 at gradient 10 μM [81] |
| Arginine Chloride | OprE | Pseudomonas aeruginosa | ≤1 | ≈0.1 at gradient 10 μM [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghai, I. Understanding Drug Permeability in Pseudomonas aeruginosa. Life 2025, 15, 1705. https://doi.org/10.3390/life15111705
Ghai I. Understanding Drug Permeability in Pseudomonas aeruginosa. Life. 2025; 15(11):1705. https://doi.org/10.3390/life15111705
Chicago/Turabian StyleGhai, Ishan. 2025. "Understanding Drug Permeability in Pseudomonas aeruginosa" Life 15, no. 11: 1705. https://doi.org/10.3390/life15111705
APA StyleGhai, I. (2025). Understanding Drug Permeability in Pseudomonas aeruginosa. Life, 15(11), 1705. https://doi.org/10.3390/life15111705






