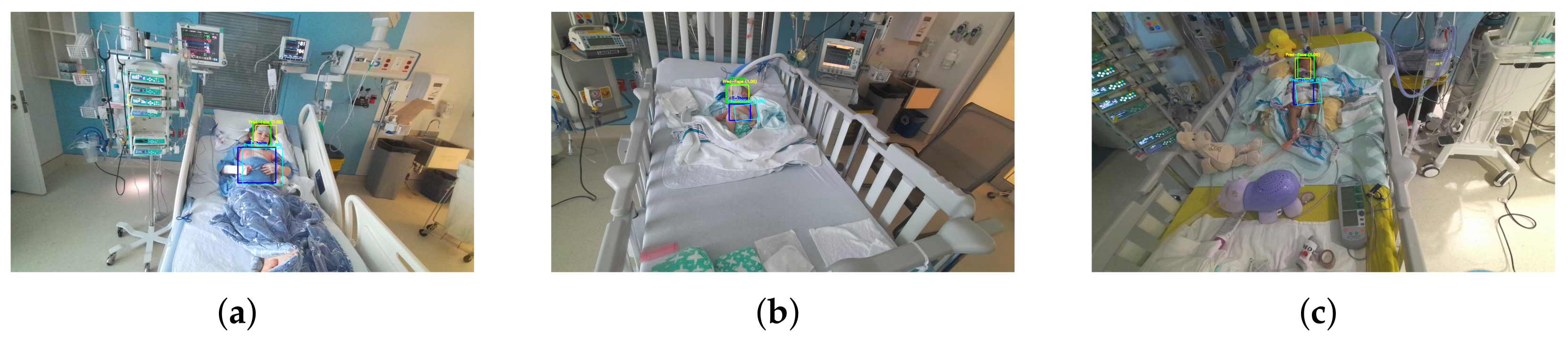
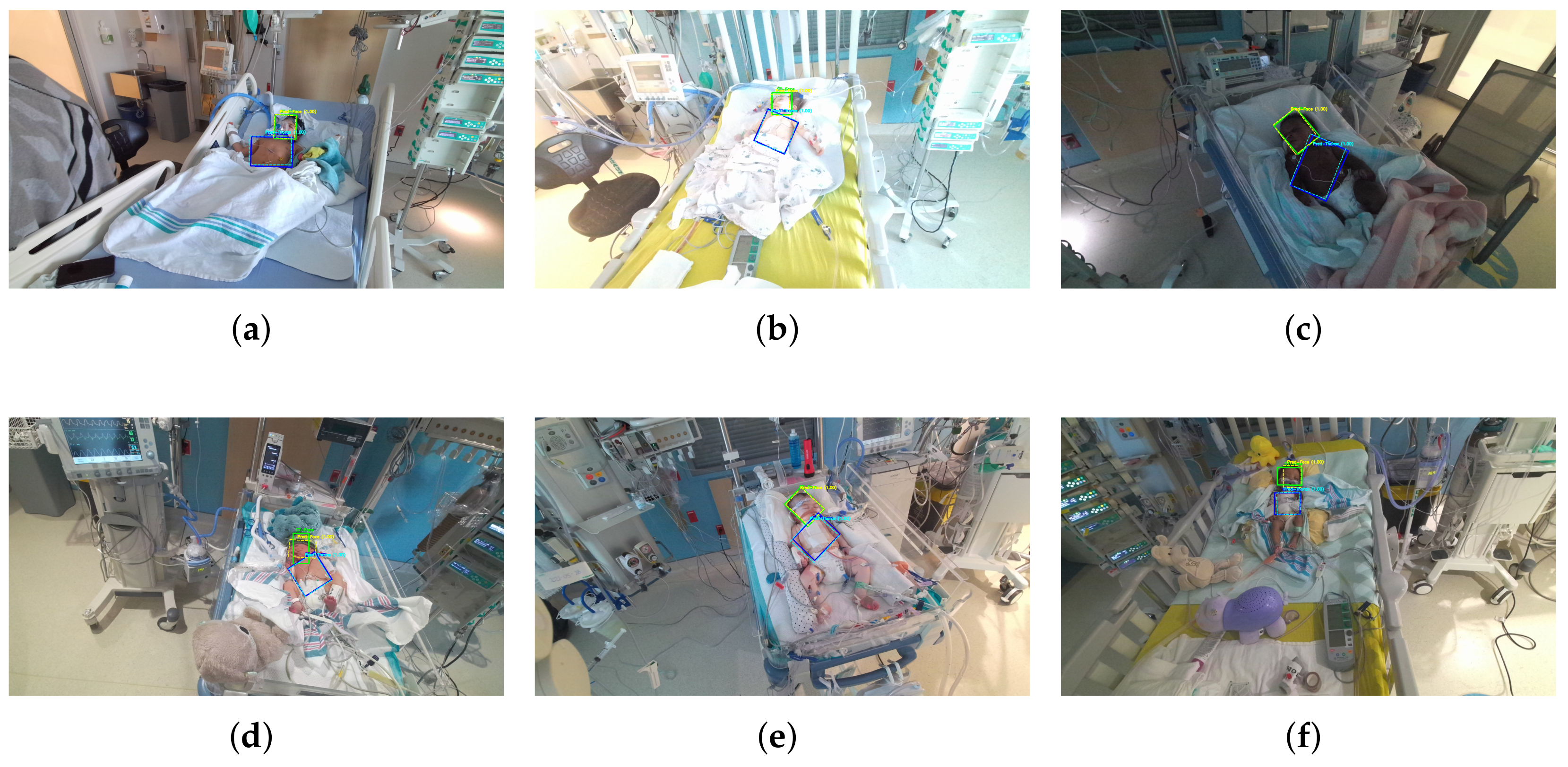
1. Introduction
Monitoring vital signs in pediatric patients within Pediatric Intensive Care Units (PICUs) is essential due to their fragile health conditions. Non-contact approaches, such as remote photoplethysmography (rPPG) and respiratory monitoring, especially for conditions like Acute Respiratory Distress Syndrome (ARDS) [
1,
2], depend on accurate detection of anatomical regions, particularly the face and thoracoabdominal areas. Accurate and anatomically consistent localization is essential for reliable vital sign estimation in PICU environments. Remote photoplethysmography (rPPG) depends on stable face crops that preserve skin-only regions, as chrominance fluctuations are easily corrupted by background leakage or bounding box drift. Even minor spatial jitter or rotation error can distort the rPPG signal, degrading heart rate accuracy and introducing artifacts into the frequency spectrum. Similarly, thoracoabdominal detection must capture the cyclic expansion of the chest along the correct orientation axis to extract respiratory motion cues. Boxes that drift or encompass non-thoracic regions, such as blankets or bedrails, risk obscuring the subtle periodic deformations that encode respiration.
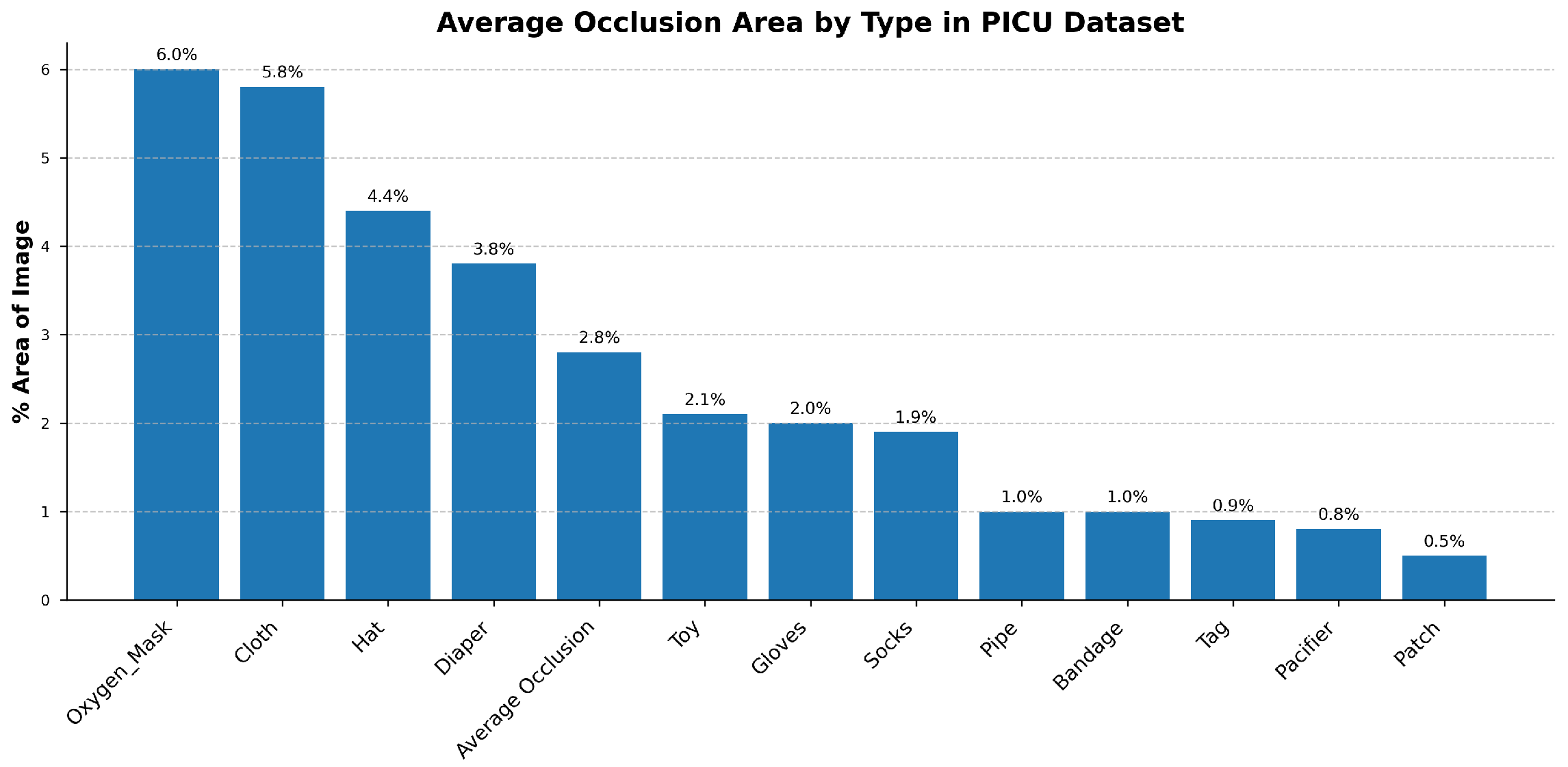
The PICU setting presents several challenges: patients are frequently obscured by medical devices, lighting conditions, and patient orientations, and there is a lack of annotated data necessary for training effective models for our specific clinical setting. Notably, no existing public video datasets capture both facial and thoracoabdominal regions simultaneously in PICU environments, necessitating the creation of our own dataset. In addition to RGB inputs, depth information provides complementary geometric context that improves robustness to occlusions and illumination variability, both of which are frequent in PICU environments. Unlike RGB, depth is invariant to lighting changes and can help distinguish foreground anatomical structures from background clutter such as tubing, blankets, or bedrails. This is particularly valuable when visual cues are weak or partially obstructed. In such cases, depth enhances the stability of region tracking and supports more reliable detection of subtle motion patterns. However, consistent acquisition and integration of depth data in real-world clinical settings remain non-trivial, requiring careful sensor placement, calibration, and synchronization with RGB streams to ensure reliable performance across patient conditions and hardware setups.
Data scarcity remains one of the main challenges in the medical domain, primarily due to strict privacy constraints and ethical considerations. Collecting large-scale, manually annotated video datasets in a clinical environment like the PICU is exceptionally difficult and costly. Furthermore, there is a significant domain gap between general-purpose videos and clinical data; features learned from datasets such as Kinetics-400 often fail to generalize to the unique PICU setting [
3], limiting model performance across different healthcare settings [
4,
5]. Downstream tasks in the PICU are especially challenging due to the subtle motion patterns and unique anatomical features of pediatric patients, where standard face detectors often fail because of underdeveloped facial structures and frequent occlusions from medical equipment [
6,
7]. To address both data limitations and domain discrepancies, self-supervised learning (SSL) provides a promising solution. Pre-training on unlabeled hospital videos allows models to learn relevant spatiotemporal features directly from the target environment, reducing dependence on manual annotations [
8]. Among SSL methods, masked autoencoders such as VideoMAE [
9] have demonstrated strong efficiency, offering robust representation learning for clinical settings where annotated data is limited.
While convolutional neural networks (CNNs) have significantly advanced static object detection and tracking, with strong performance on large-scale datasets such as COCO [
10] and PASCAL VOC [
11], these models are not designed to capture the temporal dynamics essential for physiological monitoring [
12]. Reliable signal monitoring depends on stable region-of-interest (ROI) tracking across time [
1,
13]. Frame-based detectors, including recent YOLO variants, are limited in this context for two main reasons: first, they often suffer from temporal inconsistencies such as bounding box jitter, which introduces motion artifacts into the predicted signals [
14,
15]; second, their per-frame inference leads to high computational overhead, making them less suitable for real-time applications.
The Video Vision Transformers (ViViT) model [
16,
17,
18] relies on self-attention mechanisms to capture long-range dependencies and global context. However, it is computationally expensive and often struggles to generalize, especially when trained on limited datasets. Its lack of inherent inductive biases, such as spatial locality, makes it less effective in data-constrained environments. Moreover, its resource requirements increase quadratically when processing high-resolution inputs or integrating multimodal data like RGB and depth, which makes it difficult to deploy in real-world clinical settings. In contrast, 3D convolutional neural networks (3D-CNNs) [
19,
20] possess strong inductive biases, making them more suitable for limited-data scenarios. However, their localized kernel limits their ability to model long-range spatiotemporal dependencies, which are crucial for understanding complex clinical settings.
PICU monitoring systems must process multiple patient video streams continuously on shared, resource-limited workstations, often without dedicated accelerators or cloud offloading due to privacy and maintenance constraints. Reliable bedside use therefore requires low-jitter, real-time inference with a small memory footprint while coexisting with other clinical software. Under these conditions, models whose time and memory scale quadratically with the token length L (e.g., multi-head self-attention, ) become impractical as resolution or temporal context grows. This motivates architectures with linear-time complexity () and low VRAM that maintain stable latency on longer clips and higher resolutions.
Reliable face and thoracoabdominal localization is a prerequisite for contactless vital-sign monitoring in the PICU. The face region provides the skin pixels needed for rPPG, where minute chrominance fluctuations encode heart rate; any drift or background leakage rapidly degrades signal-to-noise and produces unstable frequency peaks. The thoracoabdominal region carries respiratory motion, so bounding boxes must remain temporally stable and orientation-aware to capture cyclic expansion/deflation without being contaminated by blankets, caregiver hands, or attached devices. Axis-aligned boxes are often insufficient under infant pose changes, bed tilt, and off-axis cameras; oriented bounding boxes (OBBs) yield tighter, rotation-consistent crops, improving both rPPG sampling using skin-only pixels and respiration estimation with motion along the anteroposterior axis. These constraints, coupled with frequent occlusions, specular lighting, and the need for real-time processing on clinical hardware, motivate a detector that is robust, temporally stable, and computationally efficient for face and thoracoabdominal ROIs, precisely the focus of our approach below.
To address these challenges, we introduce Divided Space–Time (DST) Mamba, an SSM-based detector for face and thoracoabdominal regions with oriented bounding boxes (OBBs). The model is built on Selective State Space Models (SSMs) [
21] and runs in linear time
with respect to sequence length
L, which is essential for real-time monitoring. Unlike VideoMamba, which processes space and time jointly, DST decouples them: a spatial stage followed by a temporal stage. This factorization reduces cross-axis interference, preserves temporal dynamics relevant to rPPG/respiration, and enables axis-specific optimization. It also allows independent MAE pre-training for spatial masked patches and temporal masked tube objectives. To handle PICU conditions, like occlusions and low contrast, we use data-efficient masked-autoencoding pre-training and support multimodal input (RGB + depth) to improve perceptual robustness.
The primary contributions of this work are outlined as follows:
Reliable ROI detection in PICU videos: we successfully detect the face and thoracoabdominal regions with oriented boxes despite occlusions, devices, motion, and limited labels in the PICU. This robustness is achieved via a Divided Space–Time Mamba design that preserves temporal dynamics while remaining computationally efficient.
Data scarcity and domain gaps addressed: we mitigate scarce annotations and lab-to-PICU domain shift by employing self-supervised masked-autoencoder pre-training tailored to our clinical video distribution.
Multimodal robustness under occlusion: we enhance detection accuracy and reduce angle drift in occlusion-heavy scenes by integrating RGB with depth and analyzing the accuracy–complexity trade-off for deployment.
Clinical-grade efficiency and comparative gains: we achieve real-time throughput and low FLOPs while outperforming strong frame-wise and video baselines on accuracy and temporal stability through a factorized spatial-to-temporal SSM pipeline and targeted ablations.
3. Proposed Method
3.1. Overview
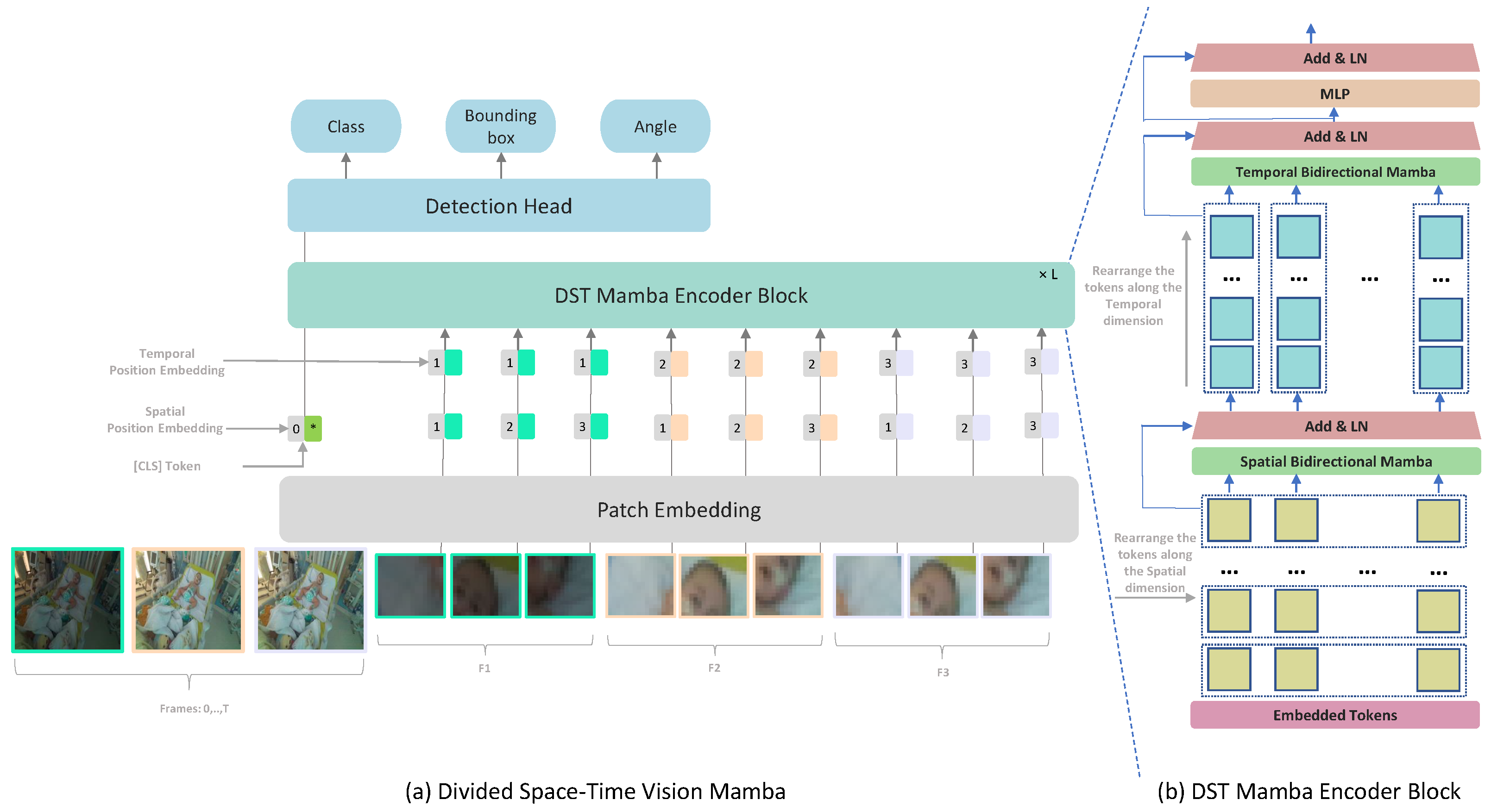
In this work, we propose a Mamba-based approach for medical video detection that reliably localizes the face and thoracoabdominal regions in PICU videos. The backbone factorizes spatiotemporal modeling by stacking Divided Space–Time (DST) Mamba blocks, which first consolidate spatial structure before modeling temporal dynamics. This sequential design allows the encoder to capture both coarse scene layouts and fine-grained motion cues, all while preserving temporal signals with linear-time complexity. On these features, a lightweight Mamba-based detection head predicts oriented bounding boxes (OBBs), yielding rotation-consistent localization under pose changes, bed tilt, and off-axis cameras, as shown in
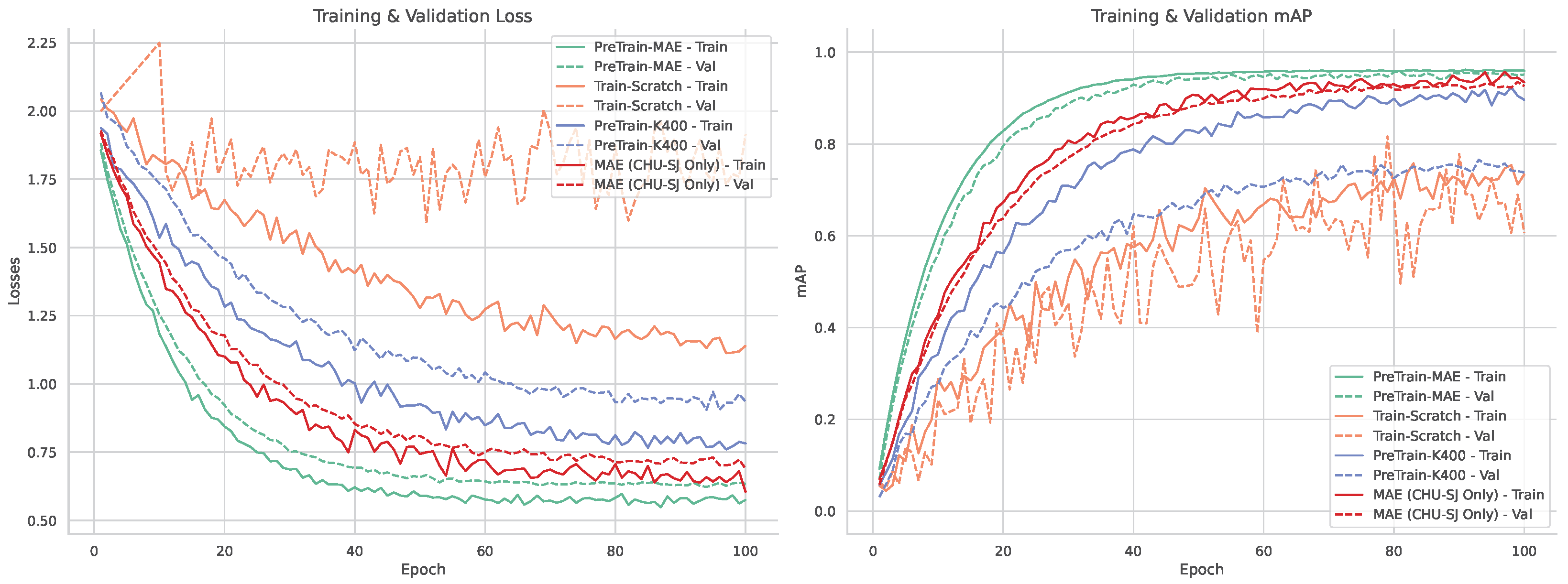
Figure 1. To address data scarcity and domain shift, we investigate two SSL pre-training techniques: Masked Autoencoders (MAE) and Unmasked Teacher (UMT). MAE reconstructs masked patches to learn robust local appearance priors, whereas UMT distills higher-level spatiotemporal structure from a teacher without masking. We quantify the contribution of SSL and depth by comparing VideoMamba and our DST-Mamba with and without pretraining and with RGB versus RGB-D input. All models are first pretrained on a combined set of CHU Sainte-Justine PICU clips and publicly available pediatric data, then fine-tuned on the PICU dataset for face and thoracoabdominal OBB detection. This comparative design targets the core clinical video challenges of data scarcity, heavy occlusions, and rapid domain shift, while favoring deployment through linear-time inference.
3.2. Preliminary Explanation: State Space Models
State Space Models (SSMs) map a 1-D function or sequence
using a hidden state
. This system is described as linear ordinary differential equations (ODEs), employing matrices
to define how the hidden state evolves and
and
for the projection of the input and the hidden state to the output:
S4 [
66] and Mamba [
21] integrate a timescale parameter
to discretize the continuous system and convert the continuous parameters
to discrete parameters
. The transformation is defined as follows:
After the discretization of
, the (
1) is transformed into:
A global convolution is employed to compute the model output:
Here
M represents the length of the input sequence
x, and
is a structured convolutional kernel.
3.3. Divided Space–Time Video Mamba
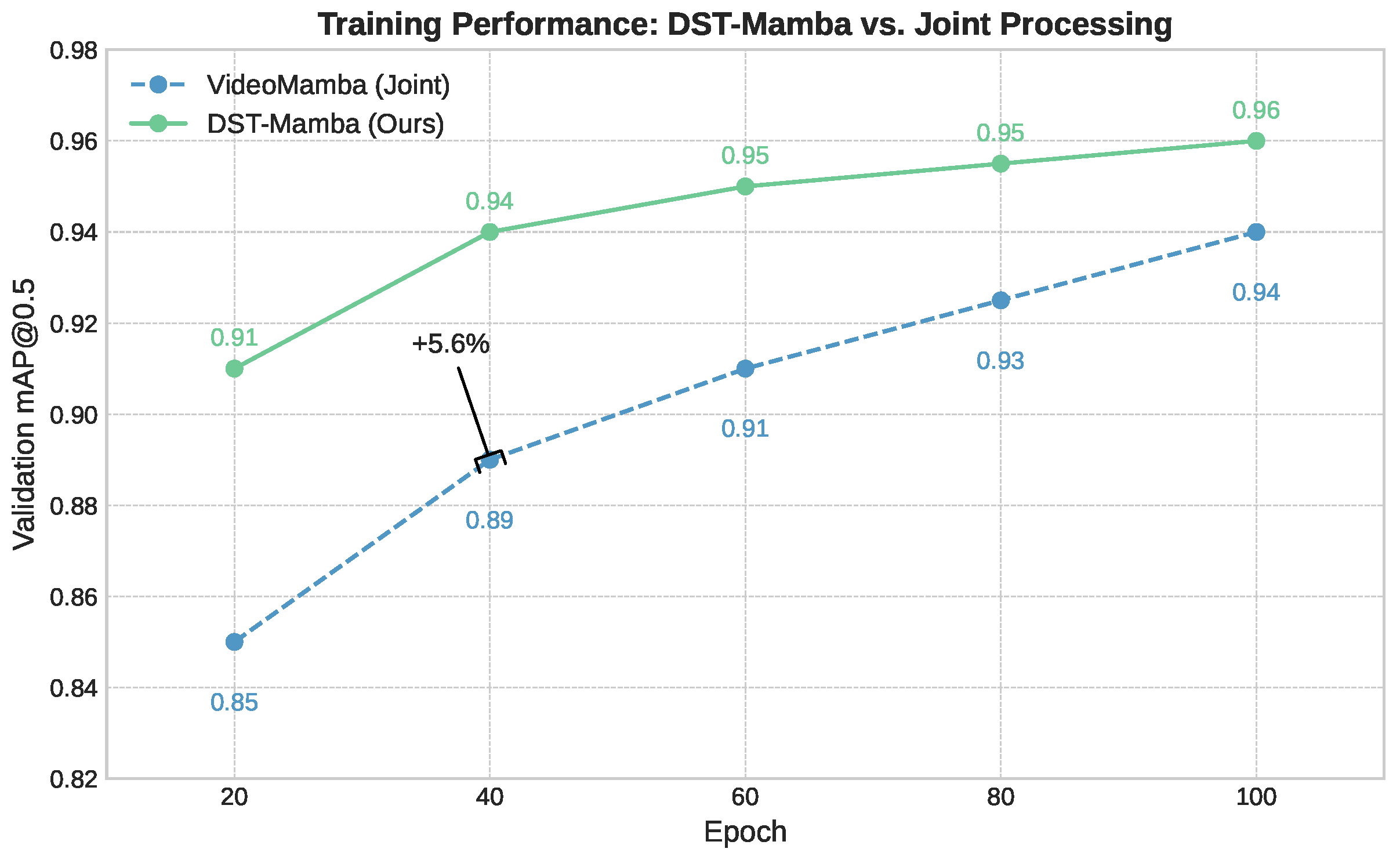
3.3.1. Baseline: Joint Spatiotemporal Processing
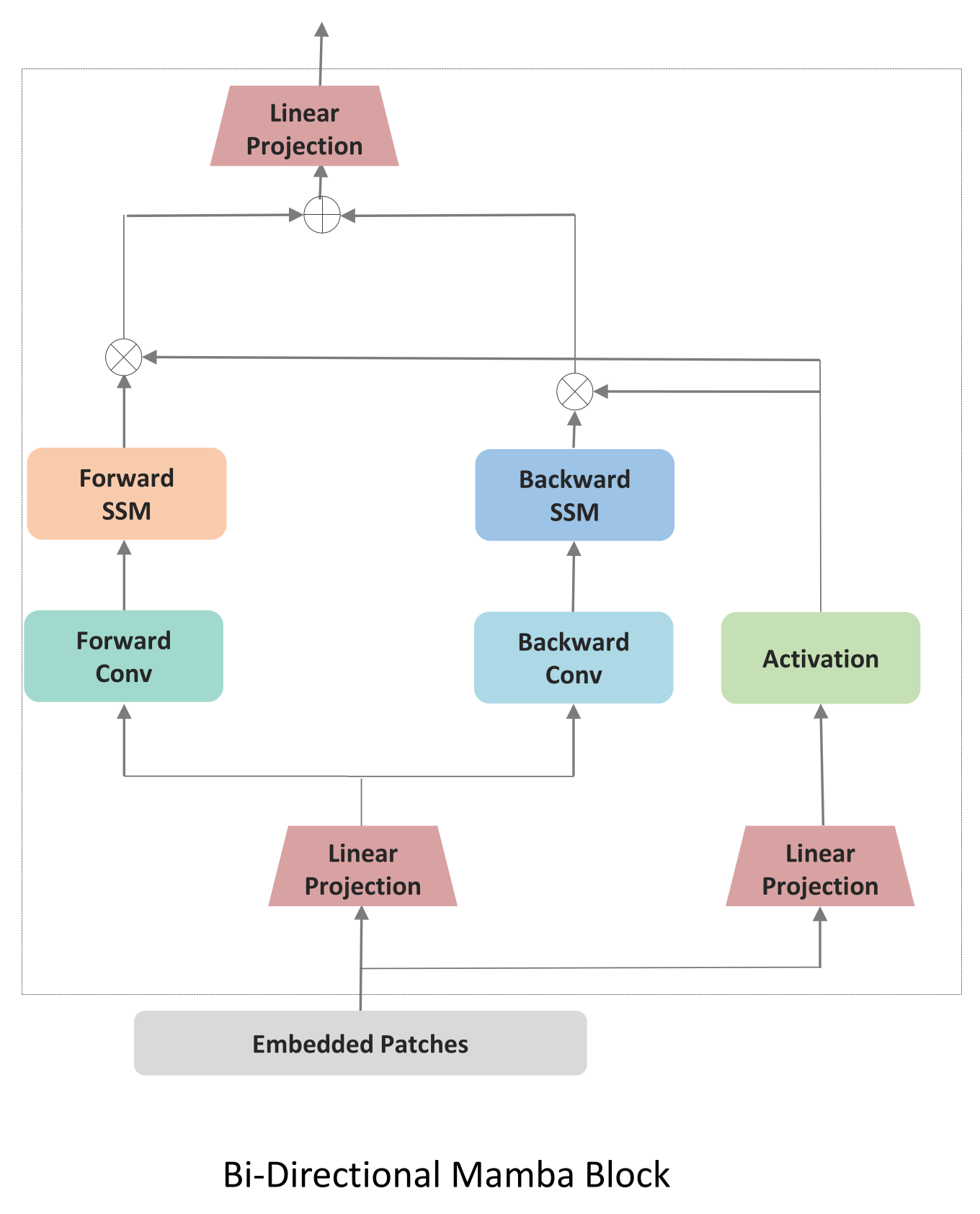
We first implemented a baseline following VideoMamba [
81] which processes spatial and temporal information jointly through a unified bidirectional scanning mechanism,
Figure 2. VideoMamba extends the Mamba state space model to video understanding by treating the entire video as a single sequence of spatiotemporal tokens. Given an input video
, where
T is the number of frames and
are spatial dimensions, VideoMamba first applies a 3D convolutional patch embedding to obtain
N spatiotemporal patches
; where
for
P is the patch size, and
D is the embedding dimension. Each token represents a local spatiotemporal cube containing information from multiple consecutive frames. VideoMamba [
81] applies a joint scanning strategy. All
N tokens are arranged in a single sequence according to a spatial-first ordering:
This sequence is then processed by bidirectional Mamba blocks:
where
,
,
are the state space parameters, and
is the time-scale parameter. The bidirectional scan enables each token to aggregate context from both past and future tokens in the sequence. All the patches are processed and then
L Bidirectional Mamba blocks are used, where a spatial-first bidirectional scan is applied.The joint approach may not allow for fine-tuning the balance between spatial and temporal processing. By processing spatial and temporal information jointly, the model might not develop specialized features for each dimension.
3.3.2. Divided Space–Time Processing
While TimeSformer [
17] employs a Divided Space–Time Multi-Head Self-Attention (MHSA), its quadratic complexity with respect to token count poses challenges for long video sequences, where token numbers grow linearly with input frames. To address this, we propose a Divided Space–Time Mamba block that models intra- and inter-frame long-range dependencies efficiently, resolving scalability issues without sacrificing performance. We refined this approach by introducing a modified Vision Mamba architecture based on a Divided Space–Time Mamba model, as illustrated in
Figure 1. By separating Vision Mamba into spatial and temporal modules, the architecture leverages specialized learning for each dimension: the spatial module captures fine-grained details within individual frames, while the temporal module tracks movement and event progression over time. This division is particularly effective for medical video detection, enabling the model to learn dynamic appearance and motion cues more efficiently.
Each frame in the input clip is divided into non-overlapping patches of size . This ensures that the N patches cover the entire frame, with N defined as . Each token is represented by , where p and t are spatial locations and a frame index. The sequence of tokens is initially arranged in , where N represents the patch position within each frame, T indexes time and D is the embedding size of each token.
The encoder blocks process temporal and spatial dimensions separately, one after the other. Each block
l, we first use
to fix the temporal dimension. Then, we perform a bidirectional scan across all frames to capture spatial dependencies:
The output of the temporal B-Mamba scan is then fed forward to compute the temporal B-Mamba encoder where all tokens are grouped based on frames
. The temporal B-Mamba block performs bidirectional selective scans across frames, ensuring that temporal dependencies are aggregated from both past and future contexts. Across both time and space dimensions, separate parameters are learned:
for the temporal component and
for the spatial component.
3.4. Pretraining Approaches
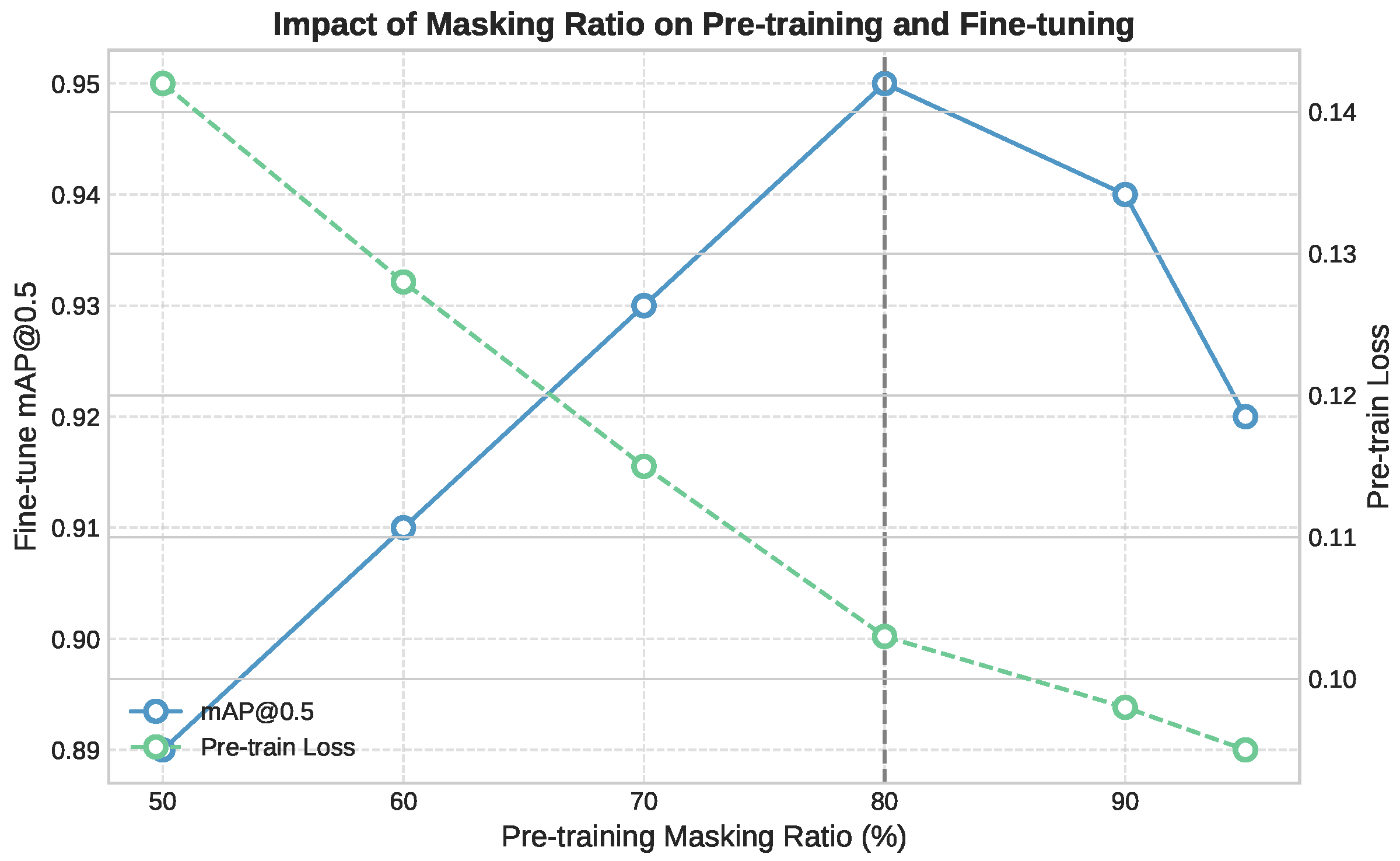
To address the scarcity of labeled data in clinical video settings, we investigated two distinct self-supervised learning (SSL) strategies for initializing our Divided Space–Time Mamba model: a Teacher–Student approach based on semantic distillation, and a fully self-supervised reconstruction strategy using masked autoencoding. The embedded tokens are passed through the encoder and decoder parts, respectively. The encoder part consists of L stacked Mamba blocks and aims to extract meaningful latent representations by processing only masked input sequences. These representations capture the context and structure of the visible data while learning to predict missing tokens. In the pretraining stage, the learnable special token is removed.
Teacher-Student SSL: We first attempted a teacher-student SSL approach using CLIP [
83] as the teacher model providing semantic guidance. Inspired by previous works [
64,
81], the decoder part aligns unmasked tokens directly with a linear projection to the teacher model. For masking strategy, we employ a frame-by-frame semantic masking approach, assigning higher probabilities to tokens that carry crucial clues [
64,
84]. In the PICU setting, this strategy resulted in unstable convergence and poor generalization, which is caused by the semantic gap between the teacher and the target domain and the mismatch between the pretraining objectives. CLIP was trained on generic web images and captions that emphasize everyday objects and scenes, while our clinical data involve subtle and domain-specific patterns such as neonatal anatomy, occlusions, and the presence of medical equipment. The teacher often highlighted irrelevant elements like monitors or cables instead of the anatomical regions required for detection. Additionally, CLIP’s training objective focuses on global image–text alignment, whereas our model requires precise spatial localization and temporal consistency. This misalignment likely led to conflicting gradients during training, especially under high masking ratios and in visually degraded frames.
Masked Autoencoders SSL: UMT relies heavily on the semantic guidance from the teacher model. If this model is pre-trained on general images that are vastly different from PICU environments, the guidance might be less relevant or even misleading. Video Masked Autoencoders’s self-supervised approach allows it to learn directly from the target domain (PICU videos) without relying on potentially mismatched external knowledge. The decoder part consists of stacked B-Mamba blocks with a final output projection to reconstruct the masked video patches. VideoMAE’s masking strategy and reconstruction objective enable it to capture domain-specific features and patterns present in PICU data, even if they’re very different from general image datasets. In the Divided Space–Time Mamba model, and to keep the structure, masked tokens are replaced with learnable parameters. These learnable embeddings act as placeholders for the missing information and are processed alongside the unmasked tokens. The learnable parameters allow gradients to flow through the masked positions, which can improve model optimization.
3.5. Depth Information Integration
To enhance the ability of the model to learn subtle anatomical features and motion cues within the complex PICU setting. We augment our Divided Space–Time Mamba architecture by introducing depth maps as an additional input channel alongside RGB frames. For each input video frame , we incorporate a corresponding depth map , resulting in a four-channel input . Each token is represented by .
Depth information is fused through early channel-level concatenation prior to tokenization, ensuring pixel-wise alignment between RGB and depth modalities. The fused 4-channel frames are processed by a shared patch-embedding layer and the same Divided Space–Time Mamba encoder, without the use of a separate fusion or attention branch.
During the pretraining phase, we apply the same masking strategies to both RGB and depth channels, encouraging the model to learn the relationships between appearance and geometric features. Our experiments demonstrate that the incorporation of depth information leads to improved performance in both pretraining and downstream tasks, particularly in scenarios requiring precise spatial understanding of the PICU environment.
3.6. Fine-Tuning
The pretrained model undergoes fine-tuning on the PICU dataset for face and thoracoabdominal detection. During fine-tuning, the decoder block is replaced with a lightweight detection head comprising three projection layers for classification, bounding box regression, and orientation angle prediction.
The detection task in PICU environments necessitates a multi-component loss function to address distinct challenges inherent to clinical video analysis. The total loss integrates four components:
where
and
balance the contribution of each component based on their typical value ranges during training.
Classification Loss (): Binary Cross-Entropy is employed for object presence prediction. This choice addresses the non-mutually exclusive nature of face and thorax detection in PICU frames, where medical equipment frequently occludes one region while leaving the other visible.
Angle Loss (): Orientation prediction utilizes Cross-Entropy loss with Circular Spatial Layout (CSL), discretizing the 180° rotation space into 180 bins. The CSL formulation addresses the periodicity of angular measurements, where standard Cross-Entropy would incorrectly treat adjacent angles (e.g., 179° and 1°) as maximally different.
Bounding Box Regression (): L1 loss optimizes the coordinate predictions for bounding box parameters . We selected L1 over L2 loss due to its robustness to annotation outliers present in clinical data.
Oriented IoU Loss (
): The rotated Intersection over Union loss directly optimizes the spatial overlap between predicted and ground truth oriented bounding boxes:
This component ensures spatial alignment beyond coordinate accuracy, particularly crucial for oriented boxes where axis-aligned IoU would penalize correctly oriented predictions. In cases involving patient rotation, bed tilt, or oblique camera angles, a predicted box may be geometrically correct yet misjudged by axis-aligned IoU metrics due to misalignment with image axes. The rotated IoU (rIoU) metric accounts for both position and orientation, providing a more accurate measure of overlap under rotation. This is particularly important for respiratory motion analysis, where chest orientation must be tracked precisely, and penalizing correctly rotated predictions would compromise model evaluation and training.
6. Discussion
Our results indicate that the Divided Space–Time (DST) Mamba architecture directly addresses the core obstacles of PICU video detection, setting a new state of the art for face and thoracoabdominal localization. By factorizing spatiotemporal modeling space-to-time, the model preserves temporal dynamics under motion and occlusions, while oriented boxes (OBBs) with circular smooth label (CSL) supervision explicitly handle orientation variability and camera skew, improving rIoU and reducing angle error. Domain-native self-supervised pretraining (MAE/UMT) mitigates data scarcity and domain shift, yielding higher mAP and more reliable convergence than training from scratch or Kinetics-400 finetuning. Integrating depth (RGB–D) further improves localization in occlusion-heavy, low-contrast scenes, with a measured compute trade-off and occasional angle-error increase that we report. Finally, the state-space formulation confers linear-time complexity and real-time, deployment-oriented efficiency (e.g., ∼23 FPS at
with ∼7.56 GFLOPs) while outperforming strong frame-wise and video baselines on accuracy and stability. Representative failure cases are presented in
Appendix A Figure A1, illustrating reduced detection accuracy under severe occlusion, low illumination, and partial patient rotation.
6.1. From High-Accuracy Detection to Clinical Reliability
The extraction of physiological signals using non-contact methods like remote photoplethysmography (rPPG) is highly sensitive to the stability and consistency of the input ROI. Frame-based detectors process each frame independently, which introduces spatiotemporal jitter and increases inference time, limiting real-time applicability in clinical settings. In contrast, our DST-Mamba model achieves a high temporal IoU of 0.95, ensuring temporally coherent and stable ROIs. This stability reduces non-physiological motion noise and improves the signal-to-noise ratio (SNR), which is essential for reliable physiological monitoring. A mean Average Precision of 0.96 reflects both technical accuracy and clinically meaningful consistency in anatomical localization across frames. This level of performance minimizes gaps in region tracking, reducing the risk of signal disruption during rPPG or respiratory extraction. In critical care settings, even short detection lapses can lead to missed events or delayed alerts, making stable and accurate detection essential for continuous, high-fidelity monitoring. Additionally, the high rotational IoU ensures precise anatomical localization, preventing signal contamination from surrounding regions and reducing the risk of false alarms in intensive care environments.
6.2. Limitations & Future Work
The results of this study must be considered in the context of several key limitations. First, the model was developed and validated using data from a single institution. Its performance on data from other PICUs, which may differ in lighting conditions, camera configurations, and clinical protocols, remains untested. To our knowledge, no other publicly available PICU video datasets exist that capture both facial and thoracoabdominal regions simultaneously, which necessitated our reliance on single-center data. To address potential site-specific bias, our framework integrates multimodal RGB–Depth inputs, which are inherently less sensitive to lighting variations and camera-specific color calibration. In addition, we perform self-supervised pre-training on a heterogeneous corpus of over 50,000 video clips comprising both real PICU recordings and synthetically generated hospital scenarios with diverse illumination, contrast, and viewpoints. This domain-diverse pretraining strategy serves as an implicit form of cross-institutional regularization and enhances the model’s robustness to unseen acquisition conditions. A multi-center validation is therefore a critical next step to assess whether the model can generalize. Although this study incorporates depth maps during training, real-time depth capture is not yet standard in most PICU monitoring systems. Future work should assess the feasibility and clinical value of integrating low-cost depth sensors at the bedside to enable robust 4D video analysis.
Second, the dataset is limited to 485 patients due to the practical and ethical challenges of data acquisition in pediatric critical care. The absence of public video datasets for this task necessitated our use of a synthetic data generation strategy. This strategy, while beneficial to pre-training performance in our experiments, is itself a limitation. We acknowledge that this method primarily provides rich spatial augmentation and does not capture true, physiologically relevant temporal dynamics. However, our results demonstrate that this spatial pre-training provides a crucial foundation, allowing the model to generalize much more effectively when subsequently fine-tuned on real clinical videos where it learns the relevant temporal patterns. Further work is required to determine if this method captures meaningful temporal dynamics or primarily provides spatial augmentation. Third, this study’s scope is confined to the detection and tracking of anatomical regions. The work does not validate whether the improved detection metrics translate to more accurate downstream vital sign extraction. Establishing this link between technical performance and clinical utility is a crucial future step.
Finally, the model has not been tested in a live clinical workflow. Any claims regarding deployment readiness are premature, as real-world use requires prospective testing, integration with hospital IT systems, and navigating regulatory pathways.
Future work involves integrating our DST-Mamba architecture with vital sign extraction algorithms for prospective clinical validation against contact monitors. To address data scarcity and foster collaboration, we will release our open-source code and augmented data generation methodology upon publication. The code will be made publicly available at:
https://github.com/mkbensalah/Divided-Space-Time-Mamba, accessed on 29 October 2025.
7. Conclusions
In this paper, we presented the Divided Space–Time Video Mamba framework for medical video detection in Pediatric Intensive Care Unit (PICU) environments. By decoupling spatial and temporal processing, our approach achieves high accuracy (0.95 mAP@0.50) while maintaining computational efficiency. The incorporation of masked autoencoder pre-training further improves performance, reaching 0.96 rIoU and 0.95 mAP@0.50, and shows improved performance on our single-center dataset. Additionally, integrating depth information enhances the model’s robustness to occlusions and variable lighting conditions. The efficiency of DST-Mamba supports its integration into real-time clinical systems. With 7.56 GFLOPs and 73M parameters, the model processes 16-frame inputs at 640 × 640 resolution in 43 ms, achieving 23 FPS. Its linear complexity ensures predictable scalability with respect to input resolution and sequence length, unlike transformer-based models that scale quadratically. This makes it suitable for deployment on edge devices such as bedside monitors or portable diagnostic tools in the PICU. Furthermore, the divided space–time structure promotes interpretability by isolating spatial and temporal contributions. This separation facilitates the analysis of detection failures and helps verify consistency across frames. The use of oriented bounding boxes produces rotation-aware outputs that align with clinical requirements, especially in respiratory monitoring where thoracic orientation directly influences signal quality. In addition, saliency-based visualizations or attribution maps can be employed to reveal the specific regions within each frame that most influence the model’s predictions. Such visual feedback allows clinicians to verify that the model attends to relevant anatomical features, rather than being misdirected by medical equipment, patient coverings, or shadows. Future work will focus on extending DST-Mamba toward cross-device generalization by evaluating its robustness across different RGB-D sensors and acquisition settings, and by incorporating domain-adaptation techniques to mitigate sensor-specific variability. We will also investigate lightweight pruning, quantization, and token-reduction strategies to enable efficient deployment on embedded and bedside monitoring systems. This study was conducted under institutional ethical approval from CHU Sainte-Justine, with all video data processed within secure research servers. Future work will explore privacy-preserving edge AI and federated learning frameworks to ensure patient data remain local while enabling continuous, on-device model adaptation for real-time bedside use.