A Novel Approach to Assessing In-Hospital Mortality After On-Pump Aortic Valve Replacement
Abstract
1. Introduction
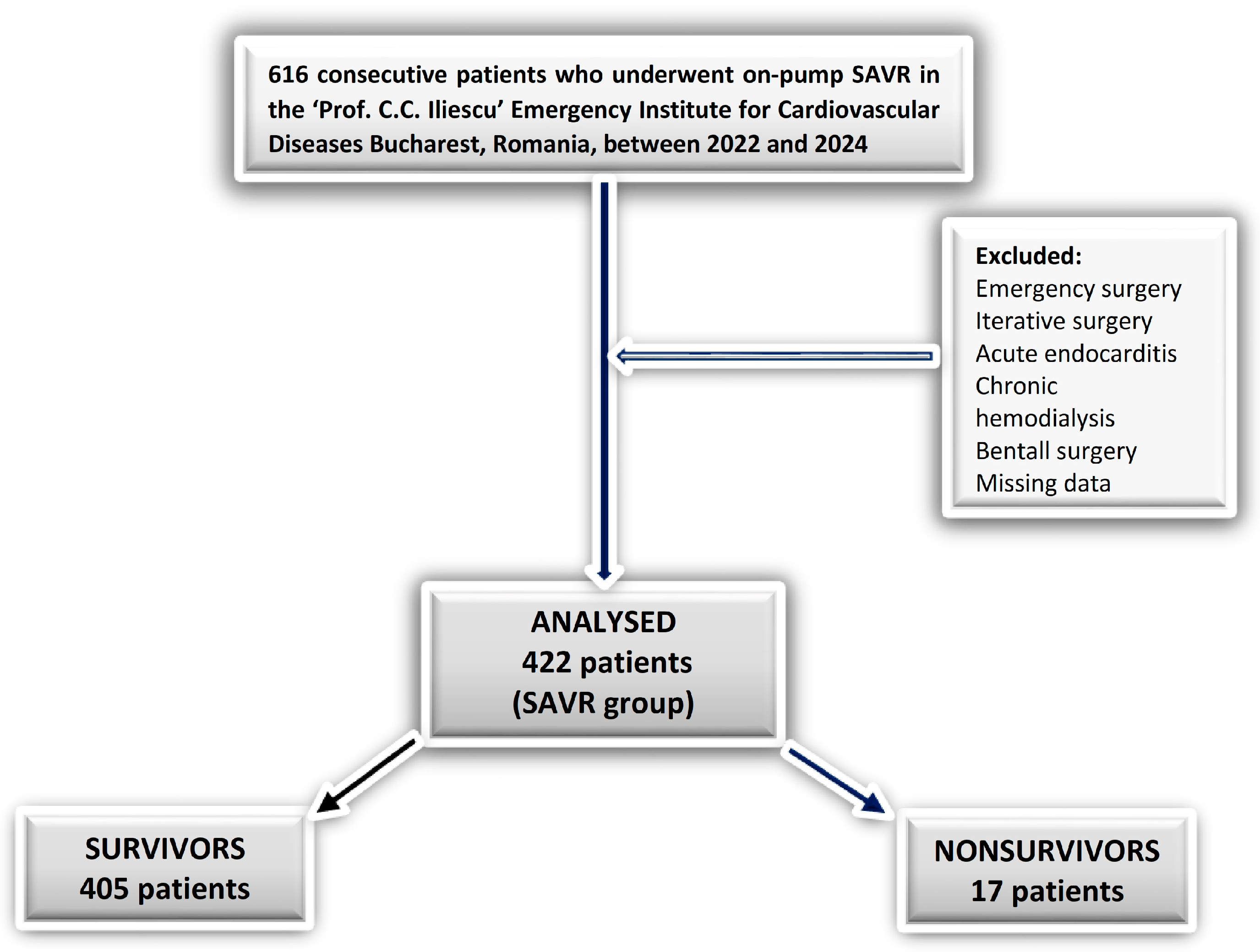
2. Materials and Methods
2.1. Study Design
2.2. Procedure
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Data Presentation
3.1.1. Preoperative Data
3.1.2. Intraoperative Data
3.1.3. Early Postoperative Data
3.1.4. Day One After Surgery Data
3.1.5. Postoperative Complications
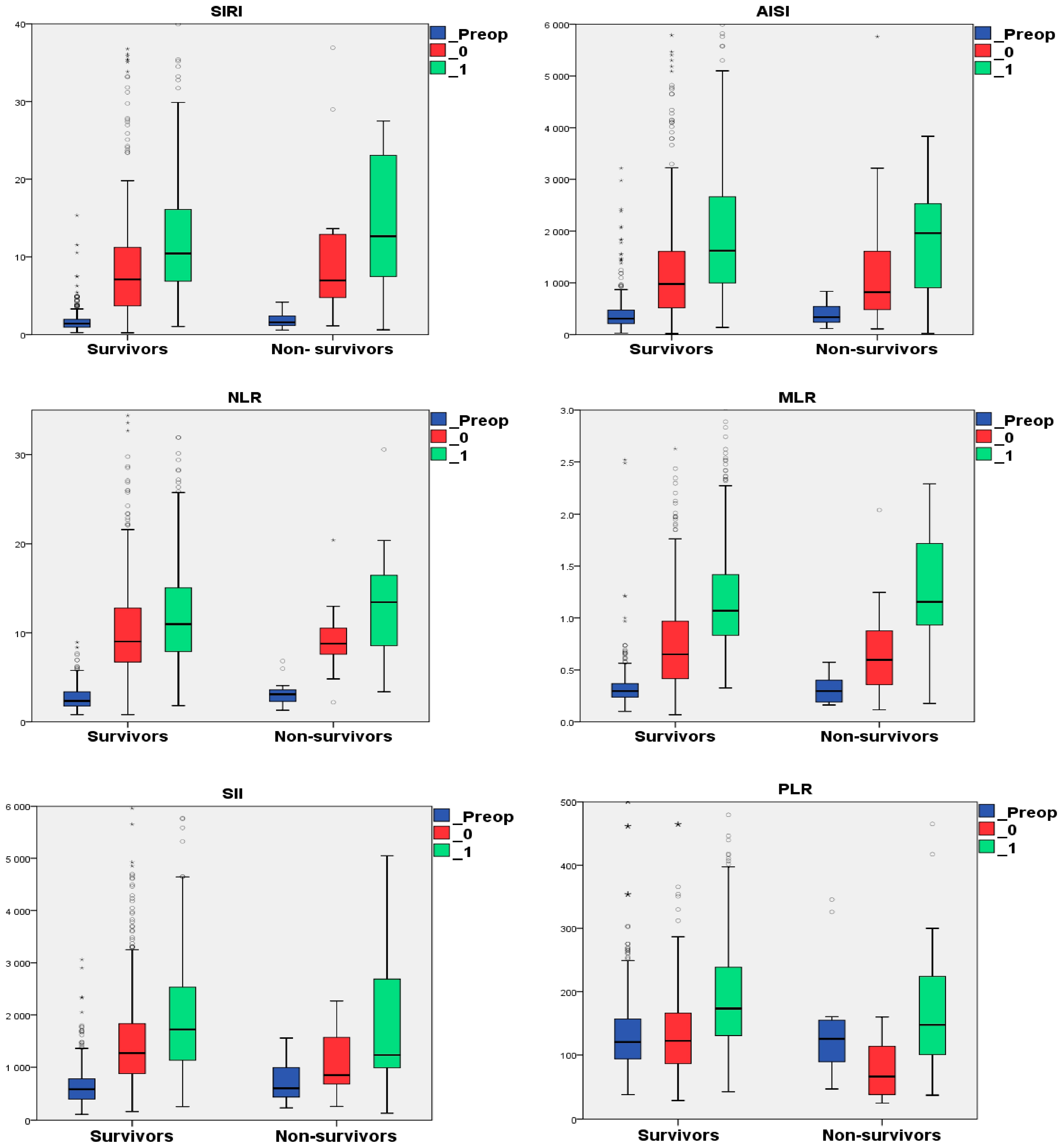
3.2. The Perioperative Dynamics of the Hematological Data and Inflammatory Indexes
3.3. The Binary Logistic Regression Analysis
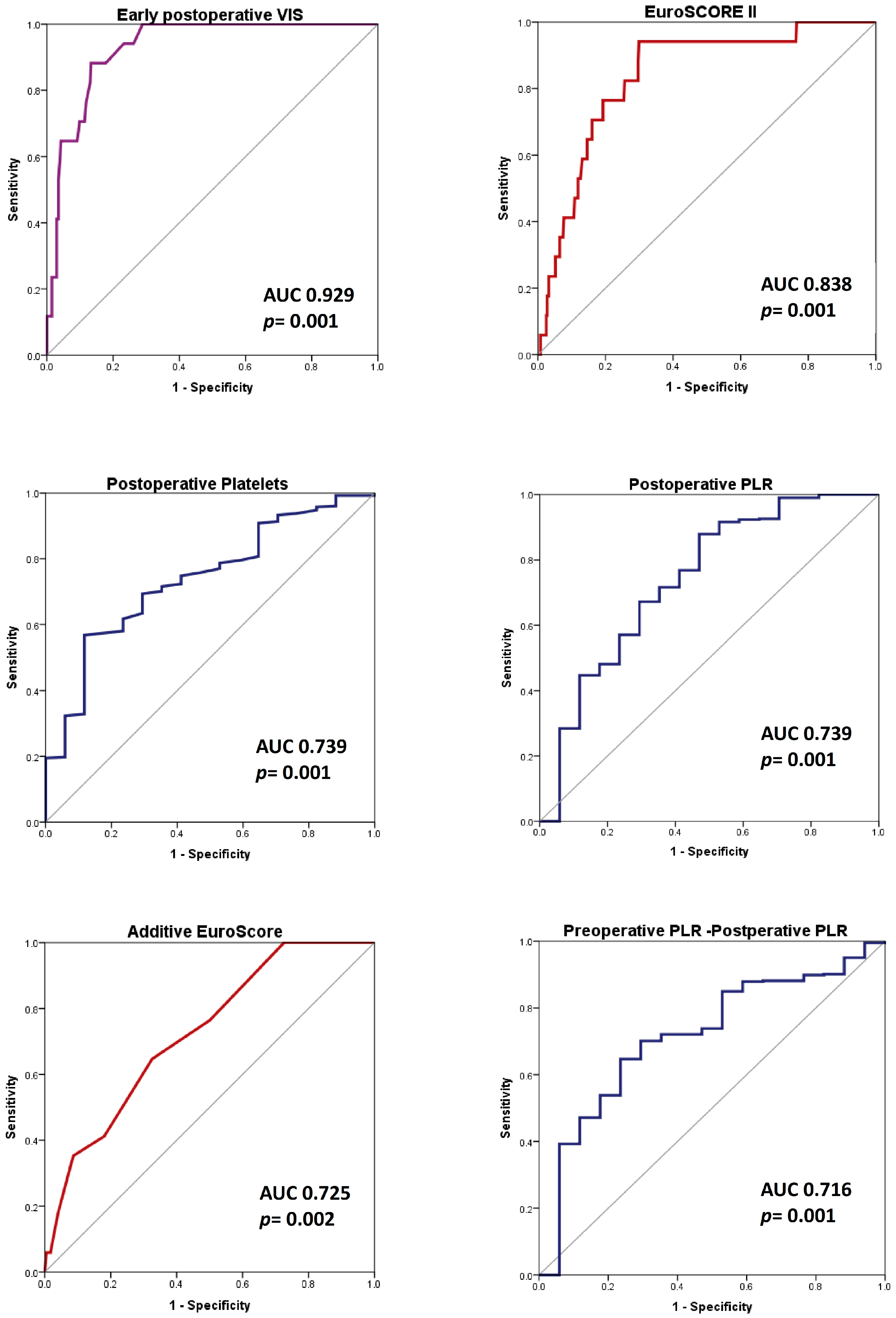
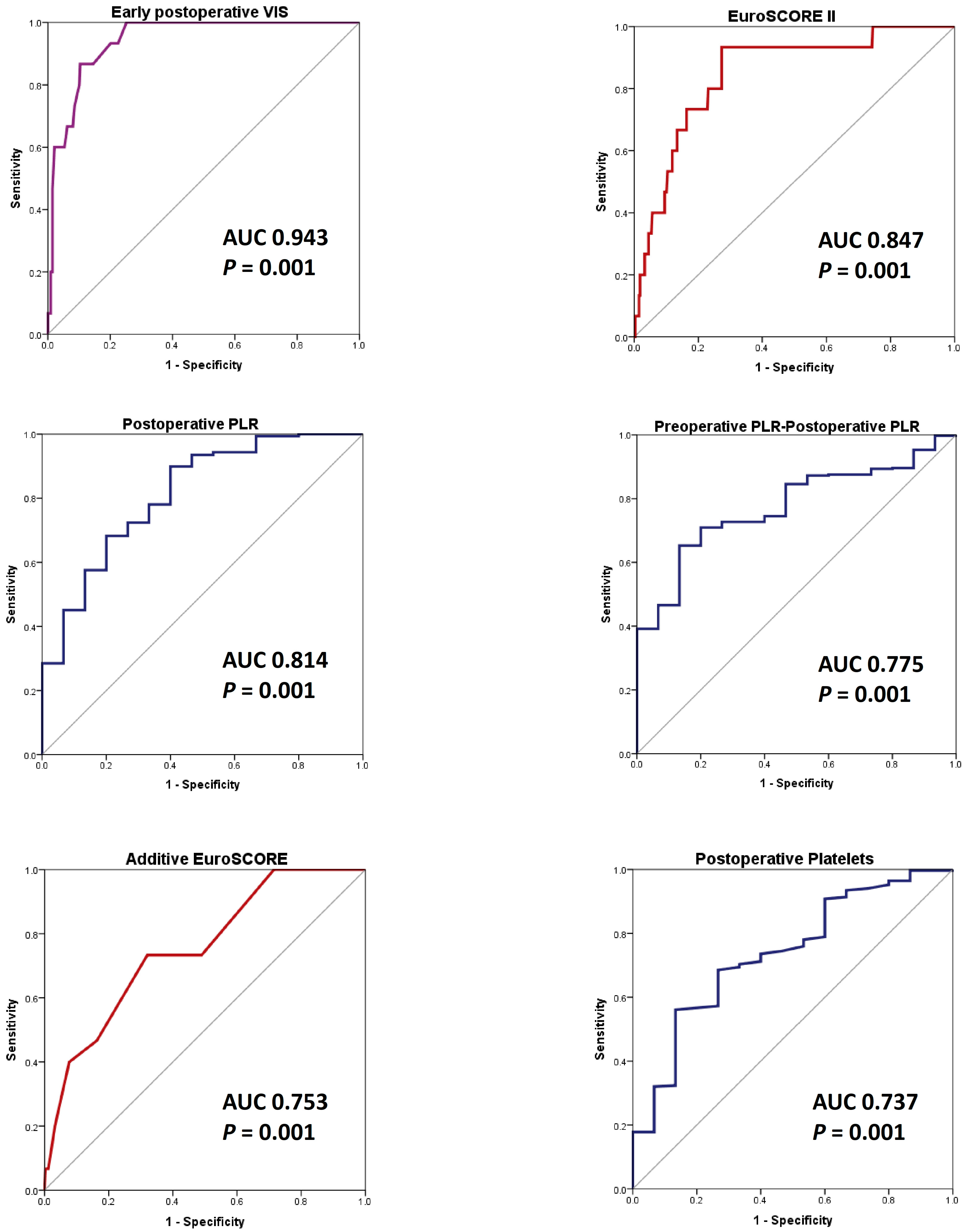
3.4. ROC Analysis for Predicting In-Hospital Death
3.4.1. SAVR Group (422 Patients)
3.4.2. AS_SAVR Group (352 Patients)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef]
- Santangelo, G.; Bursi, F.; Faggiano, A.; Moscardelli, S.; Simeoli, P.S.; Guazzi, M.; Lorusso, R.; Carugo, S.; Faggiano, P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J. Clin. Med. 2023, 12, 2178. [Google Scholar] [CrossRef]
- Drăgan, A.; Mateescu, A.D. Novel Biomarkers and Advanced Cardiac Imaging in Aortic Stenosis: Old and New. Biomolecules 2023, 13, 1661. [Google Scholar] [CrossRef]
- de Assis, L.U.; Mondellini, G.M.; van den Dorpel, M.M.; van Niekerk, J.; Van Mieghem, N.M. Incidence and Pathology of Aortic Regurgitation. J. Interv. Cardiol. 2025, 20, e07. [Google Scholar] [CrossRef]
- Ryan, C.T.; Almousa, A.; Zea-Vera, R.; Zhang, Q.; Amos, C.I.; Coselli, J.S.; Rosengart, T.K.; Ghanta, R.K. Outcomes of Aortic Valve Replacement for Chronic Aortic Insufficiency: Analysis of the Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2022, 113, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Shvartz, V.; Sokolskaya, M.; Petrosyan, A.; Ispiryan, A.; Donakanyan, S.; Bockeria, L.; Bockeri, A.O. Predictors of Mortality Following Aortic Valve Replacement in Aortic Stenosis Patients. Pathophysiology 2022, 29, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Jang, E.J.; Jo, J.W.; You, J.; Park, J.B.; Ryu, H.G. Institutional case volume and mortality after aortic and mitral valve replacement: A nationwide study in two Korean cohorts. J. Cardiothorac. Surg. 2022, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- Fudulu, D.P.; Layton, G.; Nguyen, B.; Sinha, S.; Dimagli, A.; Guida, G.; Abbasciano, R.; Viviano, A.; Angelini, G.D.; Zakkar, M. Trends and outcomes of concomitant aortic valve replacement and coronary artery bypass grafting in the UK and a survey of practices. Eur. J. Cardiothorac. Surg. 2023, 64, ezad259. [Google Scholar] [CrossRef]
- Spiliopoulos, K.; Magouliotis, D.; Angelis, I.; Skoularigis, J.; Kemkes, B.M.; Salemis, N.S.; Athanasiou, T.; Gansera, B.; Xanthopoulos, A.V. Concomitant Valve Replacement and Coronary Artery Bypass Grafting Surgery: Lessons from the Past, Guidance for the Future? A Mortality Analysis in 294 Patients. J. Clin. Med. 2024, 13, 238. [Google Scholar] [CrossRef]
- Yousef, S.; Brown, J.; Serna-Gallegos, D.; Navid, F.; Warraich, N.; Yoon, P.; Kaczorowski, D.; Bonatti, J.; Wang, Y.; Sultan, I. Impact of Aortic Root Enlargement on Patients Undergoing Aortic Valve Replacement. Ann. Thorac. Surg. 2023, 115, 396–402. [Google Scholar] [CrossRef]
- Arshad, H.B.; Minhas, A.M.K.; Khan, S.U.; Nasir, K.; Rao, N.; Thacker, S.; Butt, S.A.; Faza, N.; Little, S.H.; von Ballmoos, M.W.; et al. National Trends and Outcomes of Surgical Aortic Valve Replacement with Concomitant Mitral Valve Surgery. Cardiovasc. Revasc. Med. 2022, 40, 13–19. [Google Scholar] [CrossRef]
- Ohmes, L.B.; Kim, L.; Feldman, D.N.; Lau, C.; Munjal, M.; Di Franco, A.; Hameedi, F.; Gambardella, I.; Girardi, L.N.; Gaudino, M. Contemporary prevalence, in-hospital outcomes, and prognostic determinants of triple valve surgery: National database review involving 5234 patients. Int. J. Surg. 2017, 44, 132–138. [Google Scholar] [CrossRef]
- Laaksonen, M.; Kholova, I.; Paavonen, T.; Mennander, A. Histopathology reveals concealed aortic valve inflammation. J. Cardiothorac. Surg. 2024, 19, 41. [Google Scholar] [CrossRef]
- Driscoll, K.; Cruz, A.D.; Butcher, J.T. Inflammatory and Biomechanical Drivers of Endothelial-Interstitial Interactions in Calcific Aortic Valve Disease. Circ. Res. 2021, 128, 1344–1370. [Google Scholar] [CrossRef]
- Klauzen, P.; Basovich, L.; Shishkova, D.; Markova, V.; Malashicheva, A. Macrophages in Calcific Aortic Valve Disease: Paracrine and Juxtacrine Disease Drivers. Biomolecules 2024, 14, 1547. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, J.H. Involvement of Immune Cell Network in Aortic Valve Stenosis: Communication between Valvular Interstitial Cells and Immune Cells. Immune Netw. 2016, 16, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wallby, L.; Steffensen, T.; Broqvist, M. Role of inflammation in nonrheumatic, regurgitant heart valve disease. A comparative, descriptive study regarding apolipoproteins and inflammatory cells in nonrheumatic heart valve disease. Cardiovasc. Pathol. 2007, 16, 171–178. [Google Scholar] [CrossRef]
- Viikinkoski, E.; Aittokallio, J.; Lehto, J.; Ollila, H.; Relander, A.; Vasankari, T.; Jalkanen, J.; Gunn, J.; Jalkanen, S.; Airaksinen, J.; et al. Prolonged Systemic Inflammatory Response Syndrome After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2024, 38, 709–716. [Google Scholar] [CrossRef]
- Aljure, O.D.; Fabbro, M., II. Cardiopulmonary Bypass and Inflammation: The Hidden Enemy. J. Cardiothorac. Vasc. Anesth. 2019, 33, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Han, J.; Wang, W.; Xie, B.; Hou, X.; Jin, Z.; Chen, L.; Chen, X.; Liu, L.; Hei, F.; et al. Shanghai Medical Association, Heart and Vascular Surgery Branch. Expert consensus on perioperative inflammation management in cardiac surgery involving cardiopulmonary bypass. J. Emerg. Crit. Care Med. 2025, 9, 8. [Google Scholar] [CrossRef]
- Shvartz, V.; Sokolskaya, M.; Ispiryan, A.; Basieva, M.; Kazanova, P.; Shvartz, E.; Talibova, S.; Petrosyan, A.; Kanametov, T.; Donakanyan, S.; et al. The Role of «Novel» Biomarkers of Systemic Inflammation in the Development of Early Hospital Events after Aortic Valve Replacement in Patients with Aortic Stenosis. Life 2023, 13, 1395. [Google Scholar] [CrossRef] [PubMed]
- Gaies, M.G.; Gurney, J.G.; Yen, A.H.; Napoli, M.L.; Gajarski, R.J.; Ohye, R.G.; Charpie, J.R.; Hirsch, J.C. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr. Crit. Care Med. 2010, 11, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Oba, K.; Matsui, Y.; Morimoto, Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J. Anesth. 2018, 32, 167–173. [Google Scholar] [CrossRef]
- Tohme, J.; Lescroart, M.; Guillemin, J.; Orer, P.; Dureau, P.; Varnous, S.; Leprince, P.; Coutance, G.; Bouglé, A. Association between vasoactive-inotropic score, morbidity and mortality after heart transplantation. Interdiscip Cardiovasc. Thorac Surg. 2023, 36, ivad055. [Google Scholar] [CrossRef]
- Keleş, B.O.; Yılmaz, E.T.; Altınbaş, A.; Zengin, S.; Yılmaz, S. When is the Ideal Time to Calculate the Vasoactive Inotropic Score as a Predictor of Mortality and Morbidity in Cardiac Surgery? A Retrospective Study. Ann. Card. Anaesth. 2024, 27, 37–42. [Google Scholar] [CrossRef]
- Sun, Y.T.; Wu, W.; Yao, Y.T. The association of vasoactive-inotropic score and surgical patients’ outcomes: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Maack, C.; Eschenhagen, T.; Hamdani, N.; Heinzel, F.R.; Lyon, A.R.; Manstein, D.J.; Metzger, J.; Papp, Z.; Tocchetti, C.G.; Yilmaz, M.B.; et al. Treatments targeting inotropy. Eur. Heart J. 2019, 40, 3626–3644. [Google Scholar] [CrossRef]
- Singer, M. Catecholamine treatment for shock–equally good or bad. Lancet 2007, 370, 636–637. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Tang, B.; Yu, X.; Huang, Y. Association between perioperative plasma transfusion and in-hospital mortality in patients undergoing surgeries without massive transfusion: A nationwide retrospective cohort study. Front. Med. 2023, 10, 1130359. [Google Scholar] [CrossRef]
- Smith, M.M.; Kor, D.J.; Frank, R.D.; Weister, T.J.; Dearani, J.A.; Warner, M.A. Intraoperative Plasma Transfusion Volumes and Outcomes in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1446–1456. [Google Scholar] [CrossRef]
- Hinton, J.V.; Fletcher, C.M.; Perry, L.A.; Greifer, N.; Hinton, J.N.; Williams-Spence, J.; Segal, R.; Smith, J.A.; Reid, C.M.; Weinberg, L.; et al. Platelet versus fresh frozen plasma transfusion for coagulopathy in cardiac surgery patients. PLoS ONE 2024, 19, e0296726. [Google Scholar] [CrossRef]
- Hinton, J.V.; Xing, Z.; Fletcher, C.; Perry, L.A.; Karamesinis, A.; Shi, J.; Penny-Dimri, J.C.; Ramson, D.; Coulson, T.G.; Segal, R.; et al. Association of perioperative transfusion of fresh frozen plasma and outcomes after cardiac surgery. Acta Anaesthesiol. Scand. 2024, 68, 753–763. [Google Scholar] [CrossRef]
- Bjursten, H.; Al-Rashidi, F.; Dardashti, A.; Brondén, B.; Algotsson, L.; Ederoth, P. Risks associated with the transfusion of various blood products in aortic valve replacement. Ann. Thorac. Surg. 2013, 96, 494–499. [Google Scholar] [CrossRef]
- Fletcher, C.M.; Hinton, J.V.; Xing, Z.; Perry, L.A.; Karamesinis, A.; Shi, J.; Penny-Dimri, J.C.; Ramson, D.; Liu, Z.; Smith, J.A.; et al. Fresh frozen plasma transfusion after cardiac surgery. Perfusion 2025, 40, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.M.; Hinton, J.V.; Xing, Z.; Perry, L.A.; Greifer, N.; Karamesinis, A.; Shi, J.; Penny-Dimri, J.C.; Ramson, D.; Liu, Z.; et al. Platelet Transfusion in Cardiac Surgery: An Entropy-Balanced, Weighted, Multicenter Analysis. Anesth. Analg. 2024, 138, 542–551. [Google Scholar] [CrossRef]
- Casselman, F.; Lance, M.; Ahmed, A.; Ascari, A.; Blanco-Morillo, J.; Bolliger, D.; Eid, M.; Erdoes, G.; Gerhardus Haumann, H.; Jeppsson, A.; et al. EACTS/EACTAIC/EBCP Scientific Document Group, 2024 EACTS/EACTAIC Guidelines on patient blood management in adult cardiac surgery in collaboration with EBCP. Eur. J. Cardio-Thorac. Surg. 2024, 67, ezae352. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.; Relvas, J.H.; Persson, M.; Cabral, T.D.D.; Persson, J.E.; de Oliveira, J.S.; Bonow, P.; Freire, C.V.S.; Amaral, S. Prothrombin Complex Concentrate versus Fresh Frozen Plasma in Adult Patients Undergoing Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Chest Surg. 2024, 57, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.R.; Bronsert, M.; Reece, T.B.; Pal, J.D.; Cleveland, J.C.; Fullerton, D.A.; Gist, K.M.; Jovanovich, A.; Jalal, D.; Faubel, S.; et al. Thrombocytopenia After Cardiopulmonary Bypass Is Associated with Increased Morbidity and Mortality. Ann. Thorac. Surg. 2020, 110, 50–57. [Google Scholar] [CrossRef]
- Kertai, M.D.; Zhou, S.; Karhausen, J.A.; Cooter, M.; Jooste, E.; Li, Y.J.; White, W.D.; Aronson, S.; Podgoreanu, M.V.; Gaca, J.; et al. Platelet Counts, Acute Kidney Injury, and Mortality after Coronary Artery Bypass Grafting Surgery. Anesthesiology 2016, 124, 339–352. [Google Scholar] [CrossRef]
- Nong, Y.; Wei, X.; Lu, J.; Yu, D. The prognostic value of postoperative platelet levels in elderly patients after valve replacement surgery: A retrospective cohort study. BMC Cardiovasc. Disord. 2024, 24, 379. [Google Scholar] [CrossRef]
- Hyde, J.A.; Chinn, J.A.; Graham, T.R. Platelets and cardiopulmonary bypass. Perfusion 1998, 13, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Törnudd, M.; Ramström, S.; Kvitting, J.P.; Alfredsson, J.; Nyberg, L.; Björkman, E.; Berg, S. Platelet Function is Preserved After Moderate Cardiopulmonary Bypass Times But Transiently Impaired After Protamine. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1110–1120. [Google Scholar] [CrossRef]
- Yan, S.; Gao, S.; Lou, S.; Zhang, Q.; Wang, Y.; Ji, B. Risk Factors of Thrombocytopenia After Cardiac Surgery with Cardiopulmonary Bypass. Braz. J. Cardiovasc. Surg. 2023, 38, 389–397. [Google Scholar] [CrossRef]
- Leguyader, A.; Watanabe, R.; Berbé, J.; Boumediene, A.; Cogné, M.; Laskar, M. Platelet activation after aortic prosthetic valve surgery. Interact. Cardiovasc. Thorac. Surg. 2006, 5, 60–64. [Google Scholar] [CrossRef]
- Yanagawa, B.; Ribeiro, R.; Lee, J.; Mazer, C.D.; Cheng, D.; Martin, J.; Verma, S.; Friedrich, J.O. Canadian Cardiovascular Surgery Meta-Analysis Working Group. Platelet Transfusion in Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 111, 607–614. [Google Scholar] [CrossRef]
- Shi, S.S.; Shi, C.C.; Zhao, Z.Y.; Shen, H.Q.; Fang, X.M.; Tan, L.H.; Zhang, X.H.; Shi, Z.; Lin, R.; Shu, Q. Effect of Open Heart Surgery with Cardiopulmonary Bypass on Peripheral Blood Lymphocyte Apoptosis in Children. Pediatr. Cardiol. 2009, 30, 153–159. [Google Scholar] [CrossRef]
- Li, W.J.; Peng, Y.X.; Zhao, L.Q.; Wang, H.Y.; Liu, W.; Bai, K.; Chen, S.; Lu, Y.N.; Huang, J.H. T-cell lymphopenia is associated with an increased infecting risk in children after cardiopulmonary bypass. Pediatr. Res. 2024, 95, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, J.M.; Iglesias-González, J.L.; Lozano-Sánchez, F.S.; Palomero-Rodríguez, M.Á.; Sánchez-Conde, P. Inflammatory Response, Immunosuppression and Arginase Activity after Cardiac Surgery Using Cardiopulmonary Bypass. J. Clin. Med. 2022, 11, 4187. [Google Scholar] [CrossRef]
- Castro, R.C.C.; Costa, P.N.; Rocha, E.A.V.; Ribeiro, I.V.D.C.P.; Parreira, M.P. Lymphocyte Levels and Morbidity and Mortality in Cardiovascular Surgery with Cardiopulmonary Bypass. Braz. J. Cardiovasc. Surg. 2024, 39, e20230136. [Google Scholar] [CrossRef]
- Bayer, A.; Doğan, F.O.; Ersoy, F.; Ersoy, U. The effect of open heart surgery on circulating lymphocytes and lymphocyte subsets in pediatric patients. Turk. J. Thorac. Cardiovasc. Surg. 2009, 17, 13–17. [Google Scholar]
- Chiarelli, M.; Achilli, P.; Tagliabue, F.; Brivio, A.; Airoldi, A.; Guttadauro, A.; Porro, F.; Fumagalli, L. Perioperative lymphocytopenia predicts mortality and severe complications after intestinal surgery. Ann. Transl. Med. 2019, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Akdag, S.; Akyol, A.; Asker, M.; Duz, R.; Gumrukcuoglu, H.A. Platelet-to-Lymphocyte Ratio May Predict the Severity of Calcific Aortic Stenosis. Med. Sci. Monit. 2015, 21, 3395–3400. [Google Scholar] [CrossRef]
- Yayla, Ç.; Açikgöz, S.K.; Yayla, K.G.; Açikgöz, E.; Canpolat, U.; Kirbaş, Ö.; Öksüz, F.; Özcan, F.; Akboğa, M.K.; Topaloğlu, S.; et al. The association between platelet-to-lymphocyte ratio and inflammatory markers with the severity of aortic stenosis. Biomark. Med. 2016, 10, 367–373. [Google Scholar] [CrossRef]
- Durmaz, D.; Gündöner, S. The Relationship between Aortic Clamping Time and Platelet-lymphocyte Ratio in Isolated Coronary Artery Bypass Surgery. Koşuyolu Heart J. 2024, 27, 126–130. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Li, C.; Ma, Q.M.; Gu, Y.H.; Sheng, S.Y.; Ma, S.L.; Zhu, F. Alterations in novel inflammatory biomarkers during perioperative cardiovascular surgeries involving cardiopulmonary bypass: A retrospective propensity score matching study. Front. Cardiovasc. Med. 2024, 11, 1433011. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Y. The platelet-lymphocyte ratio is a promising predictor of early postoperative acute kidney injury following cardiac surgery: A case-control study. Ann. Transl. Med. 2021, 9, 1751. [Google Scholar] [CrossRef]
- Parlar, H.; Şaşkın, H. Are Pre and Postoperative Platelet to Lymphocyte Ratio and Neutrophil to Lymphocyte Ratio Associated with Early Postoperative AKI Following CABG? Braz. J. Cardiovasc. Surg. 2018, 33, 233–241. [Google Scholar] [CrossRef]
- Gungor, H.; Babu, A.S.; Zencir, C.; Akpek, M.; Selvi, M.; Erkan, M.H.; Durmaz, S. Association of Preoperative Platelet-to-Lymphocyte Ratio with Atrial Fibrillation after Coronary Artery Bypass Graft Surgery. Med. Princ. Pract. 2017, 26, 164–168. [Google Scholar] [CrossRef]
- Rödel, A.P.P.; Fernandes, Y.M.; Brisolara, J.V.; De Carvalho, J.A.M.; Moresco, R.N. Role of Preoperative Inflammatory Blood Cell Indexes as a Postoperative Risk Predictor Among Patients Undergoing On-Pump Cardiac Surgery. Int. J. Lab. Hematol. 2025, 47, 87–92. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Michalak, M.; Olasińska-Wiśniewska, A.; Rodzki, M.; Witkowska, A.; Gąsecka, A.; Buczkowski, P.; Perek, B.; Jemielity, M. Neutrophil Counts, Neutrophil-to-Lymphocyte Ratio, and Systemic Inflammatory Response Index (SIRI) Predict Mortality after Off-Pump Coronary Artery Bypass Surgery. Cells 2022, 11, 1124. [Google Scholar] [CrossRef]
- Drăgan, A.; Drăgan, A.Ş.; Ştiru, O. The Predictive Value of Perioperative Inflammatory Indexes in Major Arterial Surgical Revascularization from Leriche Syndrome. J. Clin. Med. 2024, 13, 6338. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Ginesu, G.C.; Tanda, C.; Feo, C.F.; Fancellu, A.; Fois, A.G.; Mangoni, A.A.; Sotgia, S.; Carru, C.; Porcu, A.; et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J. Surg. 2018, 88, 616–620. [Google Scholar] [CrossRef]
- Xu, R.; Chen, L.; Yan, C.; Xu, H.; Cao, G. Elevated Platelet-to-Lymphocyte Ratio as a Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Individuals. J. Clin. Hypertens. 2025, 27, e14980. [Google Scholar] [CrossRef]
- Zhai, G.; Wang, J.; Liu, Y.; Zhou, Y. Platelet-lymphocyte ratio as a new predictor of in-hospital mortality in cardiac intensive care unit patients. Sci. Rep. 2021, 11, 23578. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Drăgan, A.; Sinescu, I. The Role of the Cardiac Biomarkers in the Renal Cell Carcinoma Multidisciplinary Management. Diagnostics 2023, 13, 1912. [Google Scholar] [CrossRef] [PubMed]

| Variable | Survivors (n = 405) | Non-Survivors (n = 17) | p 1 |
|---|---|---|---|
| EuroSCORE 2 | 6 [4–7] | 7 [5.5–9] | 0.001 |
| EuroSCORE II 2 | 1.51 [1.07–2.45] | 3.4 [2.63–4.6] | 0.001 |
| Complex surgery 3 | 194 (47.9%) | 14 (82.35%) | 0.006 |
| VIS 2 | 4.3 [0–9] | 27 [15.5–39] | 0.001 |
| Intraop_time (h) 2 | 5 [4–5] | 6 [6–8.5] | 0.001 |
| CPB_time (min) 2 | 95 [80–156.8] | 174 [151.6–232] | 0.001 |
| ACC_time (min) 2 | 72 [60–90] | 128 [100.5–142] | 0.001 |
| RDW-SD_0 (fl) 2 | 42.3 [40–44.75] | 44.4 [41.95–48.5] | 0.010 |
| L_0 (∗103/μL) 2 | 12.74 [9.74–15.92] | 15.27 [12.85–22.74] | 0.011 |
| N_0 (∗103/μL) 2 | 10.64 [7.8–13.59] | 12.91 [9.78–18.07] | 0.018 |
| P_0 (∗103/μL) 2 | 138 [112–172] | 111 [86.5–127.5] | 0.001 |
| Lf_0 (∗103/μL) 2 | 1.16 [0.83–1.6] | 1.64 [0.98–2.58] | 0.038 |
| PLR_0 2 | 122.5 [85.83–166.33] | 65.85 [36.35–118.96] | 0.001 |
| RDW-SD_1 (fl) 2 | 43.7 [41.2–46.2] | 44.5 [43.5–48.2] | 0.037 |
| MPV_1 (fl) 2 | 11.3 [10.7–12] | 11.9 [11.05–12.8] | 0.045 |
| P_1 (∗103/μL) 2 | 155 [127–189.5] | 121 [89.5–162.5] | 0.005 |
| PLR_0-PLR_Preop 2 | 3.49 [−31.35–35.74] | −28.72 [−67.28–−8.78] | 0.003 |
| AKI 3 | 104 (25.67%) | 17 (100%) | 0.001 |
| Hemostasis R 3 | 33 (8.14%) | 10 (58.82%) | 0.001 |
| RBCs (units) 2 | 0 [0–2] | 5 [3–6] | 0.001 |
| FFP (units) 2 | 0 [0–2] | 10 [4–12] | 0.001 |
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Exp (B) | OR (CI 95%) | p | OR (CI 95%) | p | |
| Hemostasis R | 16.1 | 16.1 (5.753–45.08) | 0.001 | 0.712 | |
| Complex surgery | 5.076 | 5.076 (1.437–17.931) | 0.012 | 0.844 | |
| RBCs (units) | 1.945 | 1.945 (1.530–2.473) | 0.001 | 0.865 | |
| FFP (units) | 1.486 | 1.486 (1.302–1.697) | 0.001 | 1.335 (1.068–1.669) | 0.011 |
| Intraop_time (h) | 2.765 | 2.765 (1.892–4.040) | 0.001 | 0.115 | |
| CPB_time (min) | 1.037 | 1.037 (1.024–1.050) | 0.001 | ||
| ACC_time (min) | 1.038 | 1.038 (1.024–1.053) | 0.001 | ||
| VIS | 1.069 | 1.069 (1.042–1.097) | 0.001 | 1.058 (1.007–1.112) | 0.024 |
| LVEF (%) | 0.939 | 1.064 (1.003–1.129) | 0.041 | ||
| EuroSCORE | 1.479 | 1.479 (1.78–1.856) | 0.001 | ||
| EuroSCORE II | 1.683 | 1.683 (1.334–2.124) | 0.001 | 0.785 | |
| RDW-SD_0 (fl) | 1.099 | 1.099 (1.014–1.193) | 0.022 | 0.913 | |
| L_0 (∗103/μL) | 1.106 | 1.106 (1.031–1.186) | 0.005 | ||
| N_0 (∗103/μL) | 1.104 | 1.104 (1.022–1.192) | 0.012 | ||
| P_0 (∗103/μL) | 0.978 | 1.022 (1.008–1.036) | 0.002 | 1.033 (1.002–1.064) | 0.034 |
| Lf_0 (∗103/μL) | 2.314 | 2.314 (1.324–4.045) | 0.003 | 3.532 (1.507–8.278) | 0.004 |
| PDW_1 (fl) | 1.176 | 1.176 (1.005–1.376) | 0.043 | ||
| MPV_1 (fl) | 1.712 | 1.712 (1.1–2.664) | 0.017 | ||
| P_1 (∗103/μL) | 0.982 | 1.018 (1.006–1.029) | 0.004 | 0.113 | |
| Variable | ROC | Cut off | ||||
|---|---|---|---|---|---|---|
| AUC | p | CI 95% | Value | Ss (%) | Sp (%) | |
| VIS | 0.929 | 0.001 | 0.889–0.968 | 13.5 | 88.2 | 86.7 |
| RBCs (units) | 0.891 | 0.001 | 0.800–0.968 | 2.5 | 82.4 | 85.2 |
| CPB_time (min) | 0.871 | 0.001 | 0.762–0.980 | 160.5 | 76.5 | 91.6 |
| FFP (units) | 0.864 | 0.001 | 0.741–0.986 | 6.5 | 76.5 | 96.5 |
| Intraop_time (h) | 0.861 | 0.001 | 0.786–0.935 | 5.5 | 82.4 | 75.3 |
| ACC_time (min) | 0.839 | 0.001 | 0.740–0.938 | 109.5 | 76.5 | 85.7 |
| EuroSCORE II | 0.838 | 0.001 | 0.752–0.925 | 2.19 | 94.1 | 70.1 |
| P_0 (∗103/μL) | 0.739 | 0.001 | 0.630–0.847 | 131.5 | 56.8 | 88.2 |
| PLR_0 | 0.739 | 0.001 | 0.602–0.876 | 66.00 | 87.9 | 52.9 |
| EuroSCORE | 0.725 | 0.002 | 0.614–0.836 | 6.5 | 64.7 | 67.3 |
| PLR_Preop-PLR_0 | 0.716 | 0.001 | 0.596–0.835 | 15.56 | 76.5 | 63.5 |
| P_1 (∗103/μL) | 0.702 | 0.003 | 0.567–0.837 | 133.5 | 69.4 | 70.6 |
| RDW-SD_0 | 0.685 | 0.001 | 0.574–0.796 | 40.75 | 100 | 33.6 |
| L_0 (∗103/μL) | 0.682 | 0.010 | 0.543–0.820 | 19.55 | 47.1 | 88.9 |
| N_0 (∗103/μL) | 0.669 | 0.016 | 0.532–0.807 | 12.23 | 70.6 | 63.7 |
| RDW-SD_1 | 0.649 | 0.002 | 0.554–0.744 | 42.55 | 100 | 38.5 |
| MPV_1 (fl) | 0.644 | 0.026 | 0.517–0.770 | 10.95 | 88.2 | 37.3 |
| N_Preop (∗103/μL) | 0.633 | 0.027 | 0.515–0.752 | 4.44 | 82.4 | 46.4 |
| Variable | ROC | Cut off | ||||
|---|---|---|---|---|---|---|
| AUC | p | CI 95% | Value | Ss (%) | Sp (%) | |
| VIS | 0.943 | 0.001 | 0.905–0.980 | 13.5 | 86.7 | 89.6 |
| RBCs (units) | 0.883 | 0.001 | 0.781–0.985 | 2.5 | 80 | 86.4 |
| CPB_time (min) | 0.870 | 0.001 | 0.748–0.991 | 141.5 | 80 | 88.7 |
| Intraop_time (h) | 0.867 | 0.001 | 0.789–0.945 | 5.5 | 80 | 78.6 |
| FFP (units) | 0.849 | 0.001 | 0.713–0.986 | 6.5 | 73.3 | 97 |
| EuroSCORE II | 0.847 | 0.001 | 0.752–0.941 | 2.19 | 93.3 | 72.8 |
| ACC_time (min) | 0.837 | 0.001 | 0.726–0.947 | 109.5 | 73.3 | 88.4 |
| PLR_0 | 0.814 | 0.001 | 0.701–0.927 | 66 | 89.9 | 60 |
| PLR_Preop-PLR_0 | 0.775 | 0.001 | 0.685–0.865 | 15.56 | 86.7 | 65.3 |
| EuroSCORE | 0.753 | 0.001 | 0.632–0.874 | 6.5 | 73.3 | 68 |
| P_0 (∗103/μL) | 0.737 | 0.001 | 0.614–0.861 | 131.5 | 56.1 | 86.7 |
| L_0 (∗103/μL) | 0.729 | 0.001 | 0.592–0.866 | 19.53 | 53.3 | 88.7 |
| Lf_0 (∗103/μL) | 0.725 | 0.001 | 0.590–0.859 | 1.63 | 60 | 77.4 |
| N_0 (∗103/μL) | 0.710 | 0.002 | 0.574–0.846 | 15.72 | 53.3 | 85.5 |
| RDW-SD_0 (fl) | 0.691 | 0.001 | 0.576–0.807 | 40.75 | 100 | 33.5 |
| P_1 (∗103/μL) | 0.676 | 0.023 | 0.525–0.828 | 133.5 | 68 | 66.7 |
| N_Preop (∗103/μL) | 0.676 | 0.003 | 0.560–0.792 | 4.44 | 93.3 | 44.6 |
| SII_0 | 0.660 | 0.026 | 0.519–0.800 | 821.03 | 79.2 | 53.3 |
| L_Preop (∗103/μL) | 0.658 | 0.017 | 0.529–0.788 | 8.13 | 73.3 | 60.2 |
| RDW-SD_1 (fl) | 0.646 | 0.005 | 0.544–0.748 | 42.55 | 100 | 38.9 |
| PLR_1-PLR_0 | 0.645 | 0.014 | 0.530–0.759 | 42.31 | 80 | 49.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drăgan, A.; Drăgan, A.Ş.; Ştiru, O. A Novel Approach to Assessing In-Hospital Mortality After On-Pump Aortic Valve Replacement. Life 2025, 15, 1696. https://doi.org/10.3390/life15111696
Drăgan A, Drăgan AŞ, Ştiru O. A Novel Approach to Assessing In-Hospital Mortality After On-Pump Aortic Valve Replacement. Life. 2025; 15(11):1696. https://doi.org/10.3390/life15111696
Chicago/Turabian StyleDrăgan, Anca, Adrian Ştefan Drăgan, and Ovidiu Ştiru. 2025. "A Novel Approach to Assessing In-Hospital Mortality After On-Pump Aortic Valve Replacement" Life 15, no. 11: 1696. https://doi.org/10.3390/life15111696
APA StyleDrăgan, A., Drăgan, A. Ş., & Ştiru, O. (2025). A Novel Approach to Assessing In-Hospital Mortality After On-Pump Aortic Valve Replacement. Life, 15(11), 1696. https://doi.org/10.3390/life15111696









