Low-Cost Protocol for Quantitative Measurement of Streptococcus salivarius in Human Saliva
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sample Collection and Handling
2.3. Culture Medium Preparation and Selectivity
2.4. Serial Dilution and Culturing Protocol
2.5. Colony Identification and Confirmation
2.6. Quantification of CFU/mL
2.7. Storage Impact Assessment
3. Results
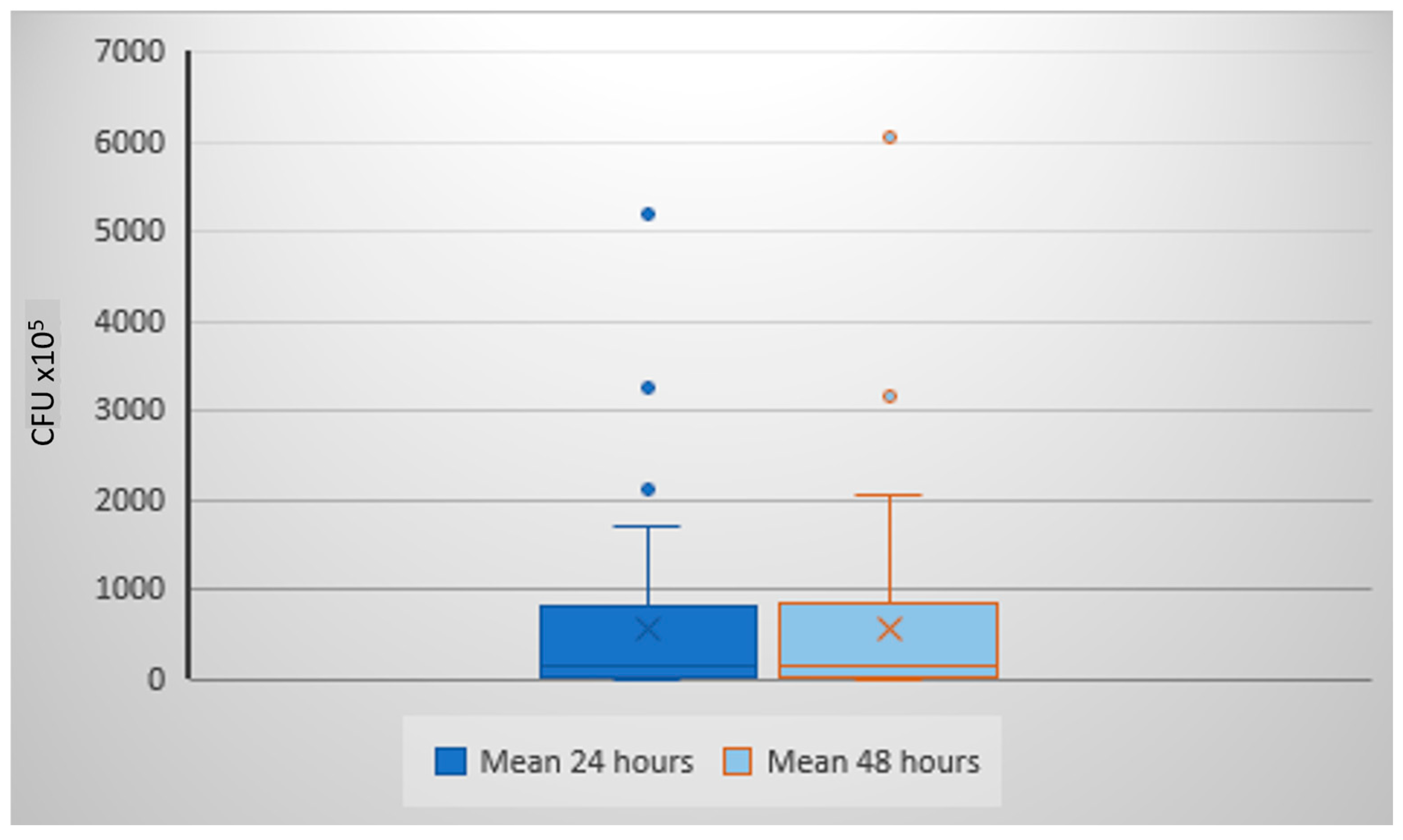
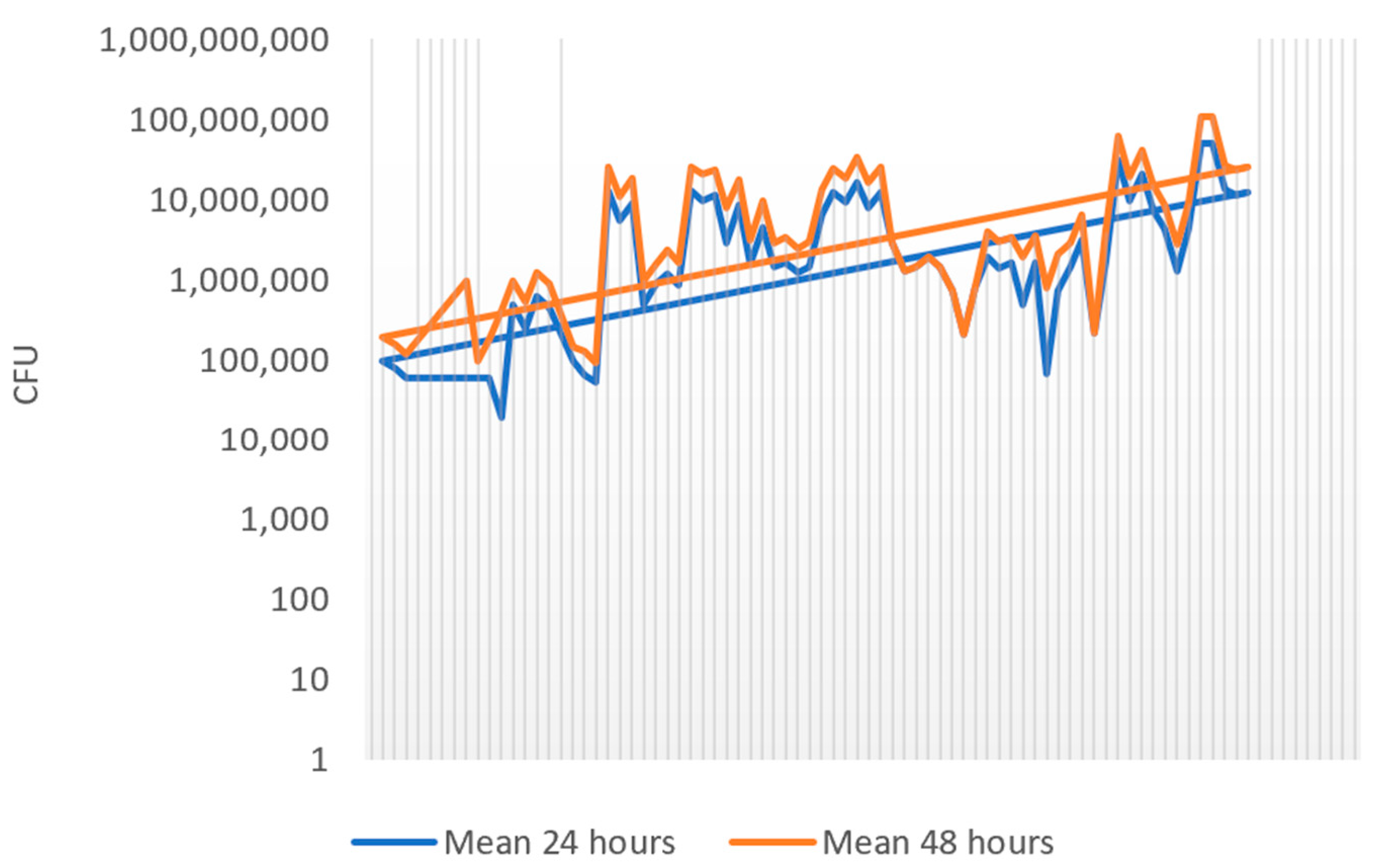
3.1. Colony Growth at 24 h vs. 48 h of Incubation
3.2. Effect of Sample Preservation on S. salivarius Viability
4. Discussion
4.1. Role of S. salivarius in Health and Disease
4.2. Validation of the Method
4.3. Comparison with Molecular Quantification Techniques
4.4. Clinical and Practical Implications
4.5. Advantages, Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S. salivarius | Streptococcus salivarius |
| CFU | colony forming unit |
| PBS | phosphate-buffered saline |
| MSA | Mitis-Salivarius Agar |
References
- Azzolino, D.; Felicetti, A.; Santacroce, L.; Lucchi, T.; Garcia-Godoy, F.; Passarelli, P.C. The emerging role of oral microbiota: A key driver of oral and systemic health. Am. J. Dent. 2025, 38, 111–116. [Google Scholar]
- Réthi-Nagy, Z.; Juhász, S. Microbiome’s Universe: Impact on health, disease and cancer treatment. J. Biotechnol. 2024, 392, 161–179. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Nilson, B.; Olaison, L.; Rasmussen, M. Clinical presentation of infective endocarditis caused by different groups of non-beta haemolytic streptococci. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 215–218. [Google Scholar] [CrossRef]
- Ahmad, S.; Song, D.; Reyes, J.V.M.; Whiting, A.; Almas, T.; Lieber, J.J. Hakuna mycotic aneurysm, Streptococcus salivarius does not always mean “no worries”. Ann. Med. Surg. 2021, 69, 102798. [Google Scholar] [CrossRef]
- Kaci, G.; Goudercourt, D.; Dennin, V.; Pot, B.; Doré, J.; Ehrlich, S.D.; Renault, P.; Blottière, H.M.; Daniel, C.; Delorme, C. Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl. Environ. Microbiol. 2014, 80, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Al-Akel, F.C.; Chiperi, L.E.; Eszter, V.K.; Bacârea, A. Streptococcus salivarius Role as a Probiotic in Children’s Health and Disease Prophylaxis-A Systematic Review. Life 2024, 14, 1613. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.B.; Oliveira, C.S.; Tavaria, F.K. Novel Strategies for Preventing Dysbiosis in the Oral Cavity. Front. Biosci. (Elite Ed.) 2023, 15, 23. [Google Scholar] [CrossRef]
- Chandra Nayak, S.; Latha, P.B.; Kandanattu, B.; Pympallil, U.; Kumar, A.; Kumar Banga, H. The Oral Microbiome and Systemic Health: Bridging the Gap Between Dentistry and Medicine. Cureus 2025, 17, e78918. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Iwasaki, S.; Také, A.; Uojima, H.; Horio, K.; Sakaguchi, Y.; Gotoh, K.; Satoh, T.; Hidaka, H.; Tanaka, Y.; Hayashi, S.; et al. Quantification of Streptococcus salivarius using the digital polymerase chain reaction as a liver fibrosis marker. World J. Hepatol. 2025, 17, 102027. [Google Scholar] [CrossRef] [PubMed]
- Al-Melh, M.A.; Bhardwaj, R.G.; Pauline, E.M.; Karched, M. Real-time polymerase chain reaction quantification of the salivary levels of cariogenic bacteria in patients with orthodontic fixed appliances. Clin. Exp. Dent. Res. 2020, 6, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liao, D.; Li, C.; Chen, L.; Wang, J.; Gan, T.; Luo, H.; Wu, N.; He, J. Protective effect of Streptococcus salivarius K12 against Mycoplasma pneumoniae infection in mice. Nan Fang Yi Ke Da Xue Xue Bao 2024, 44, 2300–2307. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhang, H.; Zheng, X.; Wang, J.; Jia, X.; Peng, X.; Xie, Q.; Zou, J.; Zheng, L.; et al. Probiotic Streptococcus salivarius K12 Alleviates Radiation-Induced Oral Mucositis in Mice. Front. Immunol. 2021, 12, 684824. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Verma, D.; Griswold, K.E.; Bailey-Kellogg, C. Building blocks and blueprints for bacterial autolysins. PLoS Comput. Biol. 2021, 17, e1008889. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Joris, B.; Charlier, P.; Foster, S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008, 32, 259–286. [Google Scholar] [CrossRef]
- Reith, J.; Mayer, C. Peptidoglycan turnover and recycling in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2011, 92, 1–11. [Google Scholar] [CrossRef]
- Higuchi, R.; Dollinger, G.; Walsh, P.S.; Griffith, R. Simultaneous amplification and detection of specific DNA sequences. Nat. Biotechnol. 1992, 10, 413–417. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ohmori, T.; Hara, M.; Takada, A.; Shojo, H.; Adachi, N.; Saito, K. A simple identification method of saliva by detecting Streptococcus salivarius using loop-mediated isothermal amplification. J. Forensic Sci. 2011, 56 (Suppl. 1), S158–S161. [Google Scholar] [CrossRef] [PubMed]
- Whale, A.S.; Huggett, J.F.; Cowen, S.; Speirs, V.; Shaw, J.; Ellison, S.; Foy, C.A.; Scott, D.J. Comparison of microfluidic digital PCR and conventional quantitative PCR for measuring copy number variation. Nucleic Acids Res. 2012, 40, e82. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Hofmann, M.; Schulz-Weidner, N.; Krämer, N.; Hain, T. The Bacterial Oral Microbiome in Children with Congenital Heart Disease: An Extensive Review. Pathogens 2023, 12, 1269. [Google Scholar] [CrossRef]
- Epprecht, J.; Ledergerber, B.; Frank, M.; Greutmann, M.; van Hemelrijck, M.; Ilcheva, L.; Padrutt, M.; Stadlinger, B.; Özcan, M.; Carrel, T.; et al. Increase in Oral Streptococcal Endocarditis Among Moderate-Risk Patients: Impact of Guideline Changes on Endocarditis Prevention. JACC Adv. 2024, 3, 101266. [Google Scholar] [CrossRef]
- Selvaraj, V.; Maheshwari, Y.; Hajeri, S.; Yokomi, R. A rapid detection tool for VT isolates of Citrus tristeza virus by immunocapture-reverse transcriptase loop-mediated isothermal amplification assay. PLoS ONE 2019, 14, e0222170. [Google Scholar] [CrossRef]
- Folwaczny, M.; Bauer, F.; Grünberg, C. Significance of oral health in adult patients with congenital heart disease. Cardiovasc. Diagn. Ther. 2019, 9 (Suppl. 2), S377–S387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Whiley, R.A.; Beighton, D. Emended descriptions and recognition of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus as distinct species. Int. J. Syst. Bacteriol. 1991, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D.; Butler-Wu, S.M. Emerging and Future Applications of Matrix-Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry in the Clinical Microbiology Laboratory: A Report of the Association for Molecular Pathology. J. Mol. Diagn. 2016, 18, 789–802. [Google Scholar] [CrossRef]
- Abdelbary, M.M.H.; Abdallah, R.Z.; Na, H.S. Editorial: The oral-gut axis: From ectopic colonization to within-host evolution of oral bacteria. Front. Cell. Infect. Microbiol. 2024, 14, 1378237. [Google Scholar] [CrossRef] [PubMed]

| Dilution | Colony Count Plate 1 | Colony Count Plate 2 | Average Colony Count | CFU/mL |
|---|---|---|---|---|
| 10−5 | x | y | T = (x + y)/2 | T × 105 |
| 10−6 | n | m | O = (n + m)/2 | O × 106 |
| 10−7 | z | w | V = (z + w)/2 | V × 107 |
| Average CFU/mL | (T + O + V)/3 |
| Sample No. | CFU/mL × 105 (24 h) | CFU/mL × 105 (48 h) |
|---|---|---|
| 1 | 5.5 | 5.7 |
| 2 | 45.8 | 46 |
| 3 | 86.6 | 79.5 |
| 4 | 82 | 82.5 |
| 5 | 107.5 | 137 |
| 6 | 148.5 | 152.5 |
| 7 | 170 | 182.5 |
| 8 | 193.3 | 195 |
| 9 | 237.5 | 237.5 |
| 10 | 439.6 | 466 |
| 11 | 450.3 | 525 |
| 12 | 930 | 932.5 |
| 13 | 947.5 | 972.5 |
| 14 | 1152.5 | 1227.5 |
| 15 | 1257.5 | 1297.5 |
| 16 | 1262.5 | 1302.5 |
| 17 | 2090 | 2137.5 |
| 18 | 5200 | 6050 |
| Initial CFU/mL × 105 | After 24 h at RT | After 24 h at 4 °C |
|---|---|---|
| 1302.5 | 1467.5 | not tested |
| 932.5 | 1442.5 | not tested |
| 137 | 155 | not tested |
| 46 | 9 | not tested |
| 182.5 | not tested | 1112.5 |
| 79.5 | not tested | 0 |
| 237.5 | not tested | 335 |
| 5.75 | not tested | 11 |
| Method | Sensitivity | Time to Results | Equipment/Cost | Output | Applicability |
|---|---|---|---|---|---|
| qPCR | High (10–100 genome copies) | 3–5 h | High cost, requires thermocycler | Relative or absolute DNA copies | Clinical diagnostics, research |
| dPCR | Very high (1–10 copies) | 5–6 h | Very high cost, specialized platform | Absolute DNA copies | Biomarker validation, precision diagnostics |
| LAMP | Moderate–high | 1–2 h | Moderate; isothermal device needed | Presence/absence or relative quantification | Field studies, forensic identification |
| Culture-based (this study) | Moderate (103–104 CFU/mL) | 48 h | Low; standard lab equipment | Viable CFU/mL | Routine labs, resource-limited settings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Akel, F.-C.; Chiperi, L.E.; Vas, K.E.; Szekely, E.; Mariean, C.R.; Budin, C.E.; Bacarea, A. Low-Cost Protocol for Quantitative Measurement of Streptococcus salivarius in Human Saliva. Life 2025, 15, 1695. https://doi.org/10.3390/life15111695
Al-Akel F-C, Chiperi LE, Vas KE, Szekely E, Mariean CR, Budin CE, Bacarea A. Low-Cost Protocol for Quantitative Measurement of Streptococcus salivarius in Human Saliva. Life. 2025; 15(11):1695. https://doi.org/10.3390/life15111695
Chicago/Turabian StyleAl-Akel, Flavia-Cristina, Lacramioara Eliza Chiperi, Krisztina Eszter Vas, Edit Szekely, Claudia Raluca Mariean, Corina Eugenia Budin, and Anca Bacarea. 2025. "Low-Cost Protocol for Quantitative Measurement of Streptococcus salivarius in Human Saliva" Life 15, no. 11: 1695. https://doi.org/10.3390/life15111695
APA StyleAl-Akel, F.-C., Chiperi, L. E., Vas, K. E., Szekely, E., Mariean, C. R., Budin, C. E., & Bacarea, A. (2025). Low-Cost Protocol for Quantitative Measurement of Streptococcus salivarius in Human Saliva. Life, 15(11), 1695. https://doi.org/10.3390/life15111695







