Daylight Sonography: Clinical Relevance of Color-Tinted Ultrasound Imaging
Abstract
1. Introduction
Aim
- Explain the visual physiological basis for adapting ultrasound imaging to photopic vision, highlighting the limitations of conventional grayscale B-mode in well-lit environments.
- Review the historical and technical evolution of color-coded ultrasound imaging, with an emphasis on Look-Up Table (LUT) development and photopic display algorithms.
- Assess the clinical applications of photopic ultrasound across emergency, interventional, outpatient, and mobile settings—where ambient light conditions challenge traditional imaging workflows.
- Identify common artifacts and optimization strategies related to photopic imaging, including adaptive color scaling and artifact suppression.
- Present user experience data from a pilot study comparing preferences between grayscale and photopic modalities.
- Propose integration with artificial intelligence (AI) to support real-time optimization, standardization, and interpretation of color-coded images.
- Discuss educational, ergonomic, and practical implications, including training needs and potential improvements in visual fatigue and workflow efficiency.
- Outline future directions for research, standardization, and clinical validation of daylight-compatible ultrasound imaging.
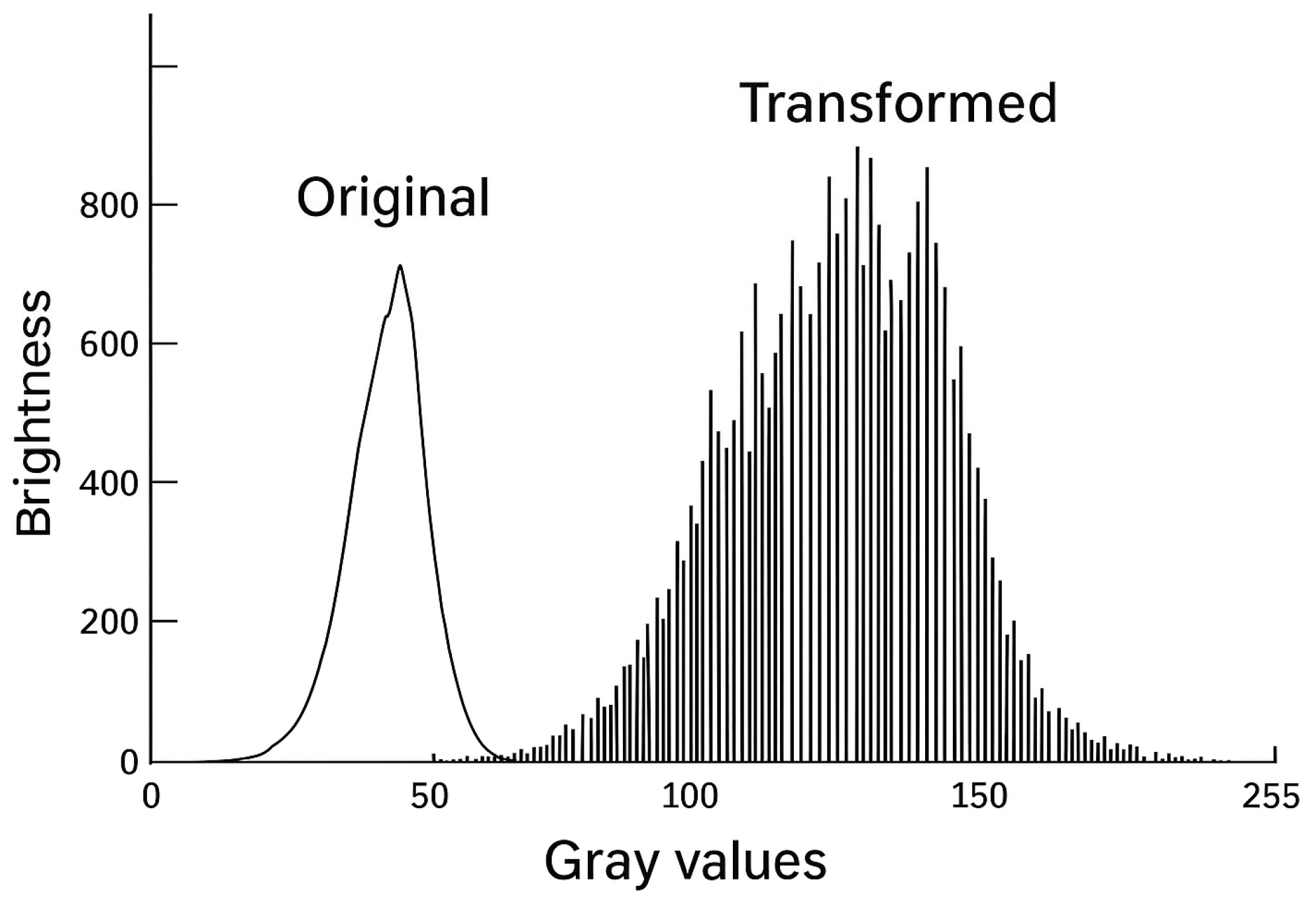
2. Visual Physiology and the Need for Colorization
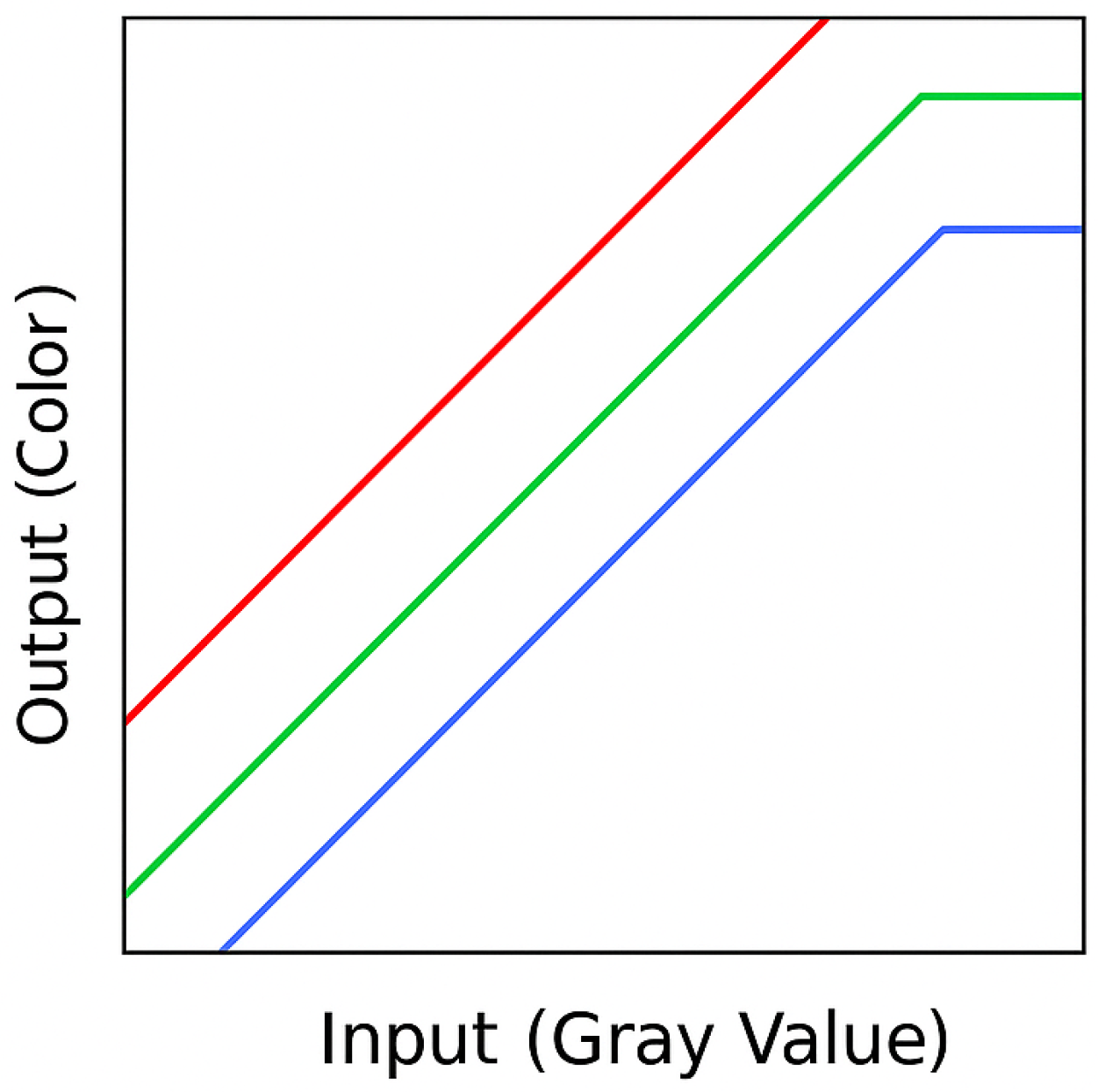
3. Technical Foundations and Evolution
Scotopic Vision and Shades of Gray
4. Clinical Implementation and Significance
- The use of Handheld Ultrasound Devices, e.g., in the emergency and intensive care setting. POCUS is increasingly performed in uncontrolled lighting environments. Photopic imaging allows reliable assessment without requiring dark rooms [59]. POCUS examinations typically focus on questions that can also be adequately clarified with ultrasound devices with only moderate image quality, e.g., handheld ultrasound devices. This is particularly the case when the findings to be assessed are represented by large impedance differences in the ultrasound image (detection of intracavitary fluid, hydronephrosis or filled urinary bladder). In those cases, the advantages of photopic imaging are obvious, whereas the discrimination of small differences in echogenicity and B-mode ultrasound quality is less important.
- Interventional Procedures: Enhanced tissue and needle visibility in color-tinted images improve safety and accuracy in ultrasound-guided interventions.
- Outpatient and ambulatory Care: Eliminating the need for darkened rooms enables better integration of ultrasound into routine patient care.
- Supervised ultrasound training: Hands-on training and live demonstrations require daylight conditions so that trainees can observe how the trainers’ right hand handles the probe and what his left hand does with the knobs and buttons of the US machine.
- Lecturing: Colorized ultrasound images do not need a dark background, nor does the room need to be as dark as possible, which helps prevent the audience from losing attention.
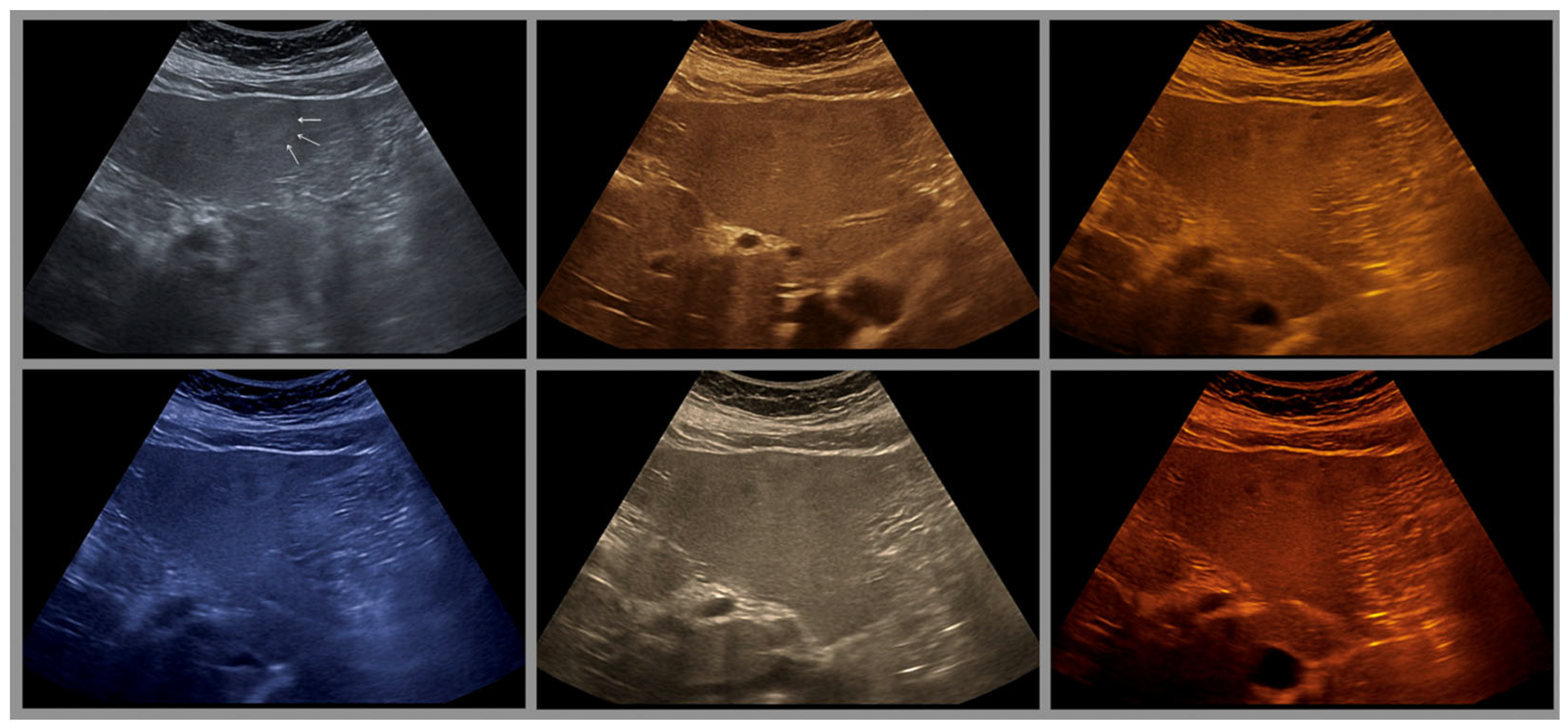
5. Artifact Management and Optimization
- Hole artifacts: Echopoor pseudolesions as a result from clustering dark gray levels.
- Echogenicity artifacts: Local glare as a result of over-enhanced brightness from grouped light gray levels.
- Veiling (obscuration or homogenization) artifacts. Mid-gray clustering that reduces depth perception.
6. Visual Adaptation and Practical Implications
- Mobile units where lighting control is limited.
- Bedside diagnostics during hospital rounds.
- Triage and field settings in disaster or military medicine.
- Human Factors and Examiner Preferences.
Is There an Ideal Color Map?
7. Integration with Advanced Ultrasound Modalities
- Contrast-Enhanced Ultrasound (CEUS): When combined with photopic imaging, CEUS benefits from improved brightness perception and reduced artifact interference.
- Photopic imaging may also hold promise for Doppler (including spectral FFT analysis) and elastography (e.g., 2D-SWE). By improving contrast perception under bright ambient light, photopic display modes could facilitate the detection of subtle vascular signals or enhance interpretation of elastographic stiffness maps. However, current implementations are limited, and systematic studies evaluating the diagnostic impact of photopic imaging in these modalities are lacking. Further research is therefore required to validate its clinical usefulness and to establish standardized settings.
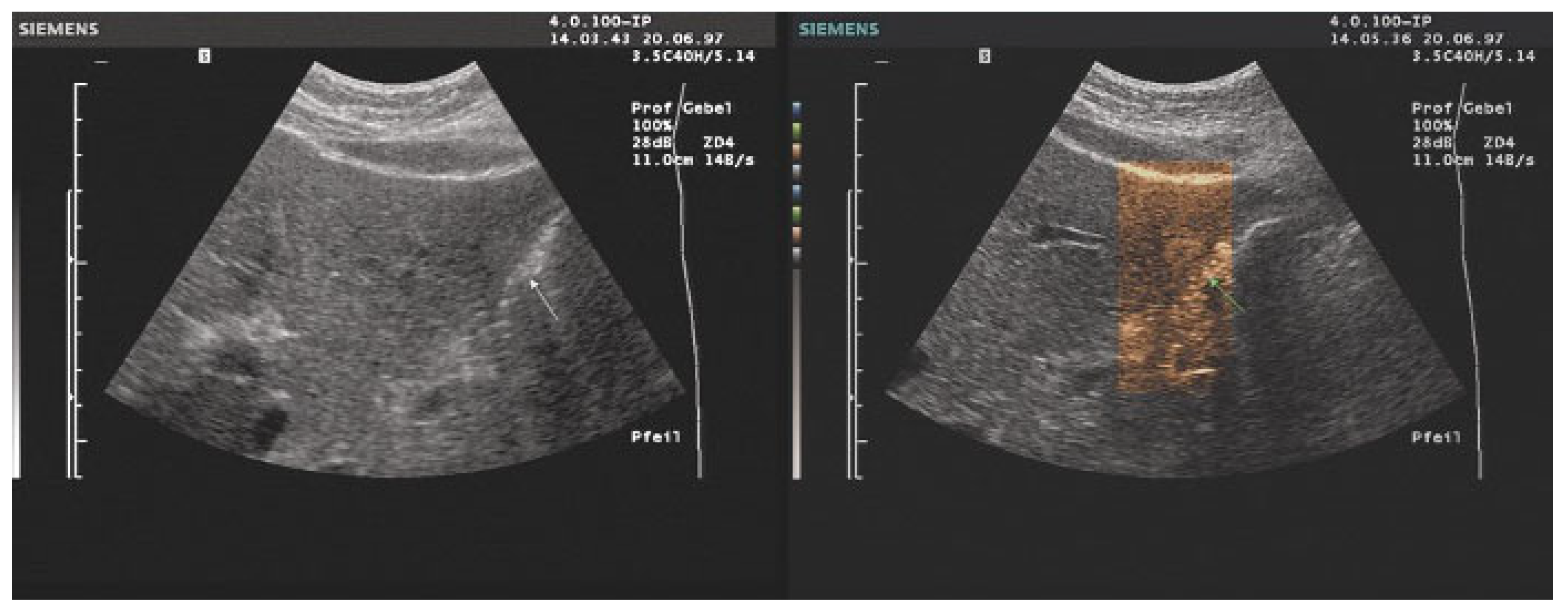
- The combination with speckle filters available in all high-end devices, similar to the low-pass filters used by Bleck et al. (1994) for tissue visualization [5], also yields significantly improved visualization results (Figure 5, Figure 6 and Figure 7). In principle, applications with new visualized texture analyses, such as random field models, are also conceivable [76,77].
8. Application in Point of Care Ultrasound (POCUS)
9. Educational and Interpretive Considerations
- LUT structure and function
- Examples of typical and atypical color mappings
- Strategies for cross-device interpretation
10. Artificial Intelligence Integration and Future Developments
10.1. AI for Real-Time LUT Optimization
- Dynamically adapting LUTs based on anatomical region, tissue type, and ambient light.
- Dynamically adjustment of color schemes to optimize lesion detection or tissue characterization for particular pathologies, thereby providing real-time, disease-tailored visualization
- Learning from expert-reviewed image datasets to optimize contrast and color mapping for specific clinical contexts (e.g., liver lesions, vascular pathology).
- Reducing artifacts such as over-saturation or misleading hue transitions through self-correcting algorithms.
- Future developments may also include disease-specific color adaptation, potentially enabled by AI, to tailor LUTs according to pathology and thereby further enhance diagnostic utility.
10.2. Automated Artifact Detection and Suppression
- Detect common photopic-specific artifacts (e.g., hole, veiling, or saturation artifacts).
- Provide real-time alerts or automatic correction before image storage or interpretation.
- Improve visual clarity and prevent diagnostic error, especially for novice users.
10.3. Standardization Across Platforms
- Translate color mappings across manufacturers for consistent cross-platform imaging.
- Provide vendor-agnostic post-processing that harmonizes image appearance for teaching, research, and telemedicine.
10.4. AI-Augmented Education and Simulation
- Color-tinted ultrasound requires tailored training. AI can enhance education through
- Interactive simulators that adapt LUT difficulty based on user proficiency.
- Real-time feedback and scoring during mock exams or image labeling exercises.
- Case-based reasoning systems that help trainees learn from misinterpretations.
10.5. Future Perspectives
- Multimodal fusion (e.g., combining photopic B-mode with elastography, CEUS, or Doppler).
- Personalized imaging presets, calibrated to user visual preferences or pathologies of interest.
- Integration into wearable or head-mounted displays for hands-free ultrasound interpretation in emergency or surgical contexts.
11. Telemedicine and Remote Consultation
- Pre-analyze acquired images to flag abnormalities for remote reviewers.
- Compress and transmit optimized, artifact-free colorized images with diagnostic overlays.
- Facilitate global access to expert-level interpretation, especially in low-resource or field environments.
12. Teaching
13. Advantages, Limitations and Future Directions
13.1. Advantages of Photopic
- Lower MI and TI with lower transmit power
- Better contrast resolution with lower gain
- Improved visual perception
- Shorter visual reaction times
- Improved depth perception
- Only minor change in gain setting and TGC required
- Better differentiation and identification of small lesions or tissue changes
- No adaptation of the eye to different light conditions required
- Can be used together with THI
- Improved fatty liver diagnostics [84]
- Advantages of CEUS (ECI): Stable vesicles and therefore longer examination time, less contrast medium required, optimally (quantitatively) evaluable CEUS images due to lower saturation during peak amplification.
13.2. Disadvantages of Photopic Imaging
- Lack of standardization: Different vendors use non-uniform terms (Photopic Mode, TINT, B-color) and LUT designs.
- Training gaps: Limited formal instruction in color-tinted image interpretation.
- Incomplete validation: Few comparative studies quantitatively assess diagnostic performance, fatigue reduction, or time savings.
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bleck, J.S.; Gebel, M.; Witt, B.; Schmitt, K.J.; Breitkopf, P.; Westhoff-Bleck, M.; Wagner, S.; Göke, M.; Caselitz, M.; Schneider, A.; et al. Sonography under Daylight Conditions. Ultraschall Med. 1998, 19, 259–264. [Google Scholar] [CrossRef]
- Bleck, J. Parametrischer Ultraschall in der Inneren Medizin am Beispiel der Leber (Venia Legendi); Medizinische Hochschule Hannover: Hannover, Germany, 1998. [Google Scholar]
- Bleck, J.S.; Gebel, M.; Witt, B.; Schmitt, K.J.; Abel, J.; Westhoff-Bleck, M.; Thiesemann, C.; Wagner, S.; Göke, M.; Benter, T.; et al. Optimierung der Darstellung von Ultraschallbildern durch Einsatz histogrammgesteuerter, farbiger, artefaktfreier, tageslichtfähiger Look-Up-Tabellen. Ultraschall Klin Prax. 1995, 9, 204–207. [Google Scholar]
- Witt, B. Artefaktfreie Tageslichtsonographie. Ph.D. Thesis, Medizinische Hochschule Hannover (MHH), Hannover, Germany, 1997. [Google Scholar]
- Bleck, J.S.; Gebel, M.; Hebel, R.; Wagner, S.; Schmidt, K.; Kruip, S.; Westhoff-Bleck, M.; Wolf, M.; Thiesemann, C.; Manns, M. Tissue characterization using intelligent adaptive filter in the diagnosis of diffuse and focal liver disease. Ultrasound Med. Biol. 1994, 20, 521–528. [Google Scholar] [CrossRef]
- Krawkow, S.W. Das Farbensehen; Akademie-Verlag: Berlin, Germany, 1955. [Google Scholar]
- Hartmann, E. Beleuchtung und Sehen am Arbeitsplatz; Wilhelm Goldmann Verlag GmbH: München, Germany, 1970. [Google Scholar]
- Schütz, E.; Caspers, H.; Speckmann, P.-J. Das Farbensehen und die Farbsinnstörung. Physiologie 1982, 16, 338–347. [Google Scholar]
- Munker, H. Sehen und Beleuchtung. In Praktische Arbeitsphysiologie; Rohmert, W., Rutenfranz, J., Eds.; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 1983; Volume 3, pp. 300–316. [Google Scholar]
- Grüsser, O.J.; Grüsser-Cornehls, U. Physiologie des Sehens. In Grundriss der Sinnesphysiologie, 5th ed.; Schmid, R.F., Ed.; Springer: Berlin and Heidelberg, Germany, 1985; pp. 174–241. [Google Scholar]
- Schmid, R.F.; Thews, G. Physiologie des Menschen; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1987; Volume 23. [Google Scholar]
- Betts, R.P. A simple gray scale to color converter. J. Med. Eng. Technol. 1979, 3, 31–33. [Google Scholar] [CrossRef]
- Cavanagh, P.; Tyler, C.W.; Favreau, O.E. Perceived velocity of moving chromatic gratings. J. Opt. Sci. Am. 1984, 1, 893–899. [Google Scholar] [CrossRef]
- Reid, J.M.; Davis, D.L.; Nation, A.W. A digital color display for multivariate information. In Ultrasound in Medicine; Springer: Boston, MA, USA, 1979; Volume 4, pp. 457–461. [Google Scholar] [CrossRef]
- Wittmann, F.; Rohler, R. Vergleichende Messungen der Kontrastübertragungsfunktion mit Sinusgittern und Rechteckgittern. Optik 1962, 19, 243–249. [Google Scholar]
- Gur, M.; Akri, V. Isoluminant stimuli may not expose the full contribution of color to visual functioning: Spatial contrast sensitivity measurements indicate interaction between color and luminance processing. Vision. Res. 1992, 32, 1253–1262. [Google Scholar] [CrossRef]
- Jameson, D. Opponent-colors theory in the light of physical findings. In Central and Peripheral Mechanisms of Color Vision; Ottoson, D., Zeki, S., Eds.; McMillan Pres: London, UK, 1985; pp. 83–102. [Google Scholar]
- Coulam, C.M.; Erickson, J.J.; Rollo, F.D.; James, A.E. The Physical Basis of Medical Imaging; Appleton-Century-Crofts: Ney York, NY, USA, 1981. [Google Scholar]
- Adams, R.; Jaffe, H.L. The photographic color display of scan data. Three years of development and experience. J. Nucl. Med. 1967, 8, 324. [Google Scholar] [PubMed]
- Coleman, D.J.; Katz, L. Color coding of B-scan ultrasonograms. Arch. Ophthalmol. 1974, 91, 429–431. [Google Scholar] [CrossRef]
- Flinn, G.S., Jr. Color encoded display of M code echocardiograms. J. Clin. Ultrasound 1976, 4, 339–341. [Google Scholar] [CrossRef]
- Flinn, G.S., Jr. Gray scale color coding of abdominal B-scan ultrasonograms. J. Clin. Ultrasound 1975, 3, 179–185. [Google Scholar] [CrossRef]
- Milan, J.; Taylor, K.J. The application of the temperature-color scale to ultrasonic imaging. J. Clin. Ultrasound 1975, 3, 171–173. [Google Scholar] [CrossRef]
- Chan, F.H.; Pizer, S.M. An ultrasonogram display system using a natural color scale. J. Clin. Ultrasound 1976, 4, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Pizer, S.M.; Zimmerman, J.B. Color display in ultrasonography. Ultrasound Med. Biol. 1983, 9, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Bleck, J.S. [New ultrasound techniques]. Orthopäde 2002, 31, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bleck, J.S.; Gebel, M.; Manns, M.P. [Quantitative sonography. Clinical role and perspectives]. Internist 2000, 41, 10–16. [Google Scholar] [CrossRef]
- Bleck, J.S.; Gebel, M.; Schmitt, K.J.; Breitkopf, P.; Sutcliffe, P. Photopic ultrasound imaging: An adaptive technique based on the physiology of vision. Electromedica 2000, 68, 59–64. [Google Scholar]
- Baum, G. Color ultrasonography for tissue differential diagnosis. In Diagnostic Ultrasound; King, D., Ed.; Mosley: St. Louis, MO, USA, 1974; pp. 282–289. [Google Scholar]
- Borcke, O.; Otto, R. Qualität des farbigen Monitorbildes im Ultraschall. In Ultraschalldiagnostik 83; Lutz, H., Ed.; Thieme: Stuttgart, NY, USA, 1983; pp. 546–550. [Google Scholar]
- Ben-Porath, M.; Clayton, G.D.; Kaplan, E. Current Status of Multi-Isotope scanning by color Modulation. J. Nucl. Med. 1967, 8, 323. [Google Scholar] [PubMed]
- Charleston, D.B.; Beck, R.N.; Wood, J.C.; Yasillo, N.J. A versatile instrument for photoscan analysis which produces color display from black and white photoscans. J. Nucl. Med. 1967, 8, 323. [Google Scholar] [CrossRef][Green Version]
- Kakehi, U.; Uchiyama, G.; Doi, O. Scan analysis with color contrast. J. Nucl. Med. 1967, 8, 324. [Google Scholar] [PubMed][Green Version]
- Kaplan, E.; Ben-Porath, M.; Clayton, G.D. Depth discrimination in color by dual channel scanning. J. Nucl. Med. 1976, 8, 323. [Google Scholar] [PubMed][Green Version]
- Holasek, E.; Jones, J.O.; Purnell, E.W.; Sokollu, A. Spectra-color ultrasonography I. Principles of a techniques for incorporation spectral information into a B-scan display. In Ultrasound in Medicine; White, D.N., Browns, R.B., Eds.; Plenum Press: New York, NY, USA; London, UK, 1977; Volume 3b, pp. 1739–1745. [Google Scholar][Green Version]
- Holasek, E.; Gans, L.A. A method for spectra-color B-scan ultrasonography. J. Clin. Ultrasound 1975, 3, 175–178. [Google Scholar] [CrossRef]
- Jones, J.P.; Holasek, E.; Purnell, E.W.; Sokollu, A. Spectra color ultrasonography. II. report of laboratory and clinical evaluation. In Ultrasound in Medicine; White, D.N., Browns, R.B., Eds.; Plenum Press: New York, NY, USA; London, UK, 1977; Volume 3b, pp. 1474–1752. [Google Scholar]
- Purnell, E.W.; Sokollu, A.; Holasek, E.; Cappaert, W.E. Clinical spectra-color ultrasonography. J. Clin. Ultrasound 1975, 3, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Masuzawa, M. Multiple frequency tomograms, simultaneous display, ultrasonic diagnostic equipment, and spectra-color ultrasonography. In Ultrasound in Medicine; White, D.N., Browns, R.B., Eds.; Plenum Press: New York, NY, USA; London, UK, 1977; Volume 3b, pp. 1759–1762. [Google Scholar]
- Yokoi, H.; Ito, K.; Yuta, S.; Matsumoto, M. Clinical applications of real time color C-mode scanning of ultrasound tomogram by digital techniques. In Ultrasound in Medicine; White, D.N., Browns, R.B., Eds.; Plenum press: New York, NY, USA; London, UK, 1977; Volume 3b, pp. 1725–1737. [Google Scholar]
- Ito, K.; Itoh, M.; Yuta, S.; Yokoi, H. Digital Ultrasonotomography by an Image Magnifying Technique and Computer-Accessed Image Processing; World Federation for Ultrasound in Medicine and Biology: London, UK, 1976; pp. 1697–1706. [Google Scholar]
- Yokoi, H.; Tatsumi, T.; Ito, K. Quantitave colour-ultrasonography by means of computer-aided simultaneous tomogram. Ultrasonics 1975, 13, 219–224. [Google Scholar] [CrossRef]
- Ito, K.; Yokoi, H.; Tatsumi, T. Quantitative colour ultrasonography, computer aided simultaneous tomogram method. In Ultrasonics in Medicine; de Vlirger, M., White, D.N., McCready, V.R., Eds.; Exerpta Medica: Amsterdam, The Netherlands, 1974; pp. 366–372. [Google Scholar]
- Yokoi, H.; Ito, K. Real time colour coding of ultrasound tomograms and their enhancement by digital techniques. In Application of Optical Instrumentation in Medicine III; Holmes, J.H., Ed.; SPIE: San Francisco, CA, USA, 1974; Volume 47. [Google Scholar] [CrossRef]
- Ito, K.; Yokoi, H.; Tatsumi, T. Quantized Ultrasonic Diagnostic Equipment; 2nd World Congress on Ultrasound in Medicine: Rotterdam, The Netherlands, 1973; p. 45. [Google Scholar]
- Yokoi, H.; Tatsumi, T.; Ito, K. Quantized Color Ultrasonography; 2nd World Congress on Ultrasonics in Medicine: Rotterdam, The Netherlands, 1973. [Google Scholar]
- Yokoi, H.; Ito, K. Ultrasonic diagnostic equipment with color display unit-for simultaneous tomogram method. Toshiba Rev. 1972, 76, 14–21. [Google Scholar]
- Yamamoto, Y.; Ito, K.; Itoh, M.; Kimura, Y. A digitized color display method for scan conversion memory. In Ultrasound in Medicine; White, D.N., Browns, R.B., Eds.; Plenum Press: New York, NY, USA; London, UK, 1977; Volume 3b, pp. 1715–1723. [Google Scholar]
- Bronson, N.R.; Pickering, N.C. Real-time color B-scan ultrasonography. J. Clin. Ultrasound 1975, 3, 191–197. [Google Scholar] [CrossRef]
- Geck, J.; Taylor, K.J.; Rosenfield, A.T. A simple method to colour modulate ultrasonic scans. J. Ultrasound Med. 1977, 3, 1753–1757. [Google Scholar]
- Huang, Z.H.; Long, W.Y.; Xie, G.Y.; Kwan, O.L.; DeMaria, A.N. Comparison of gray-scale and B-color ultrasound images in evaluating left ventricular systolic function in coronary artery disease. Am. Heart J. 1992, 123, 395–402. [Google Scholar] [CrossRef]
- Toma, S.K.; Seeds, J.W.; Cefalo, R.C. High-resolution computerized color transformation of real-time gray scale ultrasound images. J. Clin. Ultrasound 1989, 17, 353–358. [Google Scholar] [CrossRef]
- Kawamorita, T.; Yasuda, T.; Ota, T.; Iizuka, T.; Miyasaka, M.; Mamorita, N.; Handa, T. Extended just-noticeable difference for ultralow-luminance displays used in diagnostic imaging. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2024, 41, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Zander, D.; Huske, S.; Hoffmann, B.; Cui, X.W.; Dong, Y.; Lim, A.; Jenssen, C.; Lowe, A.; Koch, J.B.H.; Dietrich, C.F. Ultrasound Image Optimization (“Knobology”): B-Mode. Ultrasound Int. Open 2020, 6, E14–E24. [Google Scholar] [CrossRef]
- Kovesi, P. Good Colour Maps: How to Design Them. arXiv 2015. [Google Scholar] [CrossRef]
- Bleck, J. Computergestützte Bearbeitung von Ultraschallbildern der Leber. Ph.D. Thesis, Medizinische Hochschule Hannover (MHH), Hannover, Germany, 1988. [Google Scholar]
- Dambrosio, F.; Amy, D.; Colombo, A. B-mode color sonographic images in obstetrics and gynecology: Preliminary report. Ultrasound Obstet. Gynecol. 1995, 6, 208–215. [Google Scholar] [CrossRef]
- Marcovitz, P.A.; Bach, D.S.; Segar, D.S.; Armstrong, W.F. Impact of B-mode color encoding on rapid detection of ultrasound targets: An in vitro study. J. Am. Soc. Echocardiogr. 1993, 6, 382–386. [Google Scholar] [CrossRef]
- Horn, R.; Blaivas, M.; Wastl, D.; Michels, G.; Seibel, A.; Morf, S.; Widler, M.; Dietrich, C.F. Emergency Ultrasound in the Context of Cardiac Arrest and Circulatory Shock: “How to Avoid Cardiac Arrest”. Life 2025, 15, 646. [Google Scholar] [CrossRef]
- Lucius, C.; Jenssen, C.; Nurnberg, D.; Merkel, D.; Schreiber-Dietrich, D.G.; Merz, E.; Dietrich, C.F. [Clinical Ultrasound Part II-Sonopsychology or Psychological Interactions using Ultrasound]. Z. Gastroenterol. 2025, 63, 741–755. [Google Scholar] [CrossRef]
- Nurnberg, D.; Jenssen, C.; Lucius, C.; Klingenberg-Noftz, R.; Wustner, M.; Worlicek, H.; Merkel, D.; Eder, N.; Lo, H.; Nurnberg, M.; et al. [Clinical Ultrasound (ClinUS)-Concepts and Controversies]. Z. Gastroenterol. 2025, 63, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F. Preference of Colors Using Photopic (Pilot Study); Johann Wolfgang Goethe University: Frankfurt, Germany, 1998; p. 2. [Google Scholar]
- Fischer, T.; Filimonow, S.; Taupitz, M.; Petersein, J.; Beyersdorff, D.; Bollow, M.; Hamm, B. [Image quality and detection of pathology by ultrasound: Comparison of B-mode ultrasound with photopic imaging and tissue harmonic imaging alone and in combination]. Rofo 2002, 174, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Munch, M.; Kobialka, S.; Krauchi, K.; Steiner, R.; Oelhafen, P.; Orgul, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Blattner, P.; Oelhafen, P.; Gotz, T.; Cajochen, C. Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert? PLoS ONE 2011, 6, e16429. [Google Scholar] [CrossRef]
- Lockley, S.W.; Evans, E.E.; Scheer, F.A.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 2006, 29, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Tahkamo, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Golmohammadi, R.; Yousefi, H.; Safarpour Khotbesara, N.; Nasrolahi, A.; Kurd, N. Effects of Light on Attention and Reaction Time: A Systematic Review. J. Res. Health Sci. 2021, 21, e00529. [Google Scholar] [CrossRef]
- Costa, M.; Frumento, S.; Nese, M.; Predieri, I. Interior Color and Psychological Functioning in a University Residence Hall. Front. Psychol. 2018, 9, 1580. [Google Scholar] [CrossRef]
- Elliot, A.J.; Maier, M.A. Color psychology: Effects of perceiving color on psychological functioning in humans. Annu. Rev. Psychol. 2014, 65, 95–120. [Google Scholar] [CrossRef]
- Elliot, A.J. Color and psychological functioning: A review of theoretical and empirical work. Front. Psychol. 2015, 6, 368. [Google Scholar] [CrossRef]
- Elliot, A.J.; Maier, M.A.; Moller, A.C.; Friedman, R.; Meinhardt, J. Color and psychological functioning: The effect of red on performance attainment. J. Exp. Psychol. Gen. 2007, 136, 154–168. [Google Scholar] [CrossRef]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Hollerbach, S.; Fusaroli, P.; Lowe, A.; Koch, J.; Ignee, A.; Jenssen, C.; Dietrich, C.F. General principles of image optimization in EUS. Endosc. Ultrasound 2021, 10, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Kothe, C.; Schade, G. [Preliminary experiences with Photopic ultrasound imaging in the head and neck region]. Laryngorhinootologie 2003, 82, 127–131. [Google Scholar] [CrossRef]
- Bleck, J.S.; Gebel, M.; Schwichtenberg, A.; Klindtwork, C.; Witt, B.; Wagner, S.; Manns, M. Geräteunabhängige Standardisierung Real-Time fähiger Texturgrössen in der Sonographie. Imaging 1994, 2, 46. [Google Scholar]
- Bleck, J.S.; Ranft, U.; Gebel, M.; Hecker, H.; Westhoff-Bleck, M.; Thiesemann, C.; Wagner, S.; Manns, M. Random Field Models in the Textural Analysis of Ultrasonic images of the liver. IEEE Trans. Med. Imaging 1996, 5, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Schafer, J.M.; Dietrich, C.F. Emergency Ocular Ultrasound-Common Traumatic and Non-Traumatic Emergencies Diagnosed with Bedside Ultrasound. Ultraschall Med. 2020, 41, 618–645. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Goudie, A.; Chiorean, L.; Cui, X.W.; Gilja, O.H.; Dong, Y.; Abramowicz, J.S.; Vinayak, S.; Westerway, S.C.; Nolsoe, C.P.; et al. Point of Care Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol. 2017, 43, 49–58. [Google Scholar] [CrossRef]
- Cui, X.W.; Goudie, A.; Blaivas, M.; Chai, Y.J.; Chammas, M.C.; Dong, Y.; Stewart, J.; Jiang, T.A.; Liang, P.; Sehgal, C.M.; et al. WFUMB Commentary Paper on Artificial intelligence in Medical Ultrasound Imaging. Ultrasound Med. Biol. 2025, 51, 428–438. [Google Scholar] [CrossRef]
- Sofka, C.M.; Lin, D.; Adler, R.S. Advantages of color B-mode imaging with contrast optimization in sonography of low-contrast musculoskeletal lesions and structures in the foot and ankle. J. Ultrasound Med. 2005, 24, 215–218. [Google Scholar] [CrossRef]
- Merkel, D.; Brinkmann, E.; Kammer, J.C.; Kohler, M.; Wiens, D.; Derwahl, K.M. Comparison Between Various Color Spectra and Conventional Grayscale Imaging for Detection of Parenchymal Liver Lesions With B-Mode Sonography. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2015, 34, 1529–1534. [Google Scholar] [CrossRef]
- Merz, E.; Evans, D.H.; Dong, Y.; Jenssen, C.; Dietrich, C.F. History of ultrasound in obstetrics and gynaecology from 1971 to 2021 on occasion of the 50 years anniversary of EFSUMB. Med. Ultrason. 2023, 25, 175–188. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Shi, L.; Lowe, A.; Dong, Y.; Potthoff, A.; Sparchez, Z.; Teufel, A.; Guth, S.; Koch, J.; Barr, R.G.; et al. Conventional ultrasound for diagnosis of hepatic steatosis is better than believed. Z. Gastroenterol. 2022, 60, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
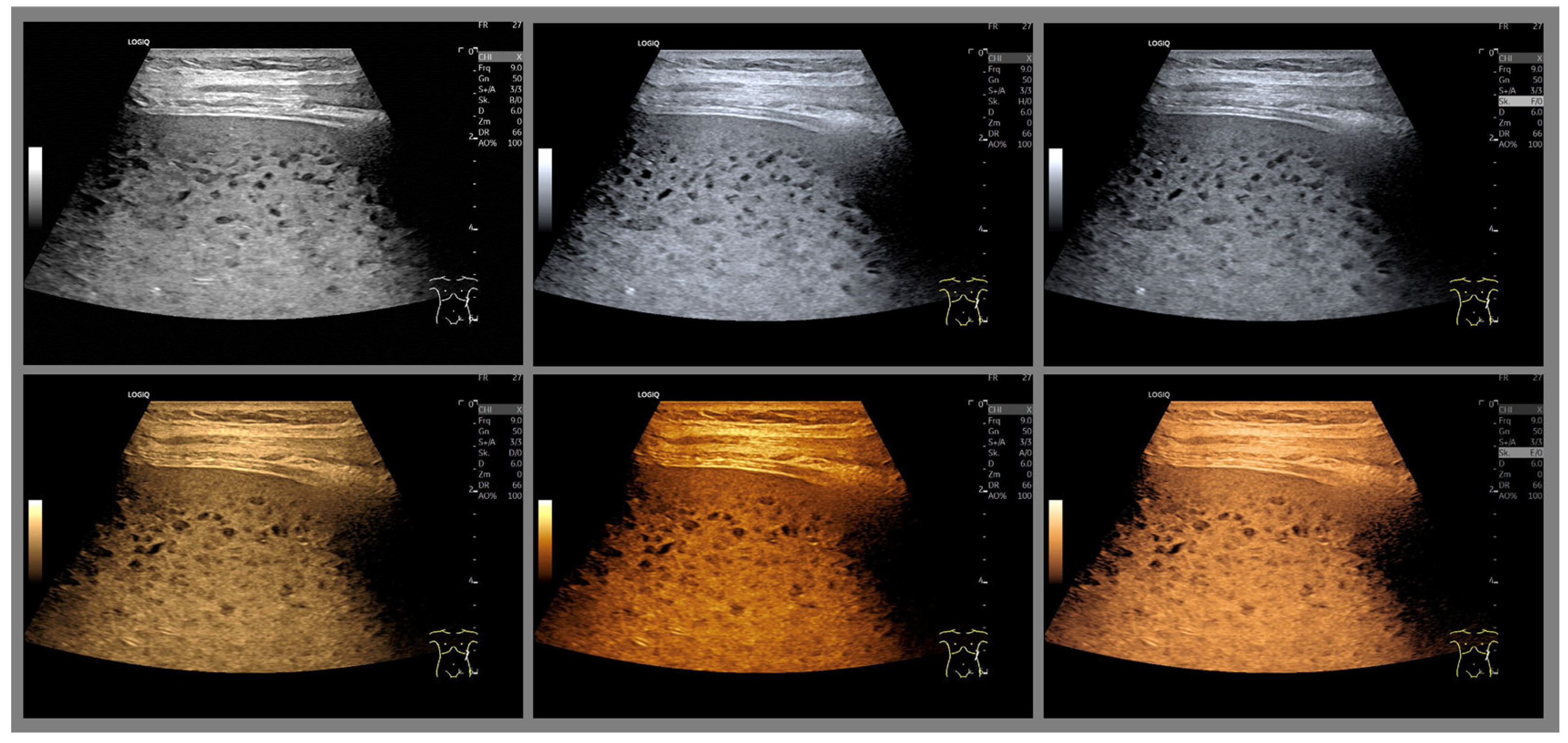
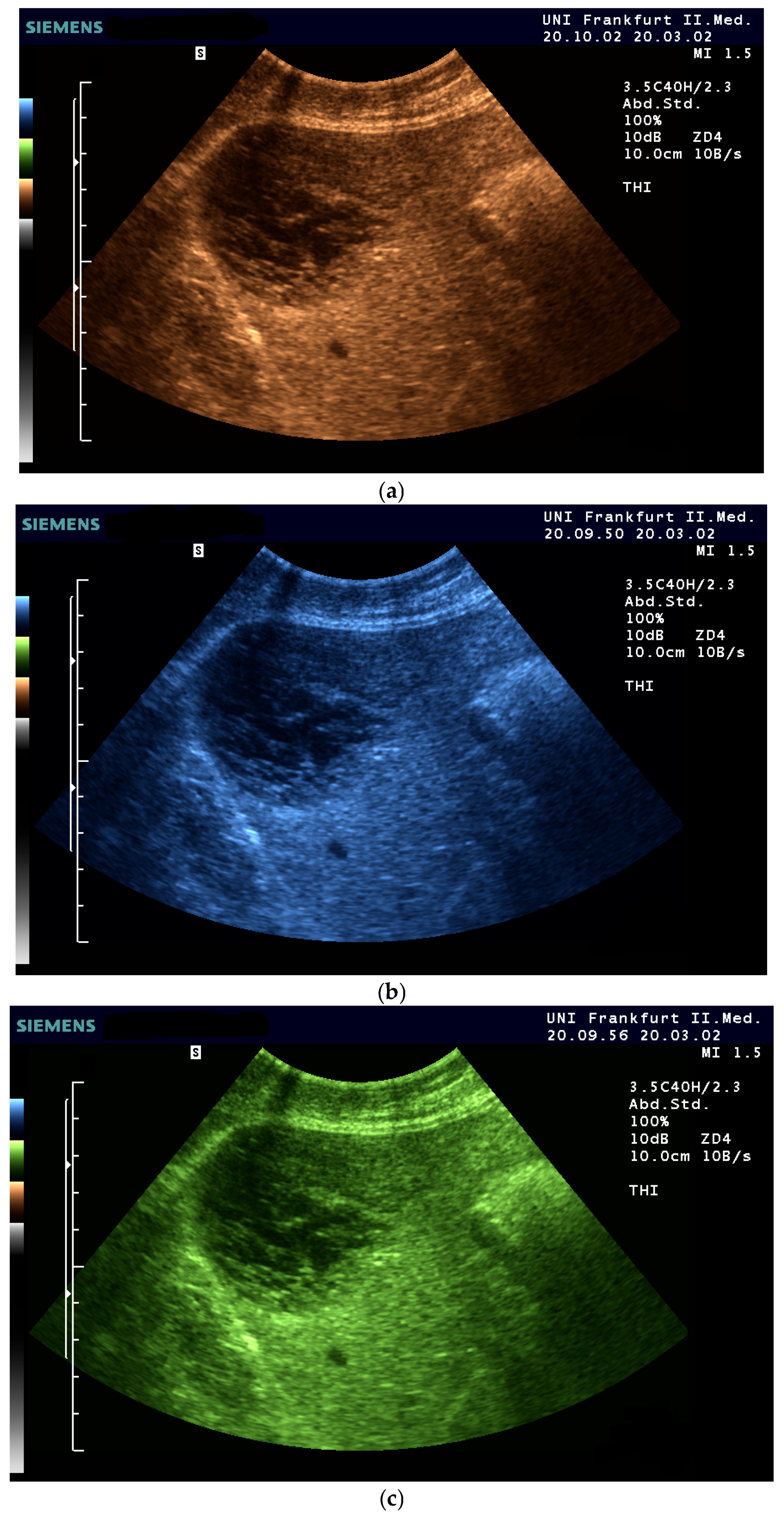
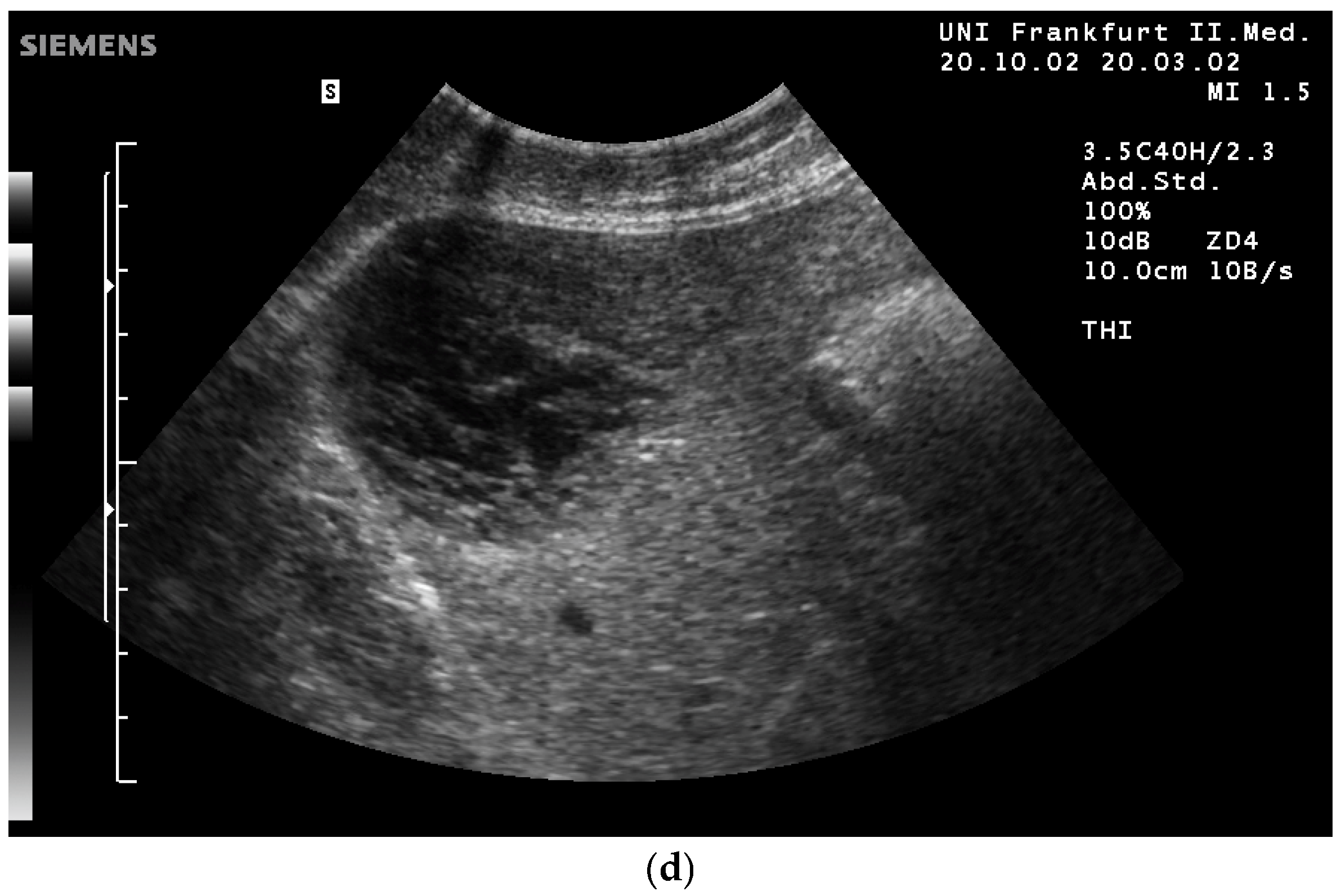

| Color | Advantages |
|---|---|
| Sepia (Yellow-Brown) | Mimics familiar tones from black-and-white photography |
| Enhances contrast while preserving anatomical detail | |
| Offers intuitive brightness mapping (lighter tones = more echogenic areas) | |
| Preferred by students and experts alike | |
| Blue | Provides excellent depth perception |
| Reduces visual fatigue by avoiding glare and high saturation | |
| Often favored by experienced examiners for high-resolution detail | |
| Facilitates alertness and performance on tasks requiring sustained attention | |
| Green (muted tones) | Offers a balanced hue that avoids excessive saturation |
| Enhances intermediate echogenicity levels without causing artifacts | |
| Especially helpful for liver and parenchymal tissue imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, C.F.; Wüstner, M.; Jenssen, C.; Merkel, D.; Bleck, J.S. Daylight Sonography: Clinical Relevance of Color-Tinted Ultrasound Imaging. Life 2025, 15, 1672. https://doi.org/10.3390/life15111672
Dietrich CF, Wüstner M, Jenssen C, Merkel D, Bleck JS. Daylight Sonography: Clinical Relevance of Color-Tinted Ultrasound Imaging. Life. 2025; 15(11):1672. https://doi.org/10.3390/life15111672
Chicago/Turabian StyleDietrich, Christoph F., Matthias Wüstner, Christian Jenssen, Daniel Merkel, and Jörg S. Bleck. 2025. "Daylight Sonography: Clinical Relevance of Color-Tinted Ultrasound Imaging" Life 15, no. 11: 1672. https://doi.org/10.3390/life15111672
APA StyleDietrich, C. F., Wüstner, M., Jenssen, C., Merkel, D., & Bleck, J. S. (2025). Daylight Sonography: Clinical Relevance of Color-Tinted Ultrasound Imaging. Life, 15(11), 1672. https://doi.org/10.3390/life15111672








