The Role of Genetic and Environmental Factors in White Leg Markings: Prevalence and Heritability Analysis in Pura Raza Española Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Traits and Database
- i.
- Dichotomous trait: classified as unaffected (class 0) or affected (class 1).
- ii.
- Discrete scale: a four-level scoring system (class 0: unaffected; class 1: white markings below the fetlock; class 2: markings above the fetlock; class 3: markings up to the cannon bone). The scale’s extremes represented the biological limits of this trait.
- A.
- Left foreleg + Right foreleg: (LF) + (RF);
- B.
- Left hindleg + Right hindleg: (LH) + (RH);
- C.
- Left legs: (LF) + (LH);
- D.
- Right legs: (RF) + (RH);
- E.
- Left foreleg + Right hindleg: (LF) + (RH);
- F.
- Right foreleg + Left hindleg: (RF) + (LH);
- G.
- Four legs: (LF) + (RF) + (LH) + (RH).
2.2. Statistical and Genetic Model
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRE | Pura Raza Española |
| ANCCE | Real Asociación Nacional de Criadores de Caballos Españoles |
| BLUPF90 | Best Linear Unbiased Prediction FORTRAN 90 |
| LF | Left foreleg |
| RF | Right foreleg |
| LH | Left hindleg |
| RH | Right hindleg |
| A | Left foreleg + Right foreleg |
| B | Left hindleg + Right hindleg |
| C | Left foreleg + Left hindleg |
| D | Right foreleg +Right hindleg |
| E | Left foreleg + Right hindleg |
| F | Right hindleg + Left hindleg |
| G | Left foreleg + Right foreleg + Left hindleg+ Right hindleg |
| ENDOG | Computer program for analyzing pedigree information |
| HPD95% | Intervals represent the range within which the true parameter values are likely to fall with 95% probability |
| SD | Standard deviation |
| CrI | Credible interval |
| σ2u | Additive genetic variance |
| σ2e | Residual variance |
| σ2m | Maternal variance |
References
- Rieder, S.; Hagger, C.; Obexer-Ruff, G.; Leeb, T.; Poncet, P. Genetic Analysis of White Facial and Leg Markings in the Swiss Franches-Montagnes Horse Breed. J. Hered. 2008, 99, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Maciel, S.V.S.A.; de Queiroz, V.H.O.; de Oliveira, C.A.A.; de Godói, F.N.; Pereira, G.L.; Curi, R.A.; Costa, R.B.; de Camargo, G.M.F. Genetic heterogeneity of white markings in Quarter Horses. Livest. Sci. 2020, 232, 103935. [Google Scholar] [CrossRef]
- Woolf, C.M. Influence of Stochastic Events on the Phenotypic Variation of Common White Leg Markings in the Arabian Horse. J. Hered. 1995, 86, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Encina, A.; Valera, M.; Ligero, M.; Rodriguez Sainz de los Terreros, A.; Sánchez-Guerrero, M.J. Characterisation of white facial markings in Pura Raza Española horses (a worldwide population genetic study). Ital. J. Anim. Sci. 2024, 23, 929–937. [Google Scholar] [CrossRef]
- Woolf, C.M. Multifactorial Inheritance of Common White Markings in the Arabian Horse. J. Hered. 1990, 81, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hauswirth, R.; Haase, B.; Blatter, M.; Brooks, S.A.; Burger, D.; Drögemüller, C.; Gerber, V.; Henke, D.; Janda, J.; Jude, R.; et al. Mutations in MITF and PAX3 cause “splashed white” and other white spotting phenotypes in horses. PLoS Genet. 2012, 8, e1002653. [Google Scholar] [CrossRef]
- Hauswirth, R.; Jude, R.; Haase, B.; Bellone, R.R.; Archer, S.; Holl, H.; Brooks, S.A.; Tozaki, T.; Penedo, M.C.; Rieder, S.; et al. Novel variants in the KIT and PAX3 genes in horses with white-spotted coat colour phenotypes. Anim. Genet. 2013, 44, 763–765. [Google Scholar] [CrossRef]
- Negro, S.; Imsland, F.; Valera, M.; Molina, A.; Solé, M.; Andersson, L. Association analysis of KIT, MITF, and PAX3 variants with white markings in Spanish horses. Anim. Genet. 2017, 48, 349–352. [Google Scholar] [CrossRef]
- Patterson Rosa, L.; Martin, K.; Vierra, M.; Lundquist, E.; Foster, G.; Brooks, S.A.; Lafayette, C. A KIT Variant Associated with Increased White Spotting Epistatic to MC1R Genotype in Horses (Equus caballus). Animals 2022, 12, 1958. [Google Scholar] [CrossRef]
- Nebe, H.D. Die Farbvererbung beim Pferd unter Besonderer Berücksichtigung der Vererbung Weisser Abzeichen. Ph.D. Thesis, Institut für Tierzucht und Haustiergenetik, Justus Liebig Universität, Giessen, Germany, 1984. [Google Scholar]
- Valera, M.; Molina, A.; Gutierrez, J.P.; Gómez, J.; Goyache, F. Pedigree analysis in the Andalusian horse: Population structure, genetic variability and influence of the Carthusian strain. Livest. Prod. Sci. 2005, 95, 57–66. [Google Scholar] [CrossRef]
- Sánchez, M.J.; Cervantes, I.; Valera, M.; Gutiérrez, J.P. Modelling genetic evaluation for dressage in Pura Raza Español horses with focus on the rider effect. J. Anim. Breed. Genet. 2014, 131, 395–402. [Google Scholar] [CrossRef]
- Solé, M.; Valera, M.; Fernández, J. Genetic structure and connectivity analysis in a large domestic livestock meta-population: The case of the Pura Raza Español horses. J. Anim. Breed. Genet. 2018, 135, 460–471. [Google Scholar] [CrossRef]
- LG_ANCCE. Purebred Spanish Horse. Breeding Program. Available online: https://www.lgancce.com/Documentacion/Normativa/Nacional/programa_cria_en.pdf (accessed on 20 September 2025).
- Sánchez, M.J.; Azor, P.J.; Molina, A.; Parkin, T.; Rivero, J.L.L.; Valera, M. Prevalence, risk factors and genetic parameters of cresty neck in Pura Raza Español horses. Equine Vet. J. 2017, 49, 196–200. [Google Scholar] [CrossRef]
- R core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 21 September 2025).
- Bürkner, P.C. brms: An R package for bayesian multilevel models using stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Misztal, I.; Tsuruta, S.; Lourenco, D.; Masuda, Y.; Aguilar, I.; Legarra, A.; Vitezica, Z. Manual for BLUPF90 Family of Programs; University of Georgia: Athens, GA, USA, 2014; Available online: https://nce.ads.uga.edu/html/projects/programs/docs/blupf90_all8.pdf (accessed on 20 September 2025).
- Gutierrez, J.P.; Goyache, F. A note on ENDOG: A computer program for analysing pedigree information. J. Anim. Breed. Genet. 2005, 122, 172–176. [Google Scholar] [CrossRef]
- Stachurska, A.; Ussing, A.P. White Markings in the Horse. Medycyna Wet. 2012, 68, 74–78. [Google Scholar]
- Bellone, R.R. Pleiotropic effects of pigmentation genes in horses. Anim. Genet. 2010, 41, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Genetics and the Behaviour of Domestic Animals, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar] [CrossRef]
- Trut, L.N. Early canid domestication: The farm-fox experiment. Am. Sci. 1999, 87, 160–169. [Google Scholar] [CrossRef]
- Dobney, K.; Larson, G. Genetics and animal domestication: New windows on an elusive process. J. Zool. 2006, 269, 261–271. [Google Scholar] [CrossRef]
- Stachurska, A.; Pieta, M.; Lojek, J.; Szulowska, J. Performance in racehorses of various colours. Livest. Sci. 2007, 106, 282–286. [Google Scholar] [CrossRef]
- Haase, B.; Signer-Hasler, H.; Binns, M.M.; Obexer-Ruff, G.; Hauswirth, R.; Bellone, R.R.; Burger, D.; Rieder, S.; Wade, C.M.; Leeb, T. Accumulating mutations in series of haplotypes at the KIT and MITF loci are major determinants of white markings in Franches-Montagnes horses. PLoS ONE. 2013, 8, e75071. [Google Scholar] [CrossRef] [PubMed]
- Perdomo-González, D.; García de Paredes, R.; Valera, M.; Bartolomé, E.; Gómez, M.D. Morpho-Functional Traits in Pura Raza Menorquina Horses: Genetic Parameters and Relationship with Coat Color Variables. Animals 2022, 12, 2319. [Google Scholar] [CrossRef]
- Brooks, S.A.; Lear, T.L.; Adelson, D.L.; Bailey, E. A chromosome inversion near the KIT gene and the Tobiano spotting pattern in horses. Cytogenet. Genome Res. 2008, 119, 225–230. [Google Scholar] [CrossRef]
- Brooks, S.A.; Palermo, K.M.; Kahn, A.; Hein, J. Impact of white-spotting alleles, including W20, on phenotype in the American Paint Horse. Anim. Genet. 2020, 51, 707–715. [Google Scholar] [CrossRef]
- Ripolles, M.; Sánchez-Guerrero, M.J.; Perdomo-González, D.I.; Azor, P.; Valera, M. Survey of risk factors and genetic characteriza tion of ewe neck in a world population of Pura Raza Español horses. Animals 2020, 10, 1789. [Google Scholar] [CrossRef]
- Ripollés-Lobo, M.; Perdomo-González, D.I.; Azor, P.J.; Valera, M. Evaluation of potential effects and genetic parameters in conformational limb defects in Pura Raza Española horses. Ital. J. Anim. Sci. 2023, 22, 407–417. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.J.; Solé, M.; Azor, P.J.; Sölkner, J.; Valera, M. Genetic and environmental risk factors for vitiligo and melanoma in Pura Raza Español horses. Equine Vet. J. 2019, 51, 606–611. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.J.; Ramos, J.; Valdés, M.; Rivero, J.L.L.; Valera, M. Prevalence, environmental risk factors and heritability of body condition in Pura Raza Español horses. Livest. Sci. 2019, 230, 103851. [Google Scholar] [CrossRef]
- Poyato-Bonilla, J.; Perdomo-González, D.I.; Sánchez-Guerrero, M.J.; Varona, L.; Molina, A.; Casellas, J.; Valera, M. Genetic inbreeding depression load for morphological traits and defects in the Pura Raza Española horse. Genet. Sel. Evol. 2020, 52, 62. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.J.; Bartolomé, E.; Valera, M. Genetic study of stress assessed with infrared thermography during dressage competitions in the Pura Raza Español horse. Appl. Anim. Behav. Sci. 2016, 174, 58–65. [Google Scholar] [CrossRef]
- Gómez, M.D.; Sánchez, M.J.; Bartolomé, E.; Cervantes, I.; Poyato-Bonilla, J.; Demyda-Peyrás, S.; Valera, M. Phenotypic and genetic analysis of reproductive traits in horse populations with different breeding purposes. Animal 2020, 14, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
| No Affected | Below Fetlock | Above Fetlock | Cannon Bone | |
|---|---|---|---|---|
| Foreleg |  |  |  |  |
| Hindleg |  | 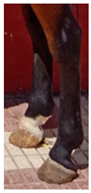 |  |  |
| White Leg Markings | Not Affected | Affected | Below Fetlock | Above Fetlock | Cannon Bone | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LF | 36,771 | (94.7) | 2054 | (5.3) | 332 | (16.2) | 1359 | (66.2) | 363 | (17.6) |
| RF | 37,021 | (95.4) | 1804 | (4.6) | 305 | (16.9) | 1172 | (65.0) | 327 | (18.1) |
| LH | 31,857 | (82.1) | 6968 | (17.9) | 846 | (12.1) | 4451 | (63.9) | 1671 | (24.0) |
| RH | 33,191 | (85.5) | 5634 | (14.5) | 714 | (12.7) | 3594 | (63.8) | 1326 | (23.5) |
| A | 37,928 | (97.7) | 897 | (2.3) | ||||||
| B | 35,273 | (90.8) | 3552 | (9.2) | ||||||
| C | 37,462 | (96.5) | 1363 | (3.5) | ||||||
| D | 37,736 | (97.2) | 1089 | (2.8) | ||||||
| E | 37,644 | (97.0) | 1181 | (3.0) | ||||||
| F | 37,621 | (96.9) | 1204 | (3.1) | ||||||
| G | 38,341 | (98.8) | 484 | (1.2) | ||||||
| White Leg Markings | σ2u | σ2m | σ2e | h2 (1) | h2observed (2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | HPD 95% | Mean | Median | HPD 95% | Mean | Median | HPD 95% | |||
| LF | 1.540 | 1.531 | 1.280–1.805 | 0.059 | 0.055 | 0.019–0.111 | 1.000 | 1.000 | 0.978–1.020 | 0.591 (0.022) | 0.187 |
| RF | 1.945 | 1.938 | 1.594–2.279 | 0.067 | 0.061 | 0.010–0.140 | 1.000 | 1.000 | 0.978–1.021 | 0.645 (0.022) | 0.180 |
| LH | 1.223 | 1.218 | 1.06–1.396 | 0.043 | 0.041 | 0.018–0.071 | 1.000 | 1.000 | 0.979–1.018 | 0.539 (0.017) | 0.515 |
| RH | 1.150 | 1.145 | 1.003–1.309 | 0.037 | 0.036 | 0.013–0.056 | 1.000 | 1.000 | 0.978–1.020 | 0.525 (0.017) | 0.418 |
| A | 1.555 | 1.350 | 0.120–3.221 | 0.610 | 0.490 | 0.009–2.103 | 1.016 | 1.001 | 0.796–1.209 | 0.472 (0.149) | 0.101 |
| B | 4.133 | 2.165 | 0.462–13.750 | 1.788 | 0.404 | 0.007–6.721 | 1.013 | 1.000 | 0.673–1.356 | 0.572 (0.102) | 0.098 |
| C | 1.687 | 1.517 | 0.042–3.940 | 0.402 | 0.234 | 0.002–1.401 | 1.168 | 1.005 | 0.439–2.387 | 0.495 (0.177) | 0.070 |
| D | 1.975 | 1.332 | 0.040–5.173 | 0.961 | 0.268 | 0.013–4.033 | 1.391 | 1.008 | 0.714–3.100 | 0.465 (0.130) | 0.245 |
| E | 1.002 | 0.986 | 0.058–1.903 | 0.272 | 0.058 | 0.004–1.300 | 1.007 | 1.001 | 0.747–1.167 | 0.410 (0.187) | 0.076 |
| F | 4.957 | 2.083 | 0.841–21.230 | 2.833 | 0.613 | 0.008–16.320 | 0.977 | 0.998 | 0.467–1.353 | 0.573 (0.075) | 0.108 |
| G | 1.161 | 0.983 | 0.421–2.461 | 0.382 | 0.211 | 0.011–1.371 | 1.150 | 1.007 | 0.522–2.248 | 0.394 (0.209) | 0.030 |
| Genetics Parameters | Left Foreleg (LF) | Right Foreleg (RF) | Left Hindleg (LH) | Right Hindleg (RH) | |
|---|---|---|---|---|---|
| Mean | 4.877 | 4.772 | 7.904 | 5.659 | |
| σ2u | Median | 4.871 | 4.781 | 7.922 | 5.657 |
| HPD 95% | 4.341–5.368 | 4.188–5.243 | 7.060–8.719 | 4.971–6.218 | |
| Mean | 0.185 | 0.130 | 0.398 | 0.273 | |
| σ2m | Median | 0.181 | 0.121 | 0.400 | 0.271 |
| HPD 95% | 0.062–0.309 | 0.014–0.273 | 0.119–0.589 | 0.146–0.410 | |
| Mean | 4.000 | 3.420 | 8.046 | 5.898 | |
| σ2e | Median | 3.968 | 3.411 | 7.794 | 5.730 |
| HPD 95% | 3.295–4.611 | 2.868–3.966 | 5.292–11.280 | 4.036–7.928 | |
| h2 | 0.514 (0.061) | 0.539 (0.028) | 0.574 (0.029) | 0.488 (0.043) | |
| White Leg Markings | Left Foreleg (SD) [HPD 95%] | Right Foreleg (SD) [HPD 95%] | Left Hindleg (SD) [HPD 95%] | Right Hindleg (SD) [HPD 95%] |
|---|---|---|---|---|
| Left Foreleg | 0.991 (0.003)[0.985–0.995] | 0.907 (0.014) [0.883–0.936] | 0.886 (0.016) [0.854–0.915] | |
| Right Foreleg | 0.988 (0.005) [0.977–0.995] | 0.887 (0.015) [0.854–0.915] | 0.863 (0.019) [0.823–0.899] | |
| Left Hindleg | 0.887 (0.026) [0.840–0.934] | 0.866 (0.021) [0.828–0.909] | 0.995 (0.002) [0.991–0.998] | |
| Right Hindleg | 0.883 (0.019) [0.848–0.920] | 0.858 (0.020) [0.820–0.896] | 0.986 (0.005) [0.975–0.993] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encina, A.; Sánchez-Guerrero, M.J.; Ligero, M.; Rodríguez-Sainz de los Terreros, A.; Valera, M. The Role of Genetic and Environmental Factors in White Leg Markings: Prevalence and Heritability Analysis in Pura Raza Española Horses. Life 2025, 15, 1661. https://doi.org/10.3390/life15111661
Encina A, Sánchez-Guerrero MJ, Ligero M, Rodríguez-Sainz de los Terreros A, Valera M. The Role of Genetic and Environmental Factors in White Leg Markings: Prevalence and Heritability Analysis in Pura Raza Española Horses. Life. 2025; 15(11):1661. https://doi.org/10.3390/life15111661
Chicago/Turabian StyleEncina, Ana, María José Sánchez-Guerrero, Manuel Ligero, Arantxa Rodríguez-Sainz de los Terreros, and Mercedes Valera. 2025. "The Role of Genetic and Environmental Factors in White Leg Markings: Prevalence and Heritability Analysis in Pura Raza Española Horses" Life 15, no. 11: 1661. https://doi.org/10.3390/life15111661
APA StyleEncina, A., Sánchez-Guerrero, M. J., Ligero, M., Rodríguez-Sainz de los Terreros, A., & Valera, M. (2025). The Role of Genetic and Environmental Factors in White Leg Markings: Prevalence and Heritability Analysis in Pura Raza Española Horses. Life, 15(11), 1661. https://doi.org/10.3390/life15111661





