The Multifaceted Role of Platelets in Atherosclerosis and Ischemic Disease: Pathogenesis, Inflammation, and Therapeutic Opportunities
Abstract
1. Introduction
2. Platelets in the Initiation and Progression of Atherosclerosis
2.1. Early Involvement in Lesion Formation
2.2. Immune Cell Interaction and Inflammation
2.3. Release of Inflammatory Factors
2.4. Monocyte-to-Foam Cell Transformation
2.5. Platelet-Derived Microparticles
2.6. Complement Activation and Amplification
3. Platelets and Plaque Instability
3.1. Mechanisms of Plaque Destabilization
3.2. Plaque Rupture and Thrombosis
3.3. Inflammation-Driven Rupture Risk
4. Therapeutic Intervention
4.1. Current Antiplatelet Strategies
4.2. Emerging Therapeutic Targets
4.3. Modulating Platelet–Immune Crosstalk
4.4. Platelet–Complement System Interventions
4.5. Nanotechnology and Precision Drug Delivery
4.6. Clinical Limitations and Translational Novelty
4.7. Clinical Trials Associated with Platelets
5. Conclusions, Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndromes |
| CAD | Coronary artery disease |
| CCL5 | C-C motif ligand 5 |
| CCR1 | C-C chemokine receptor type 1 |
| COX1 | Cyclooxygenase-1 |
| CVD | Cardiovascular disease |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| CXCL4 | C-X-C motif chemokine ligand 4 |
| ECM | Extracellular matrix |
| ECs | Endothelial cells |
| EVs | Extracellular vesicles |
| GCX | Glycocalyx |
| HMGB1 | High mobility group box protein 1 |
| IL | Interleukin |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| MI | Myocardial infarction |
| MCP1 | Monocyte chemoattractant protein-1 |
| MAC | Membrane attack complexes |
| MMPs | Matrix metalloproteinases |
| NETs | Neutrophil-extracellular traps |
| NO | Nitric oxide |
| NPA | Neutrophil–platelet aggregates |
| ox-LDL | oxidized low-density lipoprotein |
| PAD | Peripheral artery disease |
| PDT | Photodynamic therapy |
| PF4 | Platelet factor 4 |
| PMC | Platelet–monocyte complexes |
| PMPs | Platelet microparticles |
| PDGF | Platelet-derived growth factor |
| PSGL1 | P-selectin glycoprotein ligand-1 |
| RANTES | Regulated upon activation normal T expressed and secreted |
| ROS | Reactive oxygen species |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TNF-α | Tumor necrosis factor-alpha |
| TGF-β | transforming growth factor beta |
| UCNPs | Upconversion nanoparticles |
| VCAM1 | Vascular Cell Adhesion Molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VSMCs | Vascular smooth muscle cells |
| vWF | von Willebrand factor |
References
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.C.; David, S.G.; David, A.G.; Tarca, V.; Paduret, I.A.; Mindru, D.E.; Rosu, S.T.; Rosu, E.V.; Adumitrachioaiei, H.; Bernic, J.; et al. Atherosclerosis from Newborn to Adult-Epidemiology, Pathological Aspects, and Risk Factors. Life 2023, 13, 2056. [Google Scholar] [CrossRef]
- Rai, V.; Agrawal, D.K. The role of damage- and pathogen-associated molecular patterns in inflammation-mediated vulnerability of atherosclerotic plaques. Can. J. Physiol. Pharmacol. 2017, 95, 1245–1253. [Google Scholar] [CrossRef]
- Rai, V.; Rao, V.H.; Shao, Z.; Agrawal, D.K. Dendritic Cells Expressing Triggering Receptor Expressed on Myeloid Cells-1 Correlate with Plaque Stability in Symptomatic and Asymptomatic Patients with Carotid Stenosis. PLoS ONE 2016, 11, e0154802. [Google Scholar] [CrossRef]
- Rao, V.H.; Rai, V.; Stoupa, S.; Subramanian, S.; Agrawal, D.K. Tumor necrosis factor-alpha regulates triggering receptor expressed on myeloid cells-1-dependent matrix metalloproteinases in the carotid plaques of symptomatic patients with carotid stenosis. Atherosclerosis 2016, 248, 160–169. [Google Scholar] [CrossRef]
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Mauricio, D.; Castelblanco, E.; Alonso, N. Cholesterol and Inflammation in Atherosclerosis: An Immune-Metabolic Hypothesis. Nutrients 2020, 12, 2444. [Google Scholar] [CrossRef]
- Martinez Bravo, G.; Annarapu, G.; Carmona, E.; Nawarskas, J.; Clark, R.; Novelli, E.; Mota Alvidrez, R.I. Platelets in Thrombosis and Atherosclerosis: A Double-Edged Sword. Am. J. Pathol. 2024, 194, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of atherothrombosis: Mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef]
- Boulaftali, Y.; Massberg, S.; Nicolai, L. Platelets in vascular inflammation: Fire-fighters or pyromaniacs? Curr. Opin. Hematol. 2025, 32, 221–230. [Google Scholar] [CrossRef]
- Kameyoshi, Y.; Dorschner, A.; Mallet, A.I.; Christophers, E.; Schroder, J.M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J. Exp. Med. 1992, 176, 587–592. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Weber, C. Platelets as immune cells: Bridging inflammation and cardiovascular disease. Circ. Res. 2007, 100, 27–40. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, C.; Wang, S.; Jia, H.; Yu, B. TNF-alpha is a Novel Biomarker for Predicting Plaque Rupture in Patients with ST-Segment Elevation Myocardial Infarction. J. Inflamm. Res. 2022, 15, 1889–1898. [Google Scholar] [CrossRef]
- Eder, L.; Joshi, A.A.; Dey, A.K.; Cook, R.; Siegel, E.L.; Gladman, D.D.; Mehta, N.N. Association of Tumor Necrosis Factor Inhibitor Treatment with Reduced Indices of Subclinical Atherosclerosis in Patients With Psoriatic Disease. Arthritis Rheumatol. 2018, 70, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Arisato, T.; Hashiguchi, T.; Sarker, K.P.; Arimura, K.; Asano, M.; Matsuo, K.; Osame, M.; Maruyama, I. Highly accumulated platelet vascular endothelial growth factor in coagulant thrombotic region. J. Thromb. Haemost. 2003, 1, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.G.; Metharom, P.; Berndt, M.C. The functional role of platelets in the regulation of angiogenesis. Platelets 2015, 26, 199–211. [Google Scholar] [CrossRef]
- Tersteeg, C.; Heijnen, H.F.; Eckly, A.; Pasterkamp, G.; Urbanus, R.T.; Maas, C.; Hoefer, I.E.; Nieuwland, R.; Farndale, R.W.; Gachet, C.; et al. FLow-induced PRotrusions (FLIPRs): A platelet-derived platform for the retrieval of microparticles by monocytes and neutrophils. Circ. Res. 2014, 114, 780–791. [Google Scholar] [CrossRef]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Research 2018, 7, 236. [Google Scholar] [CrossRef]
- Rolling, C.C.; Barrett, T.J.; Berger, J.S. Platelet-monocyte aggregates: Molecular mediators of thromboinflammation. Front. Cardiovasc. Med. 2023, 10, 960398. [Google Scholar] [CrossRef]
- Huseynov, A.; Reinhardt, J.; Chandra, L.; Durschmied, D.; Langer, H.F. Novel Aspects Targeting Platelets in Atherosclerotic Cardiovascular Disease-A Translational Perspective. Int. J. Mol. Sci. 2023, 24, 6280. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, B.; Junkuszew, A.; Patkowski, K.; Szponder, T.; Ngoc, D.N.; Drzewiecka, B.; Sobczynska-Rak, A.; Wessely-Szponder, J. The activity of monocyte-derived macrophages after stimulation with platelet-rich and platelet-poor concentrates. Study on an ovine model of insertion of a tibial implant coated with silicon-doped diamond-like carbon. J. Vet. Res. 2024, 68, 167–174. [Google Scholar] [CrossRef]
- Kim, K.W.; Ivanov, S.; Williams, J.W. Monocyte Recruitment, Specification, and Function in Atherosclerosis. Cells 2020, 10, 15. [Google Scholar] [CrossRef]
- Martins, P.D.C.; van Gils, J.M.; Mol, A.; Hordijk, P.L.; Zwaginga, J.-J. Platelet Binding to Monocytes Induces Changes in Integrin Functionality Promoting Monocyte Adhesion and Transendothelial Migration. Do Platelets Migrate Along? Blood 2005, 106, 2214. [Google Scholar] [CrossRef]
- Badrnya, S.; Schrottmaier, W.C.; Kral, J.B.; Yaiw, K.C.; Volf, I.; Schabbauer, G.; Soderberg-Naucler, C.; Assinger, A. Platelets mediate oxidized low-density lipoprotein-induced monocyte extravasation and foam cell formation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Gavidia, L.M.; Burger, D.; Matthews, V.B.; Nolde, J.M.; Galindo Kiuchi, M.; Carnagarin, R.; Kannenkeril, D.; Chan, J.; Joyson, A.; Herat, L.Y.; et al. Role of Microparticles in Cardiovascular Disease: Implications for Endothelial Dysfunction, Thrombosis, and Inflammation. Hypertension 2021, 77, 1825–1844. [Google Scholar] [CrossRef]
- Arneth, B.; Arneth, R. Neutrophil Extracellular Traps (NETs) and Vasculitis. Int. J. Med. Sci. 2021, 18, 1532–1540. [Google Scholar] [CrossRef]
- Baaten, C.; Nagy, M.; Bergmeier, W.; Spronk, H.M.H.; van der Meijden, P.E.J. Platelet biology and function: Plaque erosion vs. rupture. Eur. Heart J. 2024, 45, 18–31. [Google Scholar] [CrossRef]
- Doring, Y.; Soehnlein, O.; Weber, C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, C.; Liu, X.; Hu, L.; Wang, Z.; Li, X.; Zhang, J.; Li, D.; Zhang, X.; Wu, M.; et al. The Role of Neutrophil Extracellular Traps in Atherosclerosis: From the Molecular to the Clinical Level. J. Inflamm. Res. 2025, 18, 4421–4433. [Google Scholar] [CrossRef]
- Kaczor, D.M.; Kramann, R.; Hackeng, T.M.; Schurgers, L.J.; Koenen, R.R. Differential Effects of Platelet Factor 4 (CXCL4) and Its Non-Allelic Variant (CXCL4L1) on Cultured Human Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2022, 23, 580. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C. Targeting Platelet in Atherosclerosis Plaque Formation: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9760. [Google Scholar] [CrossRef]
- Nording, H.M.; Seizer, P.; Langer, H.F. Platelets in inflammation and atherogenesis. Front. Immunol. 2015, 6, 98. [Google Scholar] [CrossRef]
- Nording, H.; Baron, L.; Langer, H.F. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020, 307, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Y.; Gao, M.; Han, Z.; Jiang, W.; Gu, Y.; Liu, Y. Platelet-Mimicking Therapeutic System for Noninvasive Mitigation of the Progression of Atherosclerotic Plaques. Adv. Sci. 2021, 8, 2004128. [Google Scholar] [CrossRef] [PubMed]
- Maaninka, K.; Neuvonen, M.; Kerkela, E.; Hyvarinen, K.; Palviainen, M.; Kamali-Moghaddam, M.; Federico, A.; Greco, D.; Laitinen, S.; Oorni, K.; et al. OxLDL sensitizes platelets for increased formation of extracellular vesicles capable of finetuning macrophage gene expression. Eur. J. Cell Biol. 2023, 102, 151311. [Google Scholar] [CrossRef] [PubMed]
- Maretti-Mira, A.C.; Golden-Mason, L.; Salomon, M.P.; Kaplan, M.J.; Rosen, H.R. Cholesterol-Induced M4-Like Macrophages Recruit Neutrophils and Induce NETosis. Front. Immunol. 2021, 12, 671073. [Google Scholar] [CrossRef]
- Barrett, T.J.; Schlegel, M.; Zhou, F.; Gorenchtein, M.; Bolstorff, J.; Moore, K.J.; Fisher, E.A.; Berger, J.S. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci. Transl. Med. 2019, 11, eaax0481. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef] [PubMed]
- Wraith, K.S.; Magwenzi, S.; Aburima, A.; Wen, Y.; Leake, D.; Naseem, K.M. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood 2013, 122, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.Y.; Zhi, X.; Liu, H.X.; Wang, X.; Chen, Y.Y.; Wang, L. Is the suppression of CD36 a promising way for atherosclerosis therapy? Biochem. Pharmacol. 2024, 219, 115965. [Google Scholar] [CrossRef]
- Li, N. Platelets as an inter-player between hyperlipidaemia and atherosclerosis. J. Intern. Med. 2024, 296, 39–52. [Google Scholar] [CrossRef]
- Ragni, E. Extracellular Vesicles: Recent Advances and Perspectives. Front. Biosci. (Landmark Ed.) 2025, 30, 36405. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S.; Kim, S. Shedding Light on the Cell Biology of Platelet-Derived Extracellular Vesicles and Their Biomedical Applications. Life 2023, 13, 1403. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Mohite, P.; Munde, S.; Ade, N.; Oladosu, T.A.; Chidrawar, V.R.; Patel, R.; Bhattacharya, S.; Paliwal, H.; Singh, S. Extracellular vesicles: The future of therapeutics and drug delivery systems. Intell. Pharm. 2024, 2, 312–328. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, G.; Zhang, Z.; Yu, Y.; Zeng, L.; Xu, Z.; Weng, J.; Xia, J.; Li, J.; Pathak, J.L. Apoptotic bodies: Bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J. Nanobiotechnol. 2023, 21, 218. [Google Scholar] [CrossRef]
- Patzelt, J.; Verschoor, A.; Langer, H.F. Platelets and the complement cascade in atherosclerosis. Front. Physiol. 2015, 6, 49. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Yin, W.; Grigg, S.E.; Ghebrehiwet, B. Blood platelets activate the classical pathway of human complement. J. Thromb. Haemost. 2006, 4, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Peerschke, E.I.; Yin, W.; Ghebrehiwet, B. Complement activation on platelets: Implications for vascular inflammation and thrombosis. Mol. Immunol. 2010, 47, 2170–2175. [Google Scholar] [CrossRef]
- Maffia, P.; Mauro, C.; Case, A.; Kemper, C. Canonical and non-canonical roles of complement in atherosclerosis. Nat. Rev. Cardiol. 2024, 21, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Complement activation: An emerging player in the pathogenesis of cardiovascular disease. Scientifica 2012, 2012, 402783. [Google Scholar] [CrossRef]
- Shadid, A.; Hok, K.D.; Domozhirov, A.Y.; Weng-Mills, T.; Doursout, M.F.; Banda, N.K.; Restrepo, M.I.; Shivshankar, P. Enigmatic Roles of Complement Anaphylatoxin Signaling in Health and Disease. Immune Netw. 2025, 25, e32. [Google Scholar] [CrossRef]
- Shivshankar, P.; Li, Y.D.; Mueller-Ortiz, S.L.; Wetsel, R.A. In response to complement anaphylatoxin peptides C3a and C5a, human vascular endothelial cells migrate and mediate the activation of B-cells and polarization of T-cells. FASEB J. 2020, 34, 7540–7560. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The role of oxidative stress in atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, Y.; Yang, F.; Yang, Y.; Cui, Z.; Shi, D.; Bai, R. Vascular smooth muscle cell phenotypic switching in atherosclerosis. Heliyon 2024, 10, e37727. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Munno, M.; Mallia, A.; Greco, A.; Modafferi, G.; Banfi, C.; Eligini, S. Radical Oxygen Species, Oxidized Low-Density Lipoproteins, and Lectin-like Oxidized Low-Density Lipoprotein Receptor 1: A Vicious Circle in Atherosclerotic Process. Antioxidants 2024, 13, 583. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; De Backer, D. Does disseminated intravascular coagulation lead to multiple organ failure? Crit. Care Clin. 2005, 21, 469–477. [Google Scholar] [CrossRef]
- Wienkamp, A.K.; Erpenbeck, L.; Rossaint, J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022, 13, 953129. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D.; et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132, e154225. [Google Scholar] [CrossRef]
- Zhou, P.; Li, T.; Jin, J.; Liu, Y.; Li, B.; Sun, Q.; Tian, J.; Zhao, H.; Liu, Z.; Ma, S.; et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicine 2020, 53, 102671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Lin, X.; Liang, H.; Liu, X.; Zhang, X.; Zhang, Q.; Zhou, F.; Yu, C.; Lei, L.; et al. Complement C5a induces the generation of neutrophil extracellular traps by inhibiting mitochondrial STAT3 to promote the development of arterial thrombosis. Thromb. J. 2022, 20, 24. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, M.; Liu, Q.; Liu, J.; Cui, Y. Neutrophil extracellular traps induce thrombogenicity in severe carotid stenosis. Immun. Inflamm. Dis. 2021, 9, 1025–1036. [Google Scholar] [CrossRef]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, M.; Jakowitsch, J.; Panzenbock, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef]
- Dehghani, T.; Panitch, A. Endothelial cells, neutrophils and platelets: Getting to the bottom of an inflammatory triangle. Open Biol. 2020, 10, 200161. [Google Scholar] [CrossRef]
- Morikis, V.A.; Simon, S.I. Neutrophil Mechanosignaling Promotes Integrin Engagement With Endothelial Cells and Motility Within Inflamed Vessels. Front. Immunol. 2018, 9, 2774. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mwangi, J.G.; Stanley, T.K.; Mitra, R.; Ebong, E.E. Regeneration and assessment of the endothelial glycocalyx to address cardiovascular disease. Ind. Eng. Chem. Res. 2021, 60, 17328–17347. [Google Scholar] [CrossRef]
- Wei, Y.N.; Li, M.H.; Liu, J.; Wang, J.T. Risk Factors of Clopidogrel Resistance in the Elderly Patients with Atherosclerotic Cardiovascular Disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2023, 45, 38–43. [Google Scholar] [CrossRef]
- Alhazzani, A.; Venkatachalapathy, P.; Padhilahouse, S.; Sellappan, M.; Munisamy, M.; Sekaran, M.; Kumar, A. Biomarkers for Antiplatelet Therapies in Acute Ischemic Stroke: A Clinical Review. Front. Neurol. 2021, 12, 667234. [Google Scholar] [CrossRef]
- Kadoglou, N.; Moulakakis, K.G.; Mantas, G.; Spathis, A.; Gkougkoudi, E.; Mylonas, S.N.; Kakisis, J.; Liapis, C. Novel Biomarkers and Imaging Indices for the “Vulnerable Patient” with Carotid Stenosis: A Single-Center Study. Biomolecules 2023, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Schunkert, H.; von Hundelshausen, P. Novel Approaches to Fine-Tune Therapeutic Targeting of Platelets in Atherosclerosis: A Critical Appraisal. Thromb. Haemost. 2020, 120, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Swieringa, F.; de Laat, B.; de Groot, P.G.; Roest, M.; Heemskerk, J.W.M. Reversible Platelet Integrin alphaIIbbeta3 Activation and Thrombus Instability. Int. J. Mol. Sci. 2022, 23, 12512. [Google Scholar] [CrossRef]
- Pereira-da-Silva, T.; Ferreira, V.; Castelo, A.; Caldeira, D.; Napoleao, P.; Pinheiro, T.; Ferreira, R.C.; Carmo, M.M. Soluble CD40 ligand expression in stable atherosclerosis: A systematic review and meta-analysis. Atherosclerosis 2021, 319, 86–100. [Google Scholar] [CrossRef]
- Hussain, H.; Tarantino, M.D.; Chaturvedi, S.; McCrae, K.R.; Roberts, J.C. Eculizumab for refractory thrombosis in antiphospholipid syndrome. Blood Adv. 2022, 6, 1271–1277. [Google Scholar] [CrossRef]
- Golomingi, M.; Kohler, J.; Lamers, C.; Pouw, R.B.; Ricklin, D.; Dobo, J.; Gal, P.; Pal, G.; Kiss, B.; Dopler, A.; et al. Complement inhibition can decrease the haemostatic response in a microvascular bleeding model at multiple levels. Front. Immunol. 2023, 14, 1226832. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Hou, Q.; Cao, H.; Li, C.; Peng, X.; Han, Q.; Wu, S.; Li, K. Aspirin does not confer protection against major ischemic vascular events in patients diagnosed with rheumatoid arthritis. J. Int. Med. Res. 2025, 53, 3000605251315359. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, R.; Yu, T. Photodynamic Therapy for Atherosclerosis: Past, Present, and Future. Pharmaceutics 2024, 16, 729. [Google Scholar] [CrossRef]
- Yang, C.; Mo, L.; Zhang, G.; Dai, Y.; Li, B.; Tan, Z.; Guo, Y.; Lu, S.; Hong, Y.; He, H.; et al. Advancements in dual-targeting nanoparticle strategies for enhanced atherosclerosis therapy: Overcoming limitations of single-targeting approaches. Bioact. Mater. 2026, 55, 302–333. [Google Scholar] [CrossRef] [PubMed]
- Tummala, R.; Rai, M.P. Glycoprotein IIb/IIIa Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Weber, C.; Habenicht, A.J.R.; von Hundelshausen, P. Novel mechanisms and therapeutic targets in atherosclerosis: Inflammation and beyond. Eur. Heart J. 2023, 44, 2672–2681. [Google Scholar] [CrossRef]
- Luo, W.; Wang, H.; Ohman, M.K.; Guo, C.; Shi, K.; Wang, J.; Eitzman, D.T. P-selectin glycoprotein ligand-1 deficiency leads to cytokine resistance and protection against atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 2012, 220, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhong, L.; Zhu, S.; Wang, Y.; Zheng, J.; Wang, S.; Zhang, J.; Huang, R. The P-selectin and PSGL-1 axis accelerates atherosclerosis via activation of dendritic cells by the TLR4 signaling pathway. Cell Death Dis. 2019, 10, 507. [Google Scholar] [CrossRef]
- Valenzuela, N.M.; Hong, L.; Shen, X.D.; Gao, F.; Young, S.H.; Rozengurt, E.; Kupiec-Weglinski, J.W.; Fishbein, M.C.; Reed, E.F. Blockade of p-selectin is sufficient to reduce MHC I antibody-elicited monocyte recruitment in vitro and in vivo. Am. J. Transplant. 2013, 13, 299–311. [Google Scholar] [CrossRef]
- Koenen, R.R.; von Hundelshausen, P.; Nesmelova, I.V.; Zernecke, A.; Liehn, E.A.; Sarabi, A.; Kramp, B.K.; Piccinini, A.M.; Paludan, S.R.; Kowalska, M.A.; et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat. Med. 2009, 15, 97–103. [Google Scholar] [CrossRef]
- Koenen, R.R.; Weber, C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat. Rev. Drug Discov. 2010, 9, 141–153. [Google Scholar] [CrossRef]
- Jourdi, G.; Marquis-Gravel, G.; Martin, A.C.; Lordkipanidze, M.; Godier, A.; Gaussem, P. Antiplatelet Therapy in Atherothrombotic Diseases: Similarities and Differences Across Guidelines. Front. Pharmacol. 2022, 13, 878416. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, Q.Y.; Chen, H.S. Dual Antiplatelet Therapy and Outcomes in Acute Mild to Moderate Stroke With Versus Without Large-Artery Atherosclerosis Post Hoc Analysis of ATAMIS. J. Am. Heart Assoc. 2024, 13, e036318. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Zhang, Q.J.; Li, H.; Liu, M.W. Progress Analysis of Personalized Antiplatelet Therapy in Patients with Coronary Heart Disease Undergoing Interventional Therapy. Rev. Cardiovasc. Med. 2024, 25, 462. [Google Scholar] [CrossRef] [PubMed]
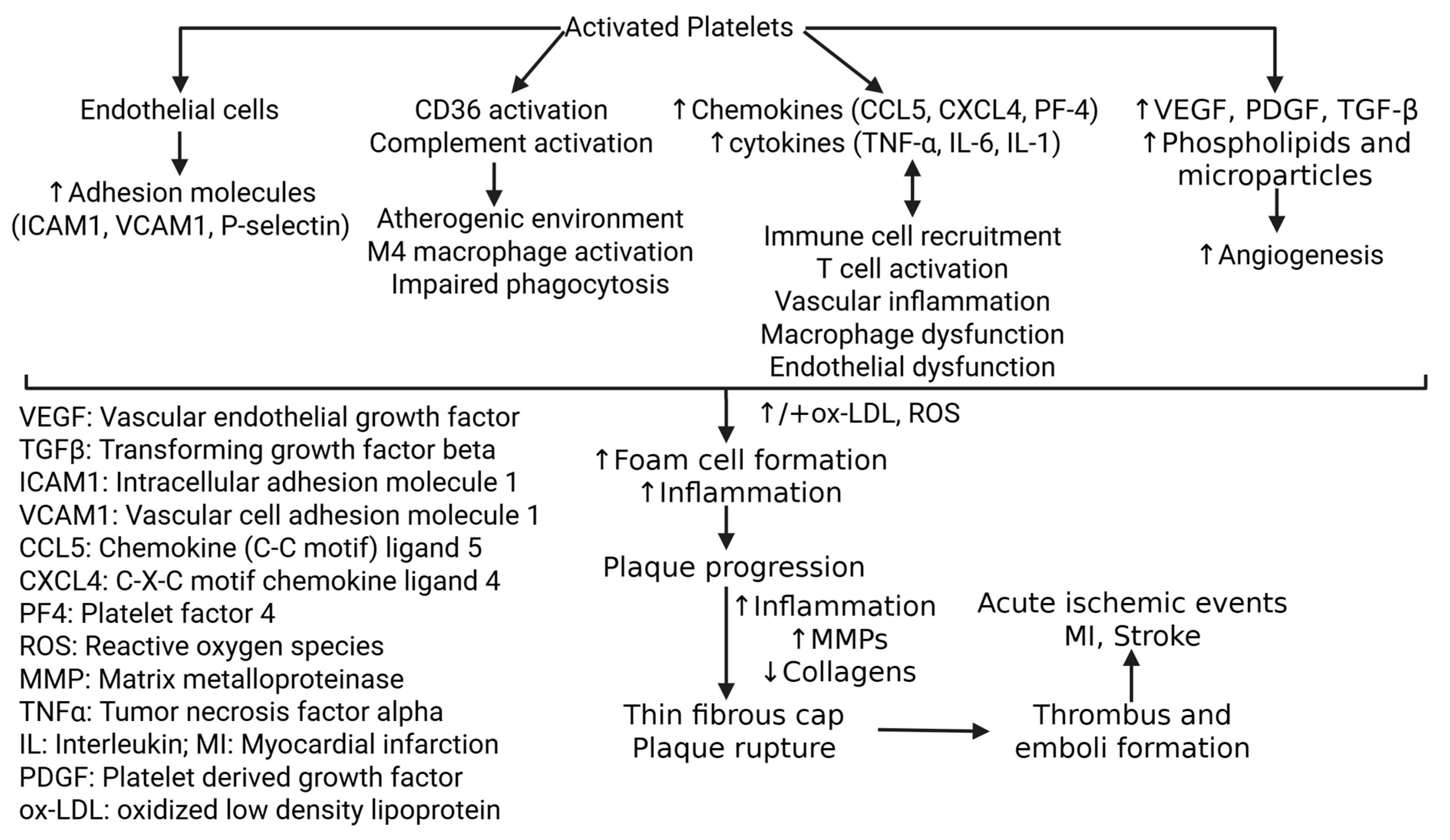
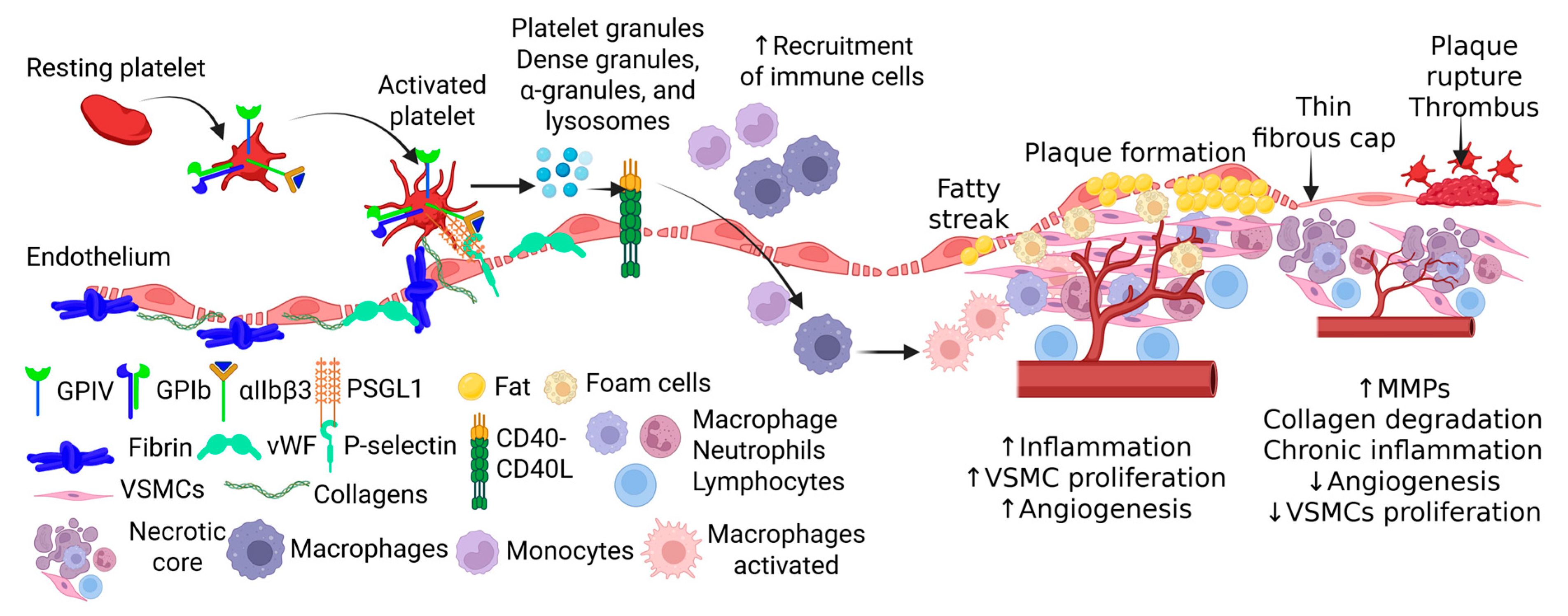

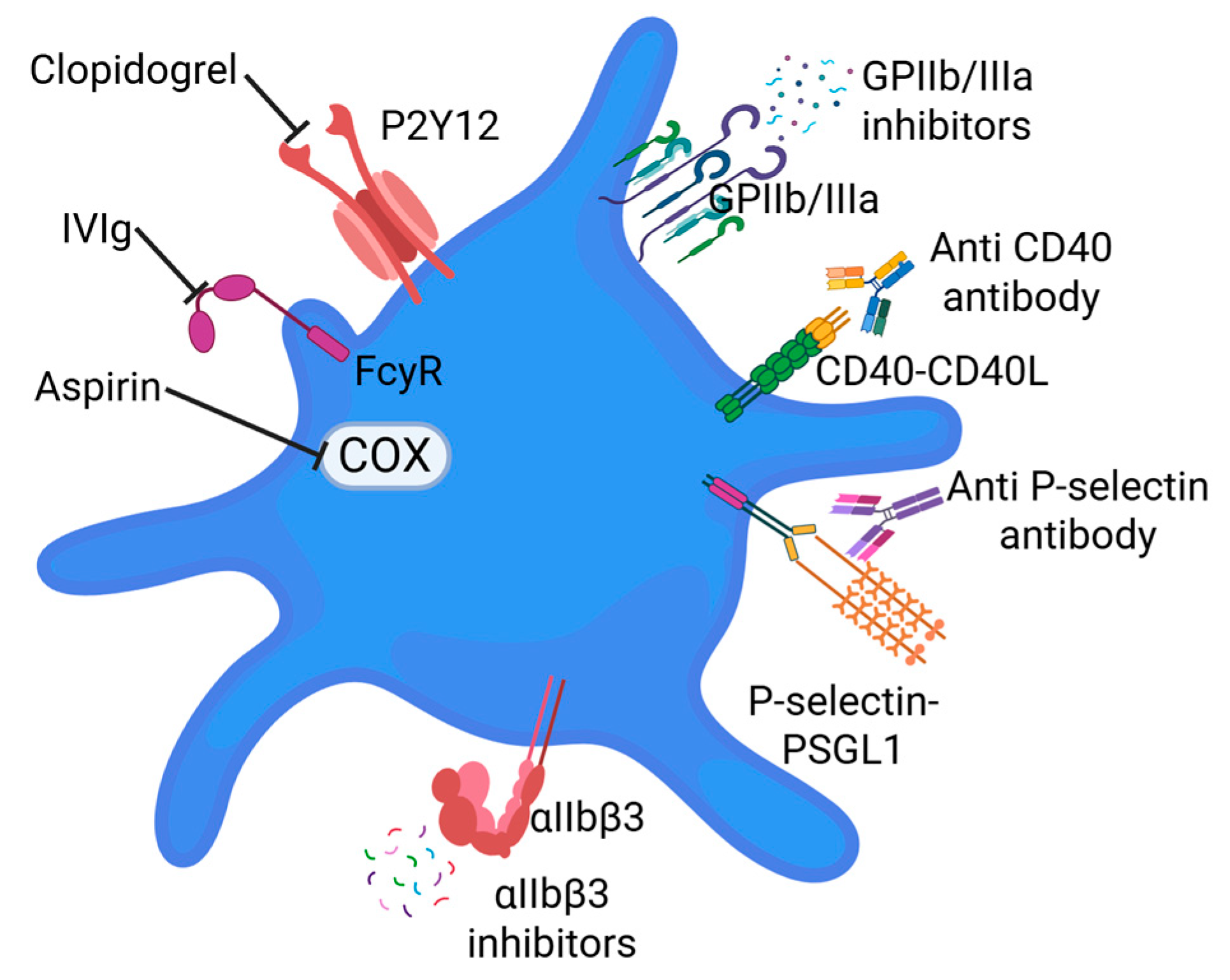
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strukel, S.; Teshome, B.; Rai, V. The Multifaceted Role of Platelets in Atherosclerosis and Ischemic Disease: Pathogenesis, Inflammation, and Therapeutic Opportunities. Life 2025, 15, 1656. https://doi.org/10.3390/life15111656
Strukel S, Teshome B, Rai V. The Multifaceted Role of Platelets in Atherosclerosis and Ischemic Disease: Pathogenesis, Inflammation, and Therapeutic Opportunities. Life. 2025; 15(11):1656. https://doi.org/10.3390/life15111656
Chicago/Turabian StyleStrukel, Sophia, Betelhem Teshome, and Vikrant Rai. 2025. "The Multifaceted Role of Platelets in Atherosclerosis and Ischemic Disease: Pathogenesis, Inflammation, and Therapeutic Opportunities" Life 15, no. 11: 1656. https://doi.org/10.3390/life15111656
APA StyleStrukel, S., Teshome, B., & Rai, V. (2025). The Multifaceted Role of Platelets in Atherosclerosis and Ischemic Disease: Pathogenesis, Inflammation, and Therapeutic Opportunities. Life, 15(11), 1656. https://doi.org/10.3390/life15111656






