Physicochemical, Functional, and Antioxidant Properties of Pectic Polysaccharides Extracted from Three Bast Fibrous Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Pectic Polysaccharides
2.3. Determination of Physicochemical Properties
2.3.1. Chemical Composition Analysis
2.3.2. Monosaccharide Composition Analysis
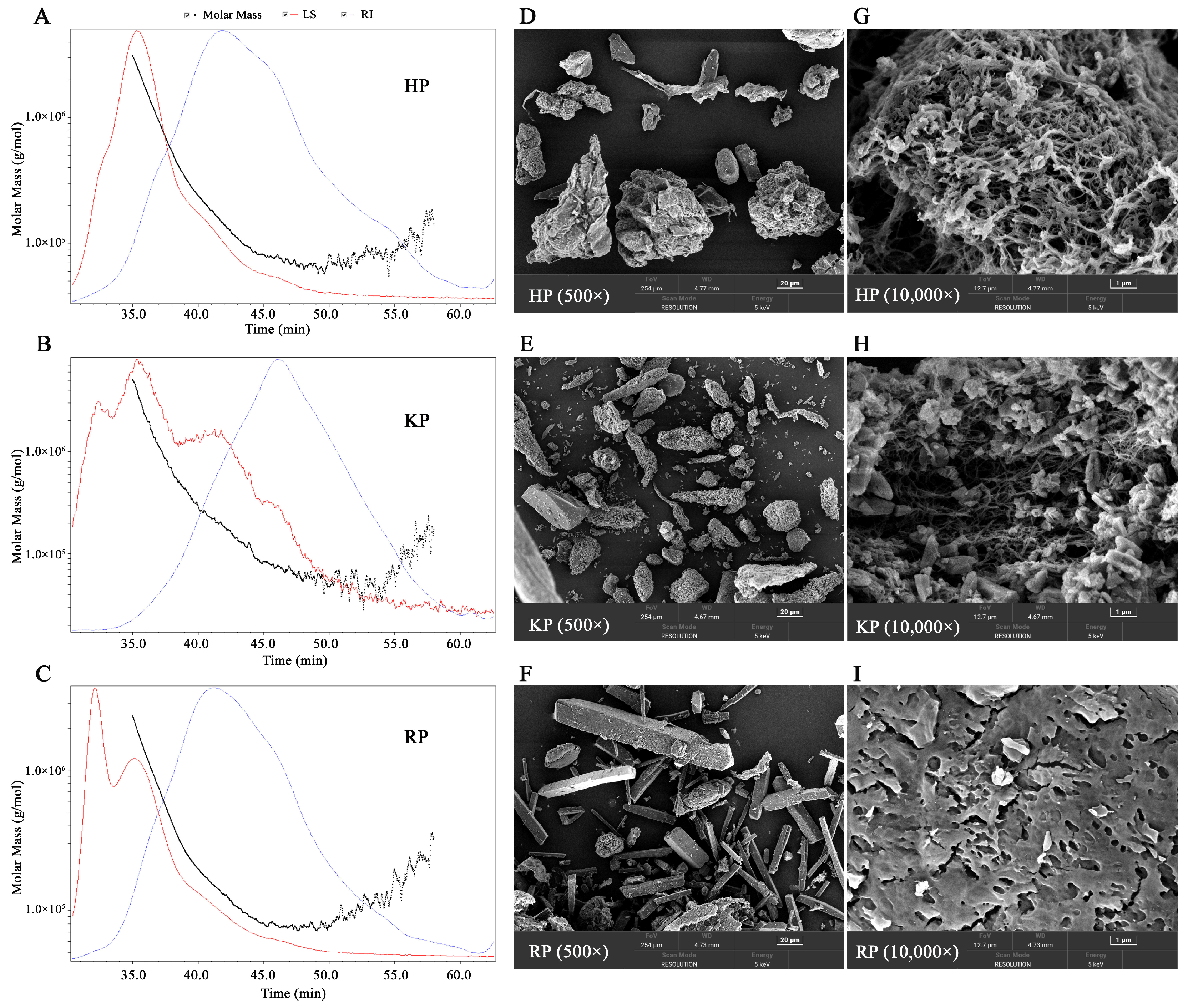
2.3.3. Molecular Weight Distribution
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Determination of Functional Properties
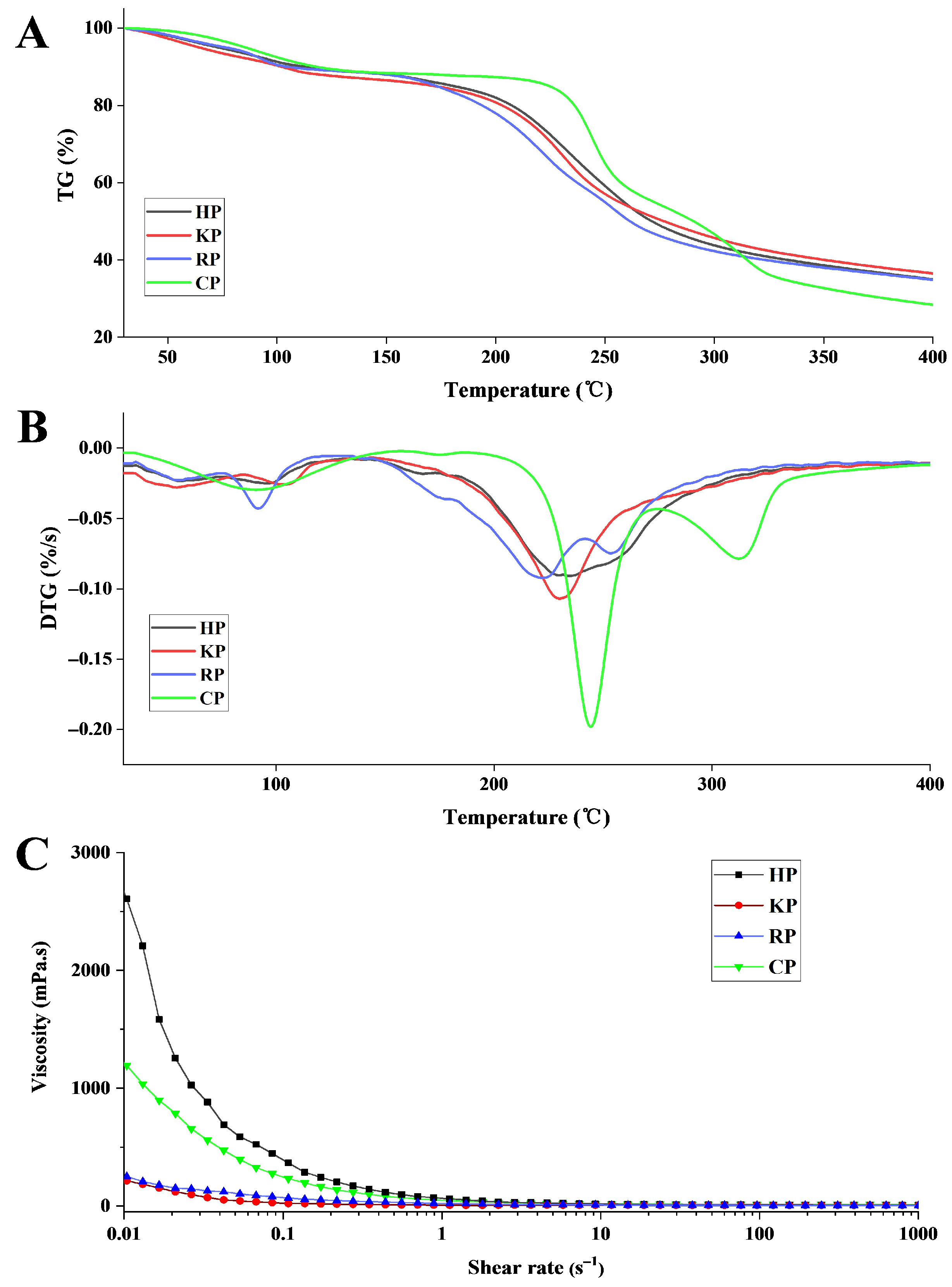
2.4.1. Thermal Analysis
2.4.2. Emulsifying Properties Evaluation
2.4.3. Rheological Behaviors
2.4.4. Adsorption Ability Determination
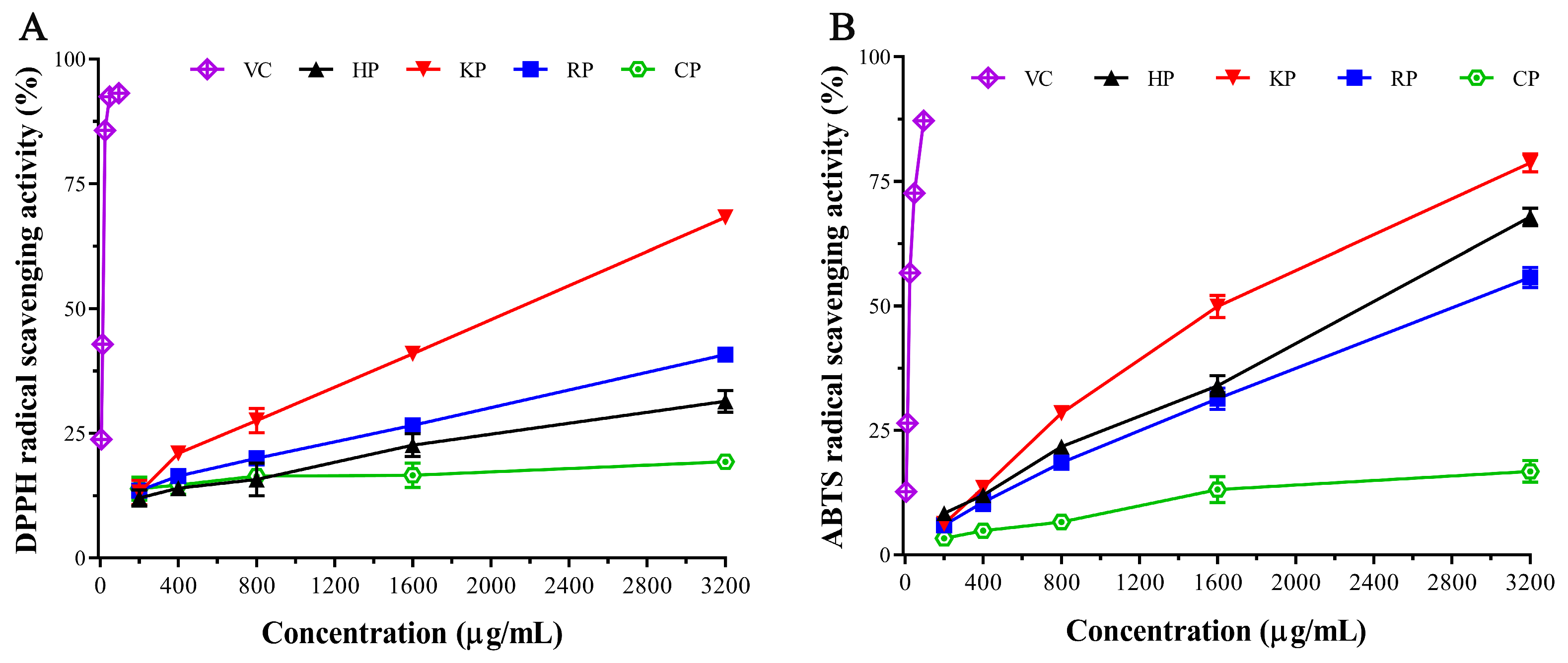
2.5. Measurement of Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
3.2. Physicochemical Properties
3.2.1. Chemical Compositions
3.2.2. Monosaccharide Composition
3.2.3. Molecular Weight Analysis
3.2.4. SEM Analysis
3.2.5. FT-IR Analysis
3.3. Functional Properties
3.3.1. Thermal Stabilities
3.3.2. Emulsifying Properties
3.3.3. Rheological Property
3.3.4. Adsorption Ability
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalita, P.; Rahman, M.T.; Haloi, P.; Bora, N.S.; Pachuau, L. Pectin in Gut Health and beyond: A Review. Int. J. Biol. Macromol. 2025, 322, 146954. [Google Scholar] [CrossRef]
- Barrera-Chamorro, L.; Fernandez-Prior, Á.; Rivero-Pino, F.; Montserrat-de La Paz, S. A Comprehensive Review on the Functionality and Biological Relevance of Pectin and the Use in the Food Industry. Carbohydr. Polym. 2025, 348, 122794. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Muflih, M.H.; Izzah, N.; Fadillah, U.; Ainani, A.F.; Dirpan, A. Pectin-Based Edible Films and Coatings: From Extraction to Application on Food Packaging towards Circular Economy—A Review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100680. [Google Scholar] [CrossRef]
- Dixit, S.S.; Muruganandam, L.; Ganesh Moorthy, I. Pectin from Fruit Peel: A Comprehensive Review on Various Extraction Approaches and Their Potential Applications in Pharmaceutical and Food Industries. Carbohydr. Polym. Technol. Appl. 2025, 9, 100708. [Google Scholar] [CrossRef]
- Peng, J.; Bu, Z.; Ren, H.; He, Q.; Yu, Y.; Xu, Y.; Wu, J.; Cheng, L.; Li, L. Physicochemical, Structural, and Functional Properties of Wampee (Clausena lansium (Lour.) Skeels) Fruit Peel Pectin Extracted with Different Organic Acids. Food Chem. 2022, 386, 132834. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, C.; Khalifa, I.; Zhu, Y.; Wang, Z.; Chen, H.; Liang, X.; Zhang, H.; Hu, L.; Yang, W. Pectin: A Review with Recent Advances in the Emerging Revolution and Multiscale Evaluation Approaches of Its Emulsifying Characteristics. Food Hydrocoll. 2024, 157, 110428. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Williams, P.A. Pectins from Food Waste: Characterization and Functional Properties of a Pectin Extracted from Broccoli Stalk. Food Hydrocoll. 2020, 107, 105930. [Google Scholar] [CrossRef]
- Wang, Z.; Song, W.; Song, H.; Huang, W.; Li, Y.; Feng, J. Effects of Extraction Methods on the Physicochemical Properties and Functionalities of Pectic Polysaccharides from Burdock (Arctium lappa L.). Int. J. Biol. Macromol. 2024, 257, 128684. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural Characterisation of Pectin Obtained from Cacao Pod Husk. Comparison of Conventional and Subcritical Water Extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Zhao, Y.; Huang, J.; Zhu, D.; Wang, S.; Zhu, L.; Chen, L.; Xu, X.; Liu, H. Chemical Structure, Chain Conformation and Rheological Properties of Pectic Polysaccharides from Soy Hulls. Int. J. Biol. Macromol. 2020, 148, 41–48. [Google Scholar] [CrossRef]
- Kian, L.K.; Saba, N.; Jawaid, M.; Sultan, M.T.H. A Review on Processing Techniques of Bast Fibers Nanocellulose and Its Polylactic Acid (PLA) Nanocomposites. Int. J. Biol. Macromol. 2019, 121, 1314–1328. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin Polymers as Wall Materials for the Nano-Encapsulation of Bioactive Compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Fang, B.; Rao, J.; Chen, B. Ferreting out the Secrets of Industrial Hemp Protein as Emerging Functional Food Ingredients. Trends Food Sci. Technol. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Hibiscus cannabinus L. (Kenaf) Studies: Nutritional Composition, Phytochemistry, Pharmacology, and Potential Applications. Food Chem. 2021, 344, 128582. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT: Production/Yield Quantities of Hemp/Kenaf/Ramie in World. 2023. Available online: http://www.fao.org/faostat/en/#home (accessed on 5 September 2025).
- Lyu, P.; Zhang, Y.; Wang, X.; Hurren, C. Degumming Methods for Bast Fibers—A Mini Review. Ind. Crops Prod. 2021, 174, 114158. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Li, X.; Liu, G.; Zhao, J. Elucidating Structure of Pectin in Ramie Fiber to Customize Enzyme Cocktail for High-Efficiency Enzymatic Degumming. Carbohydr. Polym. 2023, 314, 120954. [Google Scholar] [CrossRef]
- Vignon, M.R.; Garcia-Jaldon, C. Structural Features of the Pectic Polysaccharides Isolated from Retted Hemp Bast Fibres. Carbohydr. Res. 1996, 296, 249–260. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Liu, Y.; Zhao, X. Simultaneous Degumming and Extraction of a Nature Gum from Raw Hemp. J. Nat. Fibers 2022, 19, 2943–2952. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Li, D.; Papatheohari, Y.; Siqi, H.; Scordia, D.; Testa, G. How Kenaf (Hibiscus cannabinus L.) Can Achieve High Yields in Europe and China. Ind. Crops Prod. 2015, 68, 131–140. [Google Scholar] [CrossRef]
- Mao, K.; Chen, H.; Qi, H.; Qiu, Z.; Zhang, L.; Zhou, J. Visual Degumming Process of Ramie Fiber Using a Microbial Consortium RAMCD407. Cellulose 2019, 26, 3513–3528. [Google Scholar] [CrossRef]
- Cheng, L.; Duan, S.; Feng, X.; Zheng, K.; Yang, Q.; Liu, Z.; Peng, Y. Optimization of Pectin Extraction from Ramie by Orthogonal Methodology and Its Partial Physicochemical Properties. Nanosci. Nanotechnol. Lett. 2019, 11, 1607–1611. [Google Scholar] [CrossRef]
- Tang, J.; Qin, X.; Repo-Carrasco-Valencia, R.; Yang, X.; Deng, Y.; Hou, C.; Yang, X. Physicochemical, Functional and Antioxidant Properties of Four Polysaccharides Sequentially Extracted from Jute (Corchorus olitorius L.) Leaves. Int. J. Biol. Macromol. 2025, 323, 147223. [Google Scholar] [CrossRef] [PubMed]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of Uronic Acids without Interference from Neutral Sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2018, 101, 1466–1472. [Google Scholar] [CrossRef]
- The National Academies Press. Food Chemicals Codex, 3rd ed.; The National Academies Press: Washington, DC, USA, 1981. [Google Scholar]
- Wang, C.; Yu, Y.-B.; Chen, T.-T.; Wang, Z.-W.; Yan, J.-K. Innovative Preparation, Physicochemical Characteristics and Functional Properties of Bioactive Polysaccharides from Fresh Okra (Abelmoschus esculentus (L.) Moench). Food Chem. 2020, 320, 126647. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin Extracted from Persimmon Peel: A Physicochemical Characterization and Emulsifying Properties Evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Zhang, S.; Waterhouse, G.I.N.; Cui, T.; Sun-Waterhouse, D.; Wu, P. Pectin Fractions Extracted Sequentially from Cerasus Humilis: Their Compositions, Structures, Functional Properties and Antioxidant Activities. Food Sci. Hum. Wellness 2023, 12, 564–574. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic Extraction of Pectin from Opuntia Ficus Indica Cladodes after Mucilage Removal: Optimization of Experimental Conditions and Evaluation of Chemical and Functional Properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Wu, Z.; Chen, J.; Zhou, F.; Zhao, G. Thickening Effects of Ca2+ on Apple High-Methoxyl Pectin: Dependences on Ca2+ Concentration and the Degree of Esterification. Food Hydrocoll. 2023, 139, 108441. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and Characterization of Pectin Extracted from Sour Orange Peel by Ultrasound Assisted Method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Ruiz-Torralba, A.; Méndez-Albiñana, P.; Guerra-Hernández, E.; García-Villanova, B.; Moreno, R.; Villamiel, M.; Montilla, A. Berry Fruits as Source of Pectin: Conventional and Non-Conventional Extraction Techniques. Int. J. Biol. Macromol. 2021, 186, 962–974. [Google Scholar] [CrossRef]
- Čopíková, J.; Taubner, T.; Tůma, J.; Synytsya, A.; Dušková, D.; Marounek, M. Cholesterol and Fat Lowering with Hydrophobic Polysaccharide Derivatives. Carbohydr. Polym. 2015, 116, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, D.; Geng, Y.; Man, J.; Gao, Y.; Hang, Y.; Zheng, H.; Zhang, M. Structure, Physicochemical Characterisation and Properties of Pectic Polysaccharide from Premma Puberula Pamp. Food Hydrocoll. 2022, 128, 107550. [Google Scholar] [CrossRef]
- Aritkhodzhaev, K.A.; Arifkhodzhaev, A.O.; Yusupov, A.M. Pectin from Green Kenaf Bast. Chem. Nat. Compd. 1995, 31, 159–162. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.E.; Hosseini, S.S. Optimization of Aqueous Pectin Extraction from Citrus medica Peel. Carbohydr. Polym. 2017, 178, 27–33. [Google Scholar] [CrossRef]
- Pakarinen, A.; Zhang, J.; Brock, T.; Maijala, P.; Viikari, L. Enzymatic Accessibility of Fiber Hemp Is Enhanced by Enzymatic or Chemical Removal of Pectin. Bioresour. Technol. 2012, 107, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, C.; Chen, L.; Abbasi, A.M.; Guo, X.; Liu, R.H. Comparative Study of Phenolic Profiles, Antioxidant and Antiproliferative Activities in Different Vegetative Parts of Ramie (Boehmeria nivea L.). Molecules 2019, 24, 1551. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.G.; Gan, C.Y. Optimized Extraction and Characterization of Ramie Leaf Polysaccharides Using Deep Eutectic Solvent and Microwave: Antioxidant, Metal Chelation, and UV Protection Properties. Int. J. Biol. Macromol. 2024, 282, 136927. [Google Scholar] [CrossRef]
- Pang, Y.; Peng, Z.; Ding, K. An In-Depth Review: Unraveling the Extraction, Structure, Bio-Functionalities, Target Molecules, and Applications of Pectic Polysaccharides. Carbohydr. Polym. 2024, 343, 122457. [Google Scholar] [CrossRef]
- Guo, H.; Du, Y.; Gao, H.; Liao, Y.; Liu, H.; Wu, D.; Gan, R.; Gao, H. Isolation, Purification, Degradation of Citrus Pectin and Correlation between Molecular Weight and Their Biological Properties. LWT 2024, 196, 115837. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Vendrell-Calatayud, M.; Méndez-Albiñana, P.; Moreno, R.; Cano, M.P.; Villamiel, M. Extraction Optimization and Structural Characterization of Pectin from Persimmon Fruit (Diospyros Kaki Thunb. Var. Rojo Brillante). Carbohydr. Polym. 2021, 272, 118411. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Jin, R.; Li, S.; Yi, J.; Huang, J. Extraction and Characterization of Pectin from Premna Microphylla Turcz Leaves. Int. J. Biol. Macromol. 2019, 131, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Gu, X.; Wang, F.; Ouyang, J.; Wang, J. Purification and Structural Characterization of an α-Glucosidase Inhibitory Polysaccharide from Apricot (Armeniaca sibirica L. Lam.) Pulp. Carbohydr. Polym. 2015, 121, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Reichembach, L.H.; Lúcia de Oliveira Petkowicz, C. Pectins from Alternative Sources and Uses beyond Sweets and Jellies: An Overview. Food Hydrocoll. 2021, 118, 106824. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Huang, B.; Zhuang, Y.; Hu, Y.; Fei, P. Pectin Modified with Phenolic Acids: Evaluation of Their Emulsification Properties, Antioxidation Activities, and Antibacterial Activities. Int. J. Biol. Macromol. 2021, 174, 485–493. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S. Physiochemical, Rheological and Emulsifying Properties of Low Methoxyl Pectin Prepared by High Hydrostatic Pressure-Assisted Enzymatic, Conventional Enzymatic, and Alkaline de-Esterification: A Comparison Study. Food Hydrocoll. 2019, 93, 146–155. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Saurabh, V.; Sasi, M.; Punia, S.; Potkule, J.; Maheshwari, C.; Changan, S.; Radha; Bhushan, B.; et al. Delineating the Inherent Functional Descriptors and Biofunctionalities of Pectic Polysaccharides. Carbohydr. Polym. 2021, 269, 118319. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-Methylated Pectin from Walnut Processing Wastes as a Potential Resource: Ultrasound Assisted Extraction and Physicochemical, Structural and Functional Analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef]
- Djaoud, K.; Muñoz-Almagro, N.; Benítez, V.; Martín-Cabrejas, M.Á.; Madani, K.; Boulekbache-Makhlouf, L.; Villamiel, M. New Valorization Approach of Algerian Dates (Phoenix dactylifera L.) by Ultrasound Pectin Extraction: Physicochemical, Techno-Functional, Antioxidant and Antidiabetic Properties. Int. J. Biol. Macromol. 2022, 212, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Halake, K.; Lee, J. Antioxidant and Ion-Induced Gelation Functions of Pectins Enabled by Polyphenol Conjugation. Int. J. Biol. Macromol. 2017, 101, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-C.; Chen, X.; Wu, J.-Y. Constituents Actually Responsible for the Antioxidant Activities of Crude Polysaccharides Isolated from Mushrooms. J. Funct. Foods 2014, 11, 548–556. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of Monosaccharide Composition and Proportion on the Bioactivity of Polysaccharides: A Review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef] [PubMed]

| Sample | Hemp | Kenaf | Ramie |
|---|---|---|---|
| Image of plant and bast |  |  |  |
| Scientific name | Cannabis sativa L. | Hibiscus cannabinus L. | Boehmeria nivea L. |
| Origin | Chuxiong Prefecture, Yunnan Province, China | Yiyang City, Hunan Province, China | Yiyang City, Hunan Province, China |
| Abbreviation of extracted polysaccharides | HP | KP | RP |
| HP | KP | RP | |
|---|---|---|---|
| Yield (%) | 11.74 ± 0.25 a | 8.68 ± 0.14 b | 7.70 ± 0.31 c |
| Uronic acid (%) | 61.14 ± 0.58 a | 50.49 ± 0.80 c | 54.63 ± 0.84 b |
| DE (%) | 19.51 ± 1.75 c | 24.43 ± 1.36 b | 34.26 ± 1.89 a |
| Protein (%) | 4.82 ± 0.06 b | 2.46 ± 0.02 c | 5.93 ± 0.07 a |
| TP (mg GAE/g) | 7.12 ± 0.05 c | 21.77 ± 0.44 a | 9.85 ± 0.10 b |
| HP | KP | RP | |
|---|---|---|---|
| Monosaccharides (%) | |||
| Fuc | 1.40 ± 0.01 a | 0.81 ± 0.02 c | 1.25 ± 0.03 b |
| Rha | 23.64 ± 0.06 b | 18.84 ± 0.14 c | 41.69 ± 0.28 a |
| Ara | 26.13 ± 0.17 b | 38.46 ± 0.03 a | 15.16 ± 0.15 c |
| Gal | 23.45 ± 0.11 b | 26.35 ± 0.21 a | 26.23 ± 0.18 a |
| Glc | 5.68 ± 0.02 a | 3.29 ± 0.03 c | 4.36 ± 0.01 b |
| Xyl | 4.16 ± 0.04 a | 2.17 ± 0.05 b | 2.33 ± 0.04 b |
| Man | 3.88 ± 0.01 a | 3.28 ± 0.01 b | 2.64 ± 0.02 c |
| GalA | 9.87 ± 0.08 a | 5.04 ± 0.05 b | 4.83 ± 0.03 b |
| GlcA | 1.79 ± 0.02 a | 1.76 ± 0.01 a | 1.51 ± 0.02 b |
| Molecular weight | |||
| Mw (kDa) | 232.25 ± 4.08 b | 147.10 ± 3.32 c | 242.16 ± 6.64 a |
| Mn (kDa) | 121.48 ± 2.63 a | 78.10 ± 1.52 c | 109.33 ± 1.78 b |
| Polydispersity index | 1.91 ± 0.06 b | 1.88 ± 0.07 b | 2.22 ± 0.05 a |
| Parameters | Concentration | HP | KP | RP | CP |
|---|---|---|---|---|---|
| EC (%) | 2% | 34.38 ± 0.56 c | 41.25 ± 1.76 a | 35.21 ± 1.24 b | 33.70 ± 0.71 c |
| 4% | 37.75 ± 1.11 c | 43.76 ± 1.87 a | 40.23 ± 0.93 b | 36.88 ± 0.88 d | |
| ES (%) | 2% | 62.50 ± 1.79 a | 63.67 ± 0.65 a | 57.69 ± 1.12 b | 55.56 ±1.31 c |
| 4% | 81.70 ± 1.49 b | 82.92 ± 1.35 a | 79.69 ± 2.21 c | 67.82 ± 1.63 d | |
| WRC (g/g) | / | 6.90 ± 0.11 b | 8.19 ± 0.10 a | 4.17 ± 0.07 c | 2.39 ± 0.10 d |
| OHC (g/g) | / | 7.54 ± 0.48 b | 10.27 ± 0.32 a | 5.39 ± 0.24 c | 1.36 ± 0.11 d |
| CBC (mg/g) | / | 9.06 ± 0.41 b | 12.17 ± 0.51 a | 7.14 ± 0.30 c | 3.04 ± 0.21 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Li, X.; Xu, D.; Liu, G.; Zhang, X.; Xiong, X.; Yang, X.; Qin, X.; Deng, Y.; Hou, C.; et al. Physicochemical, Functional, and Antioxidant Properties of Pectic Polysaccharides Extracted from Three Bast Fibrous Plants. Life 2025, 15, 1618. https://doi.org/10.3390/life15101618
Tang J, Li X, Xu D, Liu G, Zhang X, Xiong X, Yang X, Qin X, Deng Y, Hou C, et al. Physicochemical, Functional, and Antioxidant Properties of Pectic Polysaccharides Extracted from Three Bast Fibrous Plants. Life. 2025; 15(10):1618. https://doi.org/10.3390/life15101618
Chicago/Turabian StyleTang, Jialing, Xi Li, Da Xu, Genggui Liu, Xiaoqin Zhang, Xiaofei Xiong, Xiai Yang, Xiaoli Qin, Yanchun Deng, Chunsheng Hou, and et al. 2025. "Physicochemical, Functional, and Antioxidant Properties of Pectic Polysaccharides Extracted from Three Bast Fibrous Plants" Life 15, no. 10: 1618. https://doi.org/10.3390/life15101618
APA StyleTang, J., Li, X., Xu, D., Liu, G., Zhang, X., Xiong, X., Yang, X., Qin, X., Deng, Y., Hou, C., & Yang, X. (2025). Physicochemical, Functional, and Antioxidant Properties of Pectic Polysaccharides Extracted from Three Bast Fibrous Plants. Life, 15(10), 1618. https://doi.org/10.3390/life15101618









