Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes, and Trehalose with Transmission Electron Microscopy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
In Vivo Study Design
3. Results
3.1. Trimix® Structure and Initial Interaction
3.2. In Vivo Results: Patient Outcomes at 30 Days
3.3. Healthy Patients (Grade 0) (Figure 12)
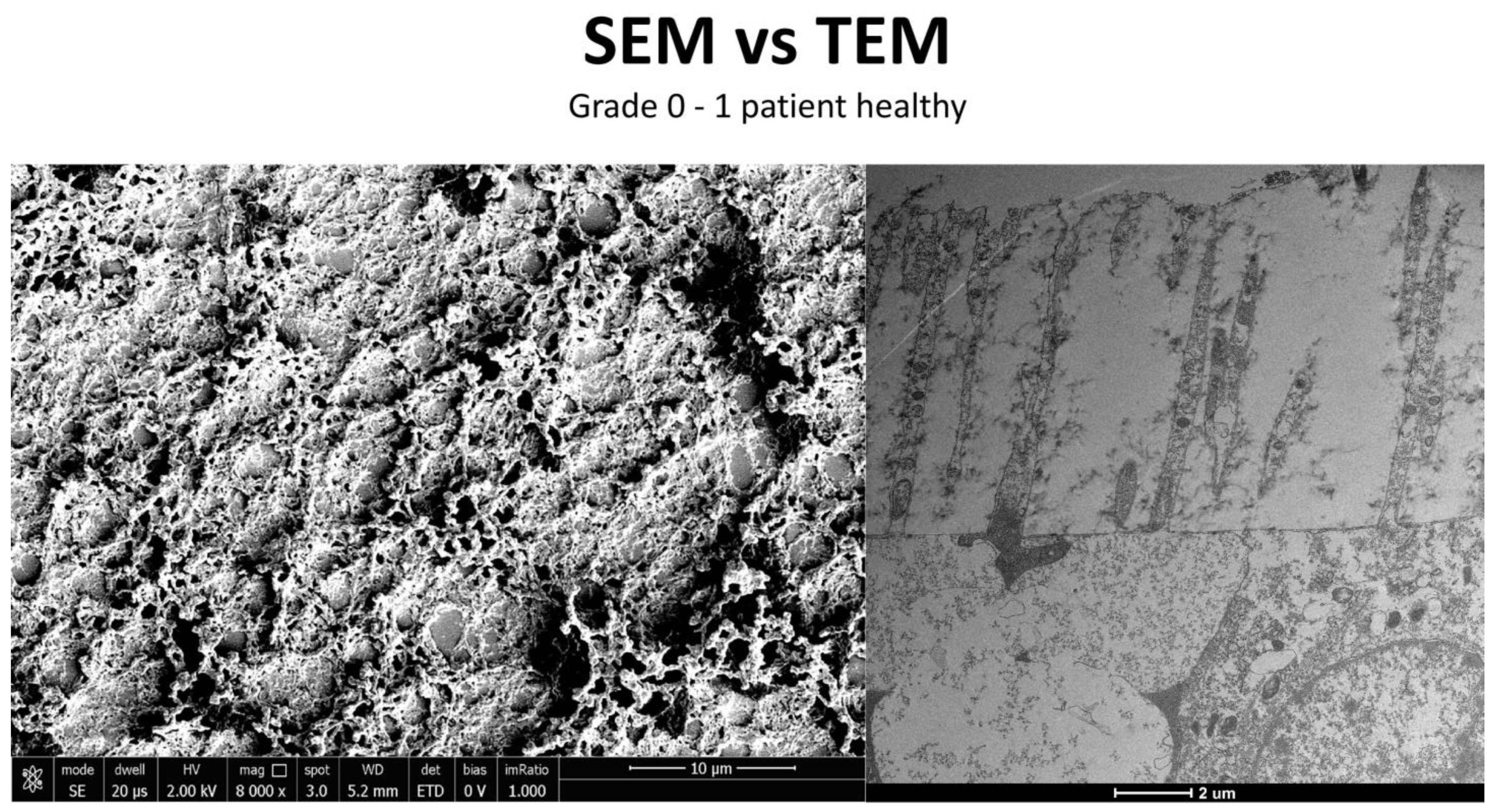

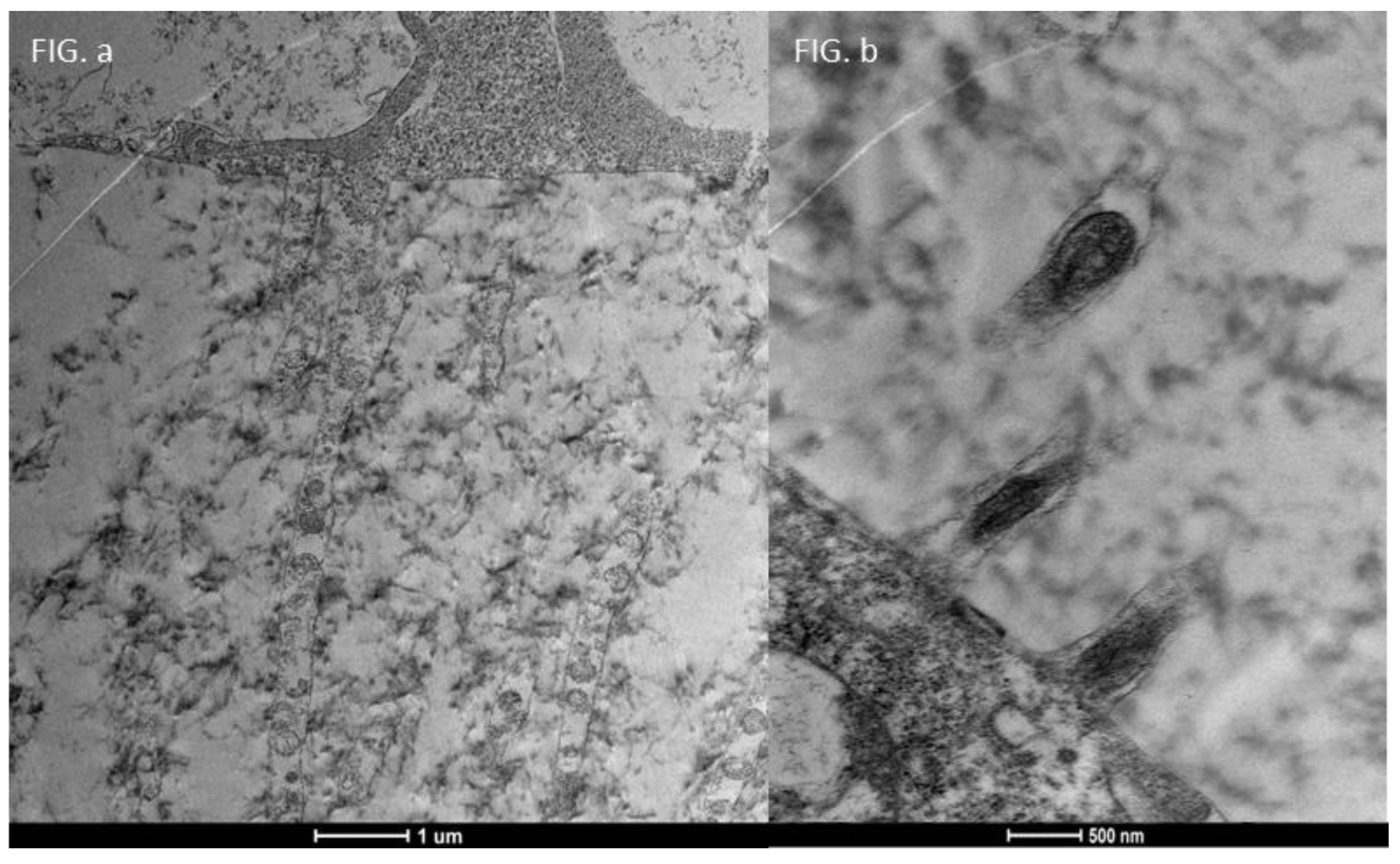
3.4. Moderate Inflammation Patients (Grade 1–2) (Figure 14)
- An increase in the number of microvilli.
- A restoration of the glycocalyx.
- Improved vesicular traffic.

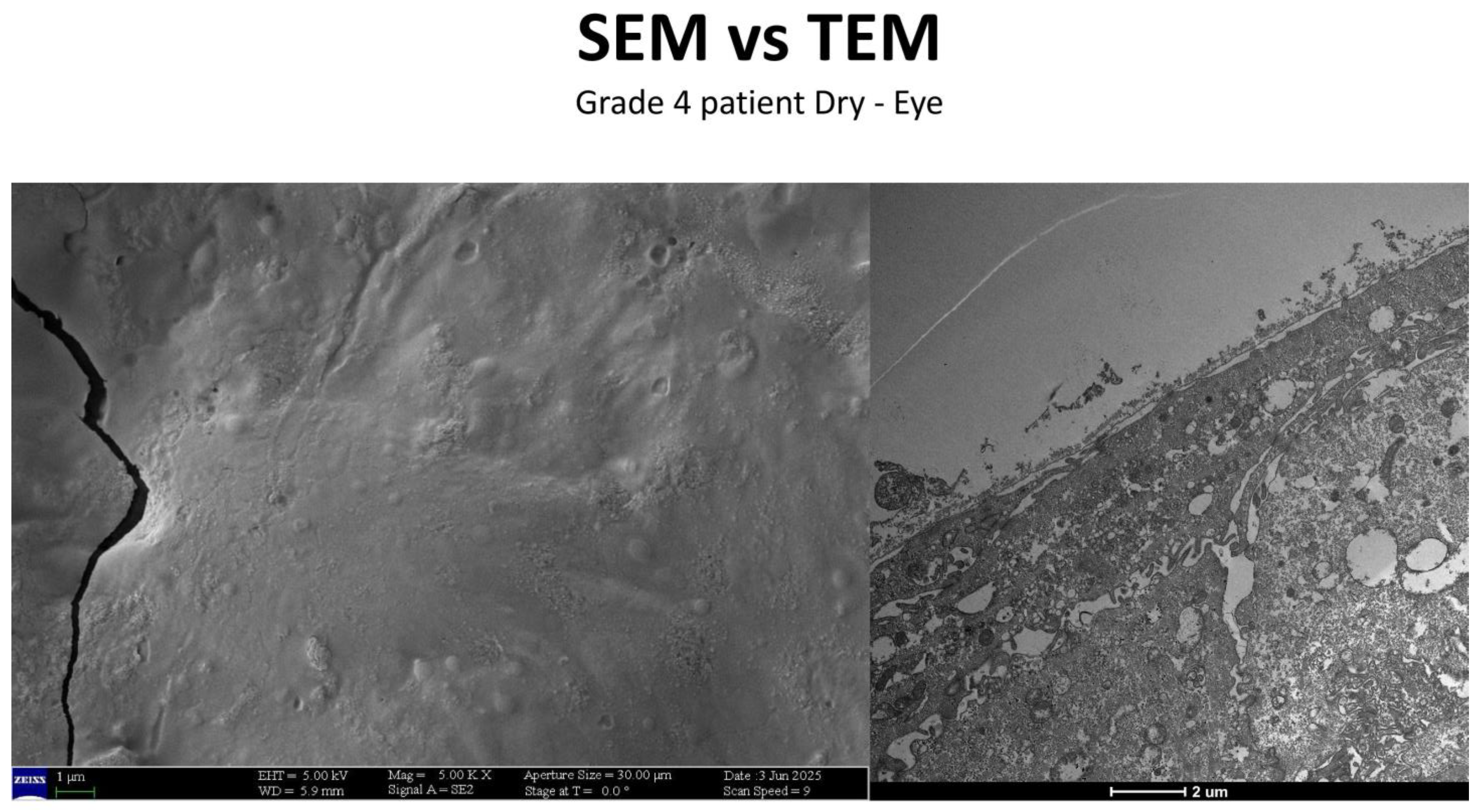
3.5. Severe Dry Eye Patients (Grade 3–4) (Figure 17)
- A slight increase in the number of microvilli.
- A presence of the glycocalyx.
- Observable vesicular transport.
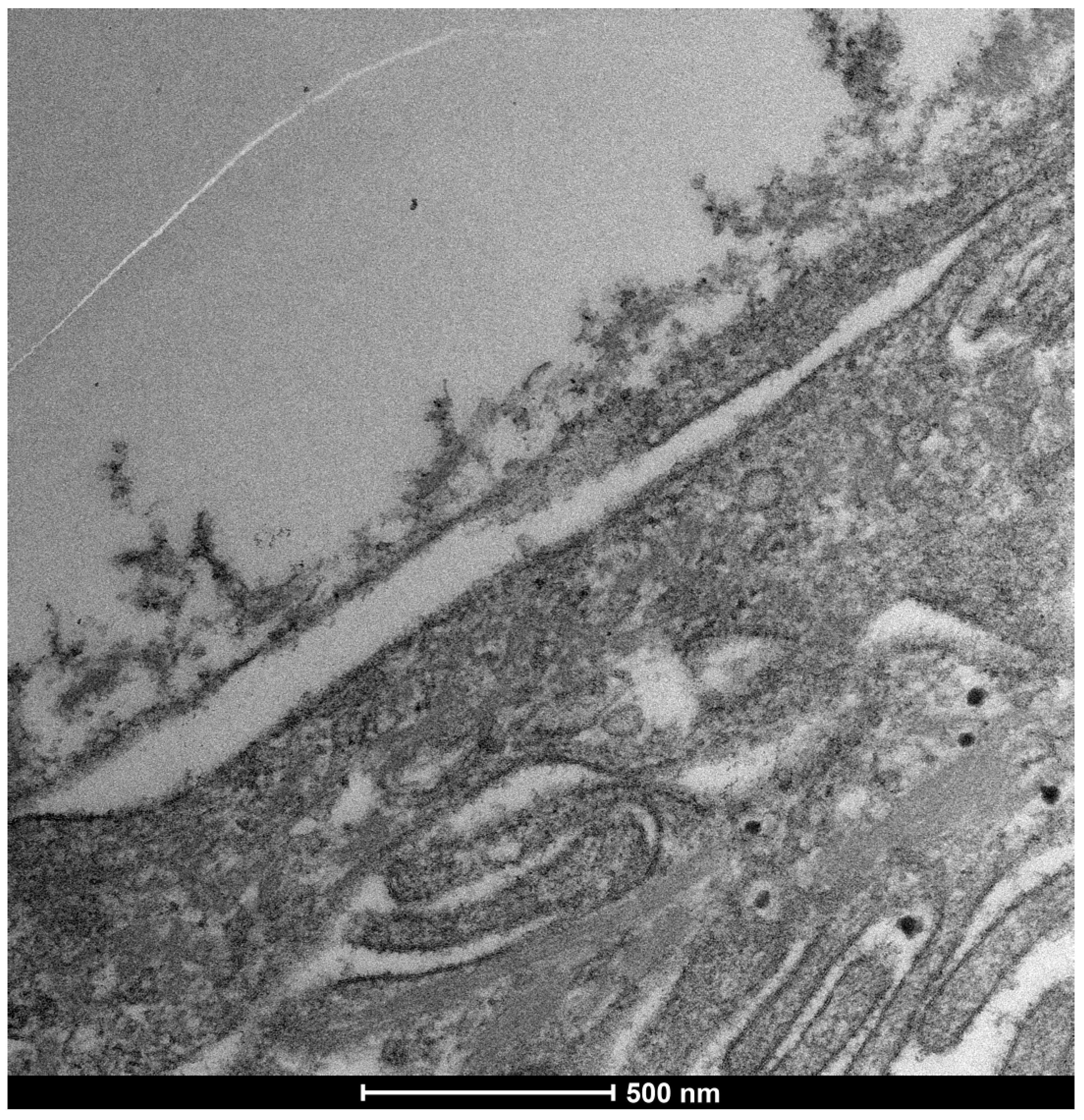
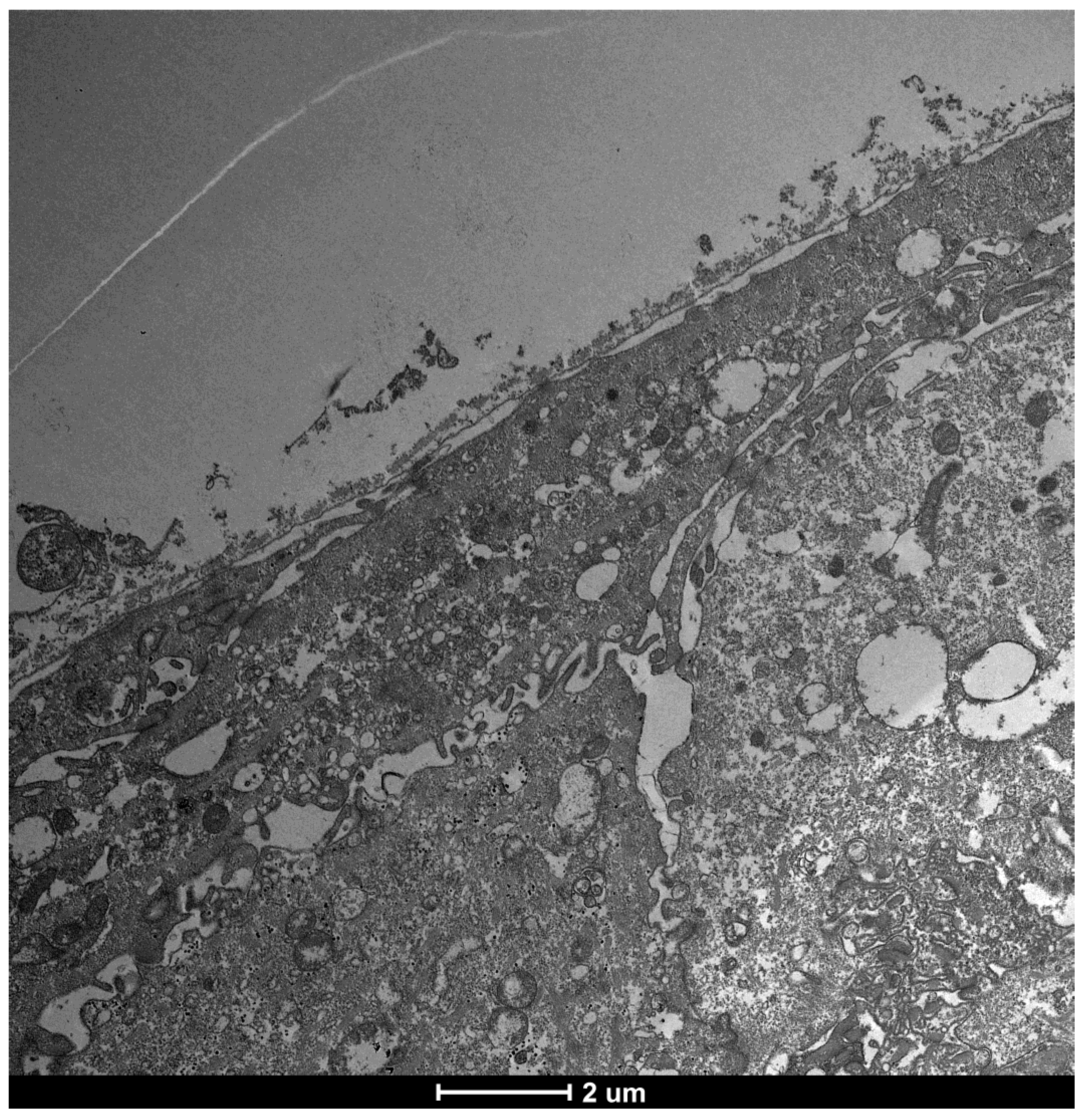
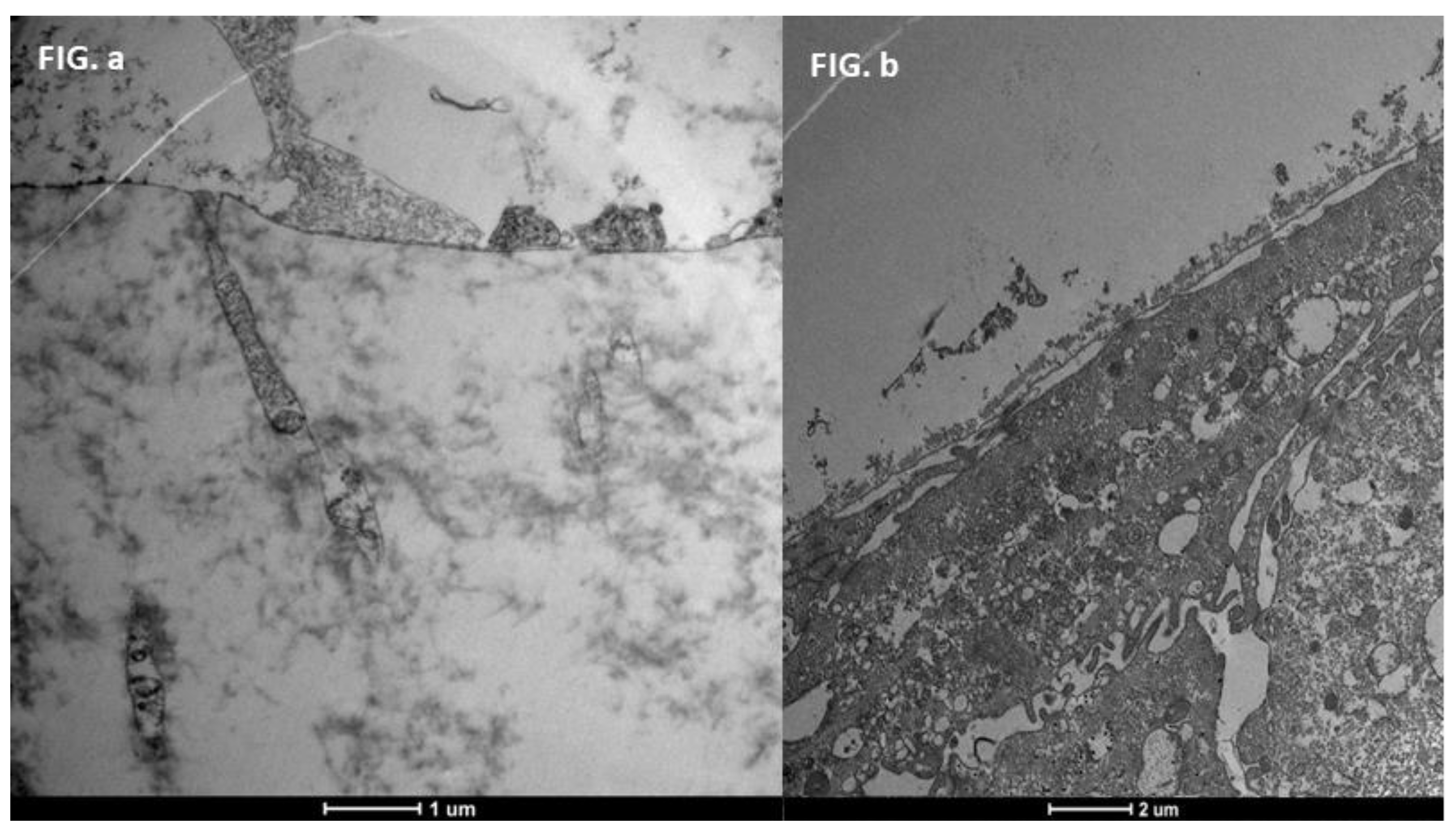
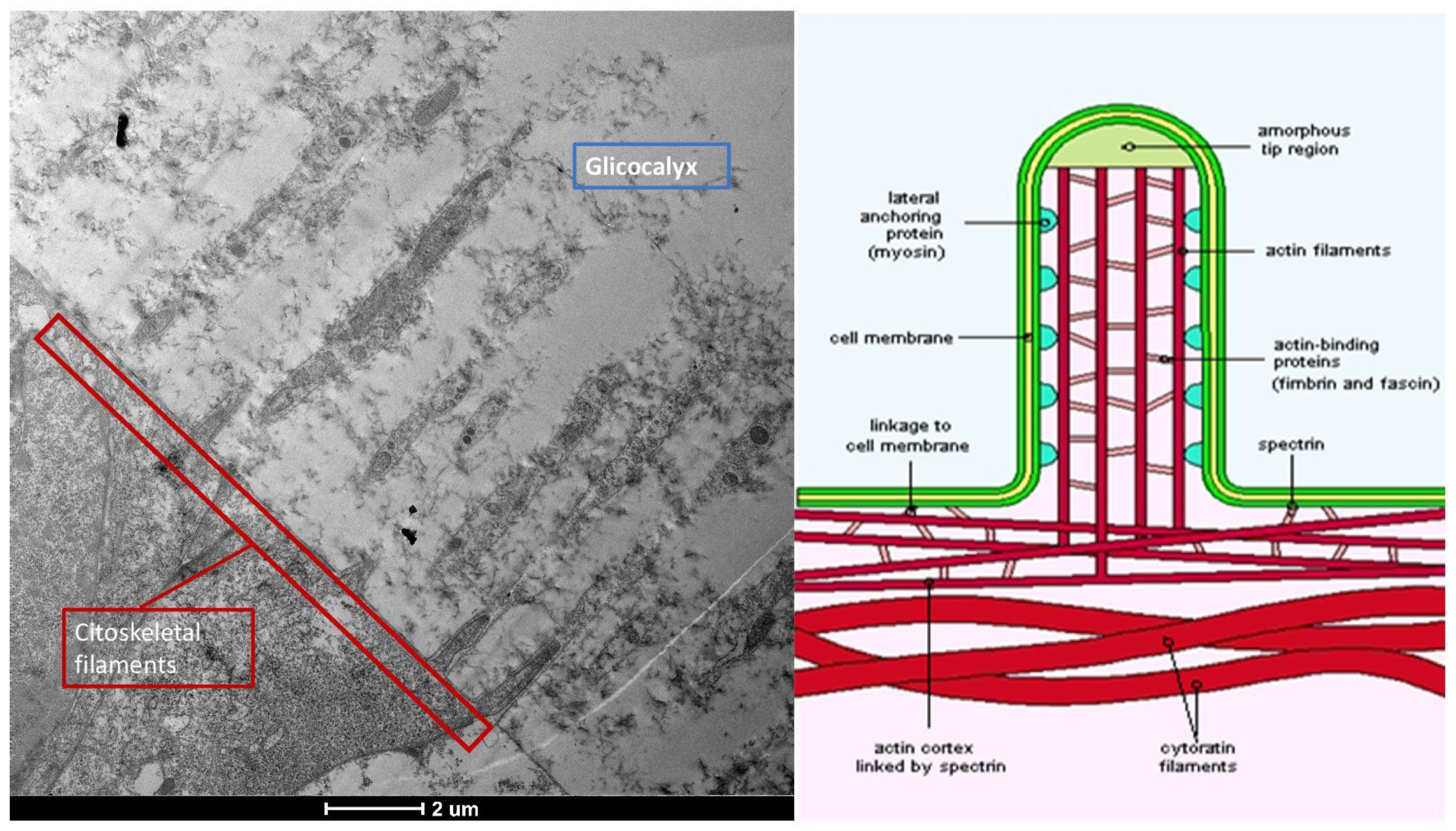
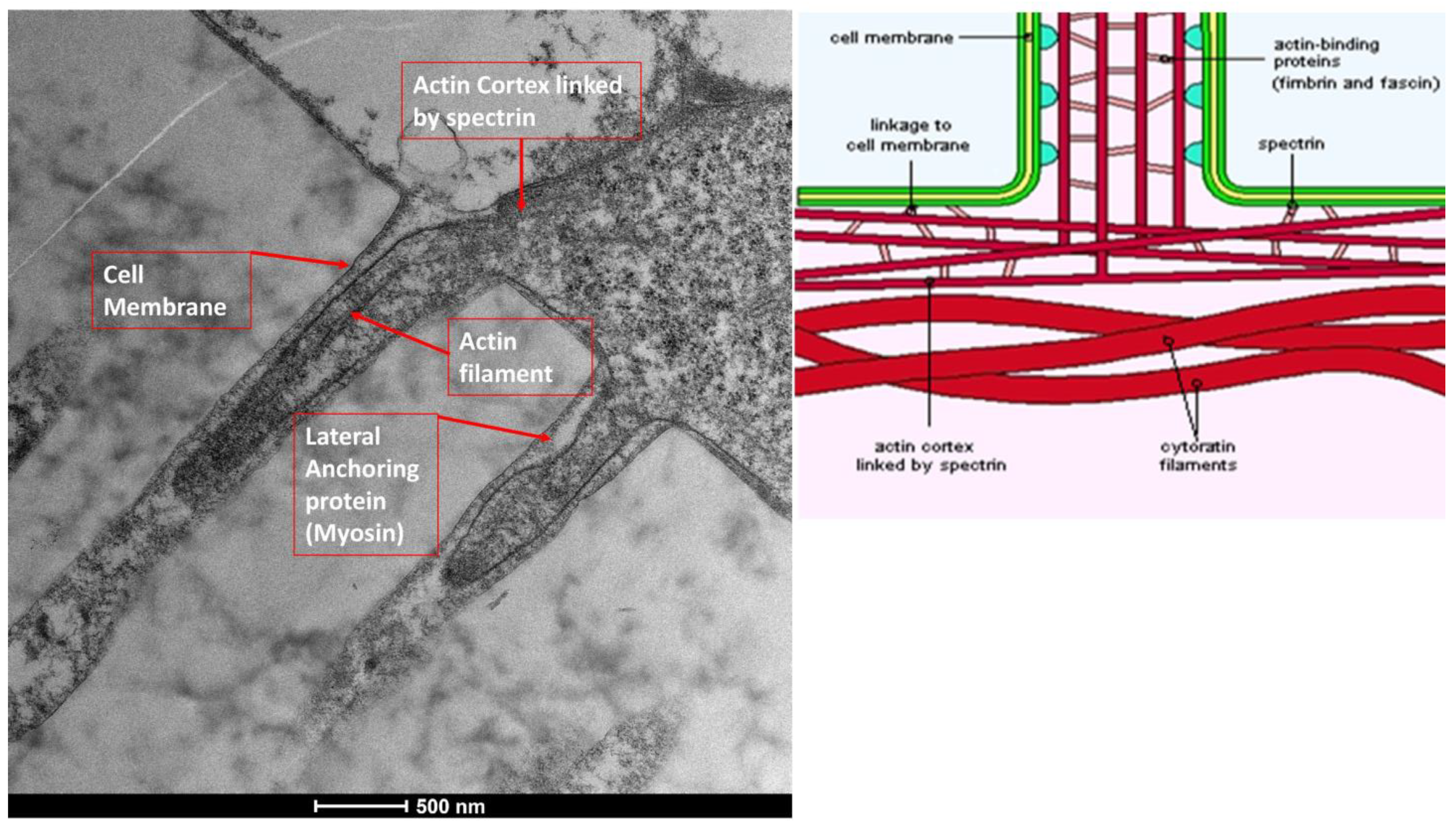
3.6. Glycocalyx Function and Structure
- Composition: Primarily composed of membrane mucins (O-glycosylated glycoproteins rich in serine, threonine, and proline) and glucidic portions of structural membrane molecules (Figure 1).
- Role in Health: In healthy cells, glycoprotein filaments of the glycocalyx contact the mucus layer in the tear film, ensuring corneo-conjunctival wettability and stable tear film formation.
- Role in Disease: In diseased cells, the impaired anchorage of mucus to the epithelium due to altered glycoprotein portions leads to a loss of wettability and prevents stable tear film formation.
4. Discussion
Mechanism of Action and Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DED | Dry-Eye Disease |
| GvHD | Chronic graft-versus-host disease |
| SPEED | Standard Patient Evaluation of Eye Dryness |
| SEM | Scanning Electron Microscopy |
| TMH | Tear meniscus height |
| NIBUT | Non-invasive tear film break-up time |
| LLT | Lipid layer thickness |
| TEM | Transmission Electron Microscopy |
| SMS | Second Mucosal System |
References
- Troisi, M.; Del Prete, S.; Troisi, S.; Marasco, D.; Rinaldi, M.; Costagliola, C. Scanning Electron Microscopy (SEM) Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes and Trehalose. Biomedicines 2024, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Koufakis, D.I.; Karabatsas, C.H.; Sakkas, L.I.; Alvanou, A.; Manthos, A.K.; Chatzoulis, D.Z. Conjunctival Surface Changes in Patients with Sjögren’s Syndrome: A Transmission Electron Microscopy Study. Investig. Opthalmol. Vis. Sci. 2006, 47, 541–544. [Google Scholar] [CrossRef]
- Tatematsu, Y.; Ogawa, Y.; Shimmura, S.; Dogru, M.; Yaguchi, S.; Nagai, T.; Yamazaki, K.; Kameyama, K.; Okamoto, S.; Kawakami, Y.; et al. Mucosal microvilli in dry eye patients with chronic GVHD. Bone Marrow Transplant. 2012, 47, 416–425. [Google Scholar] [CrossRef]
- Uchino, Y. The Ocular Surface Glycocalyx and its Alteration in Dry Eye Disease: A Review. Investig. Opthalmol. Vis. Sci. 2018, 59, DES157–DES162. [Google Scholar] [CrossRef]
- Rosati, P.; Colombo, R.; Maraldi, N. (Eds.) Istologia; Ermes: Edinburgh, Scotland, 2004; ISBN 9788870512946/8870512940. [Google Scholar]
- Hessen, M.; Akpek, E.K. Dry eye: An inflammatory ocular disease. J. Ophthalmic. Vis. Res. 2014, 9, 240–250. [Google Scholar] [PubMed Central]
- Cennamo, G.L.; Del Prete, A.; Forte, R.; Cafiero, G.; Del Prete, S.; Marasco, D. Impression cytology with scanning electron microscopy: A new method in the study of conjunctival microvilli. Eye 2008, 22, 138–143. [Google Scholar] [CrossRef]
- Grumetto, L.; Del Prete, A.; Ortosecco, G.; Barbato, F.; Del Prete, S.; Borrelli, A.; Schiattarella, A.; Mancini, R.; Mancini, A. Study on the protective Effect of a New Manganese Superoxide Desmutase on the Microvilli of Rabbit eyes exposed to UV radiation. BioMed Res. Int. 2015, 2015, 973197. [Google Scholar] [CrossRef]
- Argüeso, P.; Guzmán-Aránguez, B.L.; Azzam, D.V. Mucin expression and regulation in the ocular surface. Ocul. Surf. 2017, 15, 437–449. [Google Scholar]
- Contreras-Ruiz, L.; Masli, A.K. Immunomodulatory role of mucins in the ocular surface. Exp. Eye Res. 2015, 137, 105–112. [Google Scholar]
- Gipson, I.K. The ocular surface: The challenge of healing in a dynamic environment. Exp. Eye Res. 2004, 78, 395–403. [Google Scholar]
- Lemp, M.A.; Foulks, D.D.; Johnson, D.A.T. Management of the dry eye patient: Evidence-based recommendations. Curr. Opin. Ophthalmol. 2011, 22, 273–277. [Google Scholar]
- Mateo-Orobia, A.J.; Del Prado Sanz, E.; Blasco-Martínez, A.; Pablo-Júlvez, L.E.; Farrant, S.; Chiambaretta, F. Efficacy of artificial tears containing trehalose and hyaluronic acid for dry eye disease in women aged 42–54 versus ≥ 55 years. Cont. Lens Anterior Eye 2023, 46, 101845. [Google Scholar] [CrossRef]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef]
- Nagai, N.; Otake, H. Novel drug delivery systems for the management of dry eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as Vehicles for Topical Ophthalmic Drug Delivery and Ocular Surface Protection. Expert. Opin. Drug Deliv. 2021, 18, 819–847. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, R.; Liu, L.; Lu, Y.; Qi, J.; Wu, W. Liposomes Containing Bile Salts as Novel Ocular Delivery Systems for Tacrolimus (FK506): In Vitro Characterization and Improved Corneal Permeation. Int. J. Nanomed. 2013, 8, 1921–1933. [Google Scholar] [PubMed]
- Bonilha, V.L.; Finnemann, S.C.; Rodriguez-Boulan, E. Ezrin Promotes Morphogenesis of Apical Microvilli and Basal Infoldings in Retinal Pigment Epithelium. J. Cell Biol. 1999, 147, 1533–1548. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168. [Google Scholar] [CrossRef]
- Sánchez-González, J.M.; De-Hita-Cantalejo, C.; Sánchez-González, M.C. Crosslinked hyaluronic acid with liposomes and crocin for management symptoms of dry eye disease caused by moderate meibomian gland dysfunction. Int. J. Ophthalmol. 2020, 13, 1368–1373. [Google Scholar] [CrossRef]
- Fallacara, A.; Vertuani, S.; Panozzo, G.; Pecorelli, A.; Valacchi, G.; Manfredini, S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules 2017, 22, 2104. [Google Scholar] [CrossRef]
- Troisi, M.; Caruso, C.; D’Andrea, L.; Rinaldi, M.; Piscopo, R.; Troisi, S.; Costagliola, C. Compatibility of a New Ocular Surface Dye with Disposable and Bi-Weekly Soft Contact Lenses: An Experimental Study. Life 2024, 14, 653. [Google Scholar] [CrossRef]
- Meloni, M.; De Servi, B.; Marasco, D.; Del Prete, S. Molecular mechanism of ocular surface damage: Application to an in vitro dry eye model on human corneal epithelium. Mol. Vis. 2011, 17, 113–126. [Google Scholar] [PubMed]
- Liu, L. Development of a New Lubricant and Nutrient Tear Substitute. Ph.D. Thesis, Universität zu Lübeck, Lübeck, Germany, 2004. [Google Scholar]
- Aragona, P.; Ferreri, G.; Micali, A.; Puzzolo, D. Morphological changes of the conjunctival epithelium in contact lens wearers evaluated by impression cytology. Eye 1998, 12, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Yamada, M.; Hato, S.; Nishida, T. Fluorophotometric measurement of the precorneal residence time of topically applied hyaluronic acid. Br. J. Ophthalmol. 2008, 92, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Snibson, G.R.; Greaves, J.L.; Soper, N.D.; Tiffany, J.M.; Wilson, C.G.; Bron, A.J. Ocular surface residence times of artificial tear solutions. Cornea 1992, 11, 288–293. [Google Scholar] [CrossRef]
- Zhu, H.; Chauhan, A. Effect of viscosity on tear drainage and ocular residence time. Optom. Vis. Sci. 2008, 85, 715–725. [Google Scholar] [CrossRef]
- Casey-Power, S.; Ryan, R.; Behl, G.; McLoughlin, P.; Byrne, M.E.; Fitzhenry, L. Hyaluronic Acid: Its Versatile Use in Ocular Drug Delivery with a Specific Focus on Hyaluronic Acid-Based Polyelectrolyte Complexes. Pharmaceutics 2022, 14, 1479. [Google Scholar] [CrossRef]
- Guarise, C.; Acquasaliente, L.; Pasut, G.; Pavan, M.; Soato, M.; Garofolin, G.; Beninatto, R.; Giacomel, E.; Sartori, E.; Galesso, D. The role of high molecular weight hyaluronic acid in mucoadhesion on an ocular surface model. J. Mech. Behav. Biomed. Mater. 2023, 143, 105908. [Google Scholar] [CrossRef]
- Greaves, J.L.; Wilson, C.G.; Birmingham, A.T. Assessment of the precorneal residence of an ophthalmic ointment in healthy subjects. Br. J. Clin. Pharmacol. 1993, 35, 188–192. [Google Scholar]
- Troisi, M.; Del Prete, S.; Troisi, S.; Del Prete, A.; Bellucci, C.; Marasco, D.; Costagliola, C. The Role of Scanning Electron Microscopy in the Evaluation of Conjunctival Microvilli as an Early Biomarker of Ocular Surface Health: A Literature Review. J. Clin. Med. 2024, 13, 7569. [Google Scholar] [CrossRef]
- Hoeppener, S. Characterization of Drug Delivery Systems by Transmission Electron Microscopy. Handb. Exp. Pharmacol. 2024, 284, 191–209. [Google Scholar] [CrossRef]
- Gupta, P.; Rai, N.; Verma, A.; Gautam, V. Microscopy based methods for characterization, drug delivery, and understanding the dynamics of nanoparticles. Med. Res. Rev. 2024, 44, 138–168. [Google Scholar] [CrossRef] [PubMed]
- Troisi, M.; Troisi, S.; Strianese, D.; Rinaldi, M.; Turco, M.V.; Costagliola, C. Clinical and Biological Evaluation of NAAGA Versus Azelastine Eye Drops in Patients with Allergic Conjunctivitis and Tear Film Dysfunction: A Randomized Controlled Trial. Ophthalmol. Ther. 2025, 14, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Argüeso, P. Disrupted Glycocalyx as a Source of Ocular Surface Biomarkers. Eye Contact Lens. 2020, 46 (Suppl. S2), S53–S56. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, R.; Sharma, A. Ocular surface glycocalyx in health and disease. Front. Cell Dev. Biol. 2025, 13, 1561324. [Google Scholar] [CrossRef]
- Martinez-Carrasco, R.; Argüeso, P.; Fini, M.E. Membrane-associated mucins of the human ocular surface in health and disease. Ocul. Surf. 2021, 21, 313–330. [Google Scholar] [CrossRef]
- Vitiello, L.; Lixi, F.; Coco, G.; Giannaccare, G. Ocular Surface Side Effects of Novel Anticancer Drugs. Cancers 2024, 16, 344. [Google Scholar] [CrossRef]
- Labetoulle, M.; Benitez-del-Castillo, J.M.; Barabino, S.; Herrero Vanrell, R.; Daull, P.; Garrigue, J.-S.; Rolando, M. Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. Int. J. Mol. Sci. 2022, 23, 2434. [Google Scholar] [CrossRef]
- Labetoulle, M.; Garhöfer, G.; Ismail, D.; Garrigue, J.-S.; Amrane, M.; Guillon, M.; Aragona, P.; Baudouin, C. Review of clinical outcomes of a cationic emulsion tear substitute in patients with dry eye disease. Acta Ophthalmol. 2024, 102, 382–390. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Inferrera, L.; Spinella, R.; Postorino, E.I.; Gargano, R.; Oliverio, G.W.; Aragona, P. Clinical Efficacy, Tolerability and Safety of a New Multiple-Action Eyedrop in Subjects with Moderate to Severe Dry Eye. J. Clin. Med. 2022, 11, 6975. [Google Scholar] [CrossRef]
- Vigo, L.; Senni, C.; Pellegrini, M.; Vagge, A.; Ferro Desideri, L.; Carones, F.; Scorcia, V.; Giannaccare, G. Effects of a New Formulation of Multiple-Action Tear Substitute on Objective Ocular Surface Parameters and Ocular Discomfort Symptoms in Patients with Dry Eye Disease. Ophthalmol. Ther. 2022, 11, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Sánchez, A.; Tedesco, G.R.; Rocha-de-Lossada, C.; Russo, F.; Sánchez-González, J.M.; Borroni, D. Efficacy and safety of a multi-action tear substitute based on 0.15% cross-linked hyaluronic acid, 3% trehalose and liposomes with stearylamine: A randomized, single-mask, controlled study. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Troisi, M.; Del Prete, S.; Troisi, S.; Marasco, D.; Costagliola, C. Scanning Electron Microscopy of Conjunctival Scraping: Our Experience in the Diagnosis of Infectious Keratitis with Negative Culture Tests. Reports 2023, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Troisi, M.; Del Prete, S.; Troisi, S. Comment on Cabrera-Aguas, M.; Watson, S.L. Updates in Diagnostic Imaging for Infectious Keratitis: A Review. Diagnostics 2023, 13, 3358. [Google Scholar]
- Kwon, J.; Moghtader, A.; Kang, C.; Bibak Bejandi, Z.; Shahjahan, S.; Alzein, A.; Djalilian, A.R. Overview of Dry Eye Disease for Primary Care Physicians. Medicina 2025, 61, 460. [Google Scholar] [CrossRef]
- Aafreen, B.; Nandyala, S.; Balakrishnan, J.; Agarwal, T.; Dada, T.; Saxena, R.; Sharma, N. Preferred practice guidelines and narrative review on infectious keratitis in ocular surface diseases. Indian J. Ophthalmol. 2025, 73, 508–515. [Google Scholar] [CrossRef]
- Mateo-Orobia, A.J.; Farrant, S.; Del-Prado-Sanz, E.; Blasco-Martínez, A.; Idoipe-Corta, M.; Lafuente-Ojeda, N.; Pablo-Júlvez, L.E. A Preservative-Free Combination of Sodium Hyaluronate and Trehalose Improves Dry Eye Signs and Symptoms and Increases Patient Satisfaction in Real-Life Settings: The TEARS Study. Ophthalmol. Ther. 2024, 13, 3123–3134. [Google Scholar]
- Laihia, J.; Kaarniranta, K. Trehalose for Ocular Surface Health. Biomolecules 2020, 10, 809. [Google Scholar] [CrossRef]
- Hackshaw, A. Small studies: Strengths and limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef]
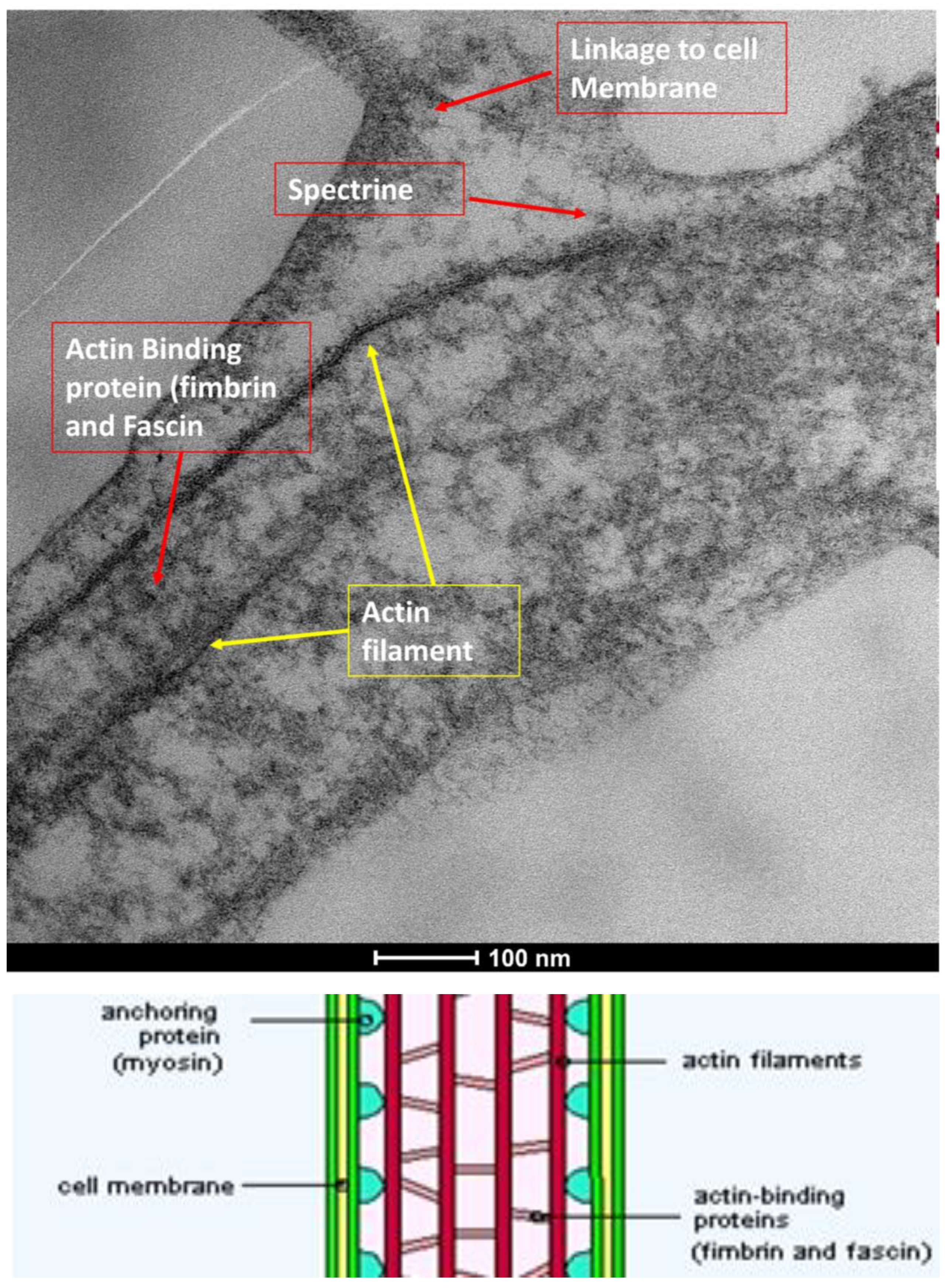
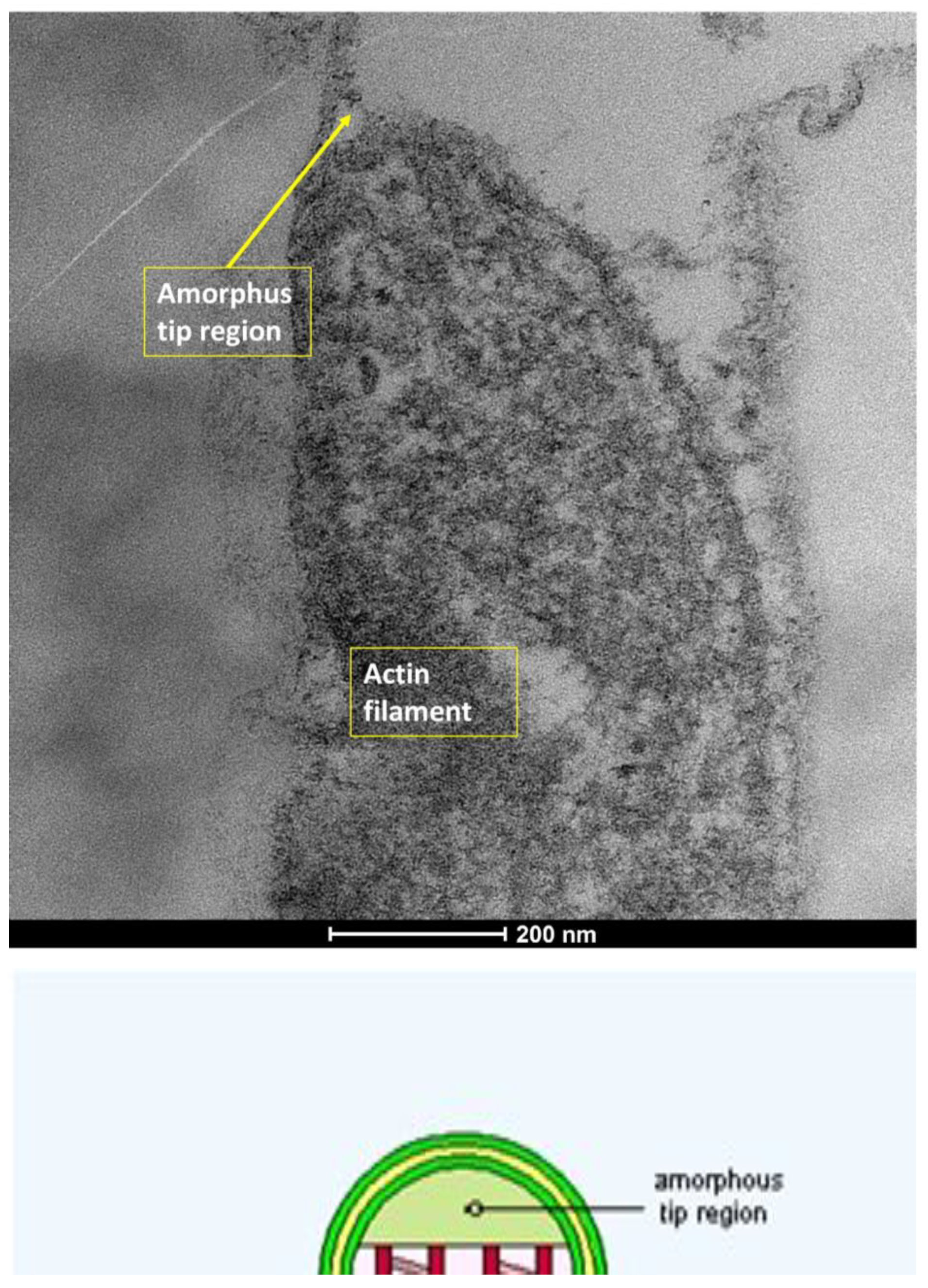
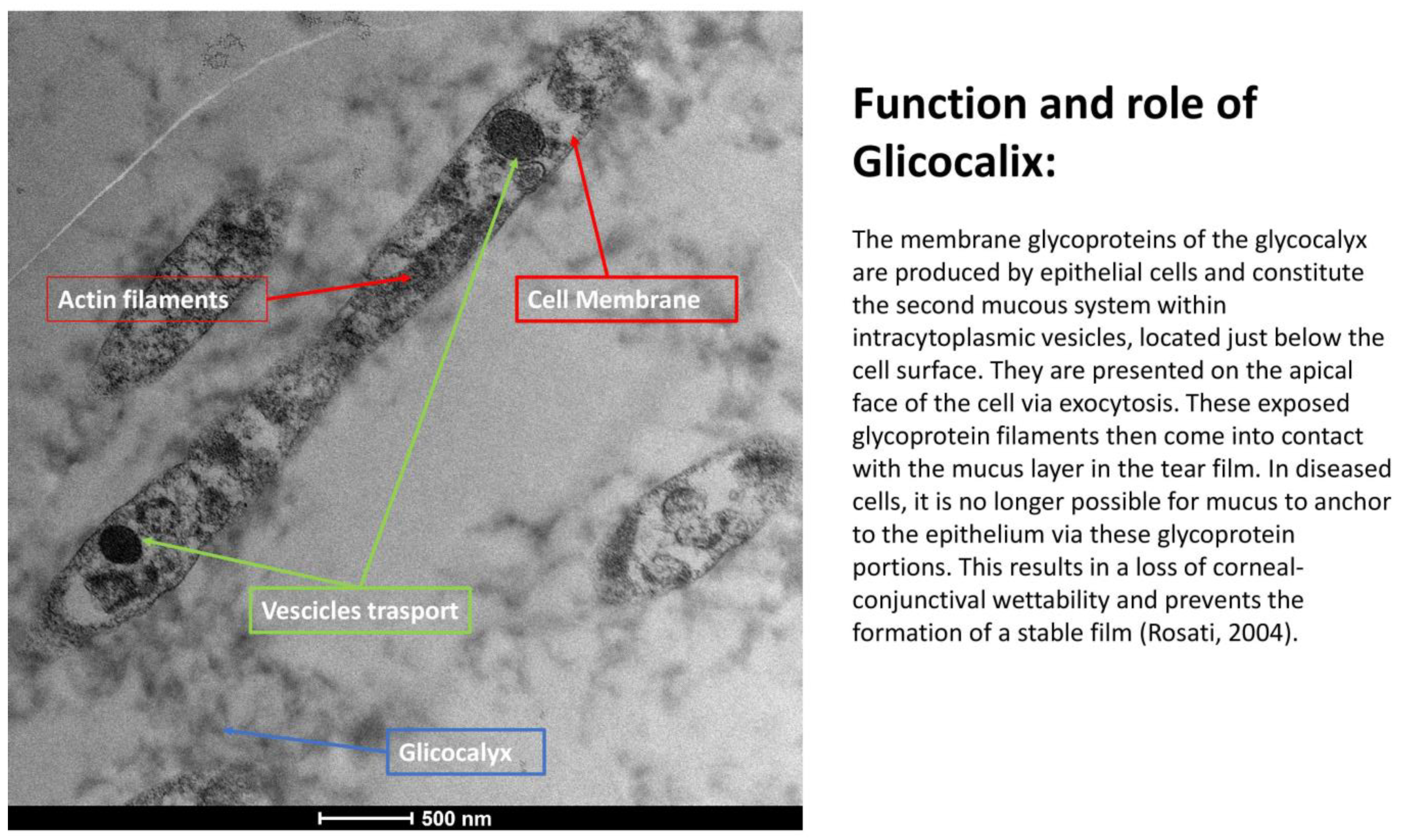
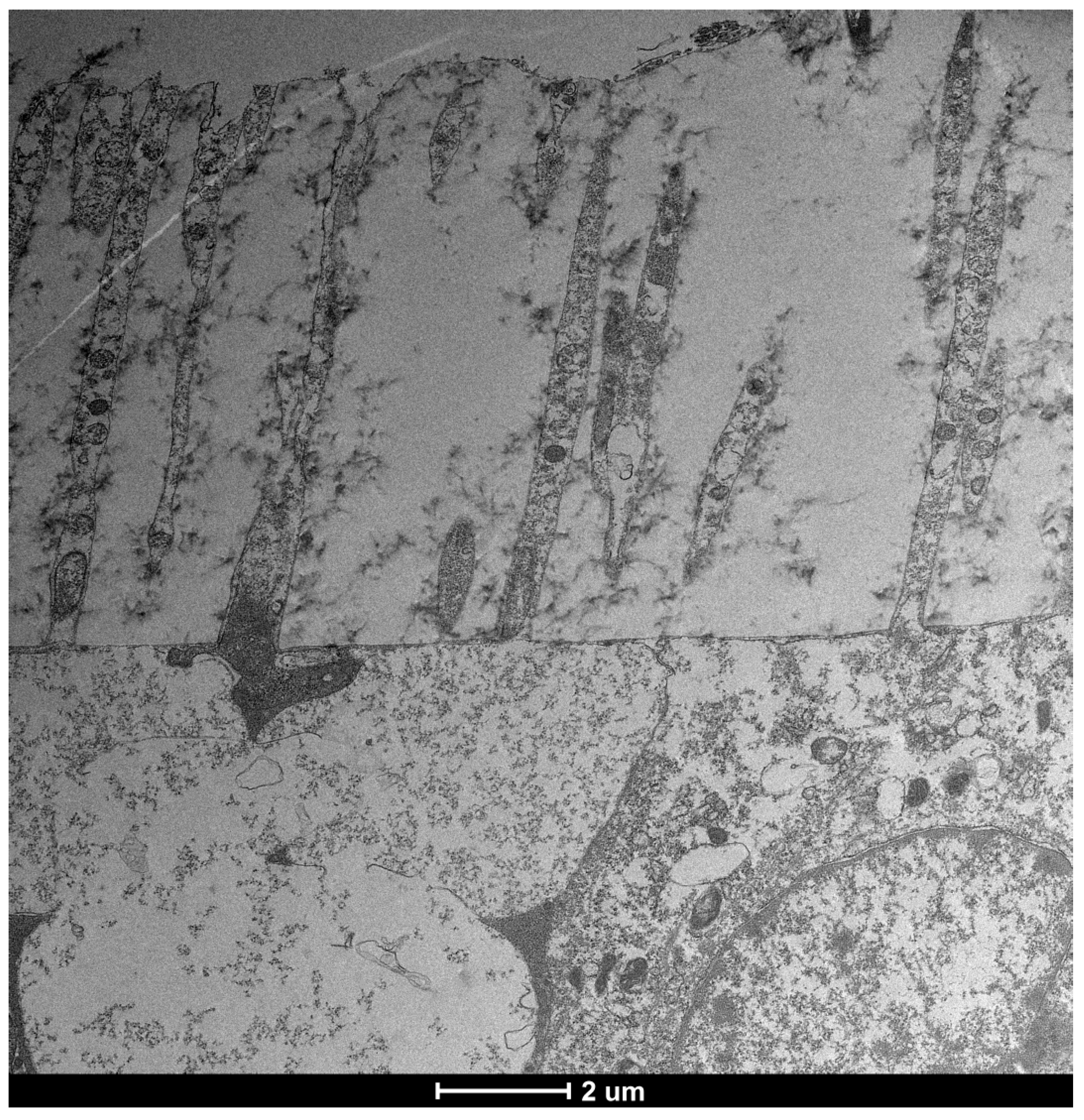
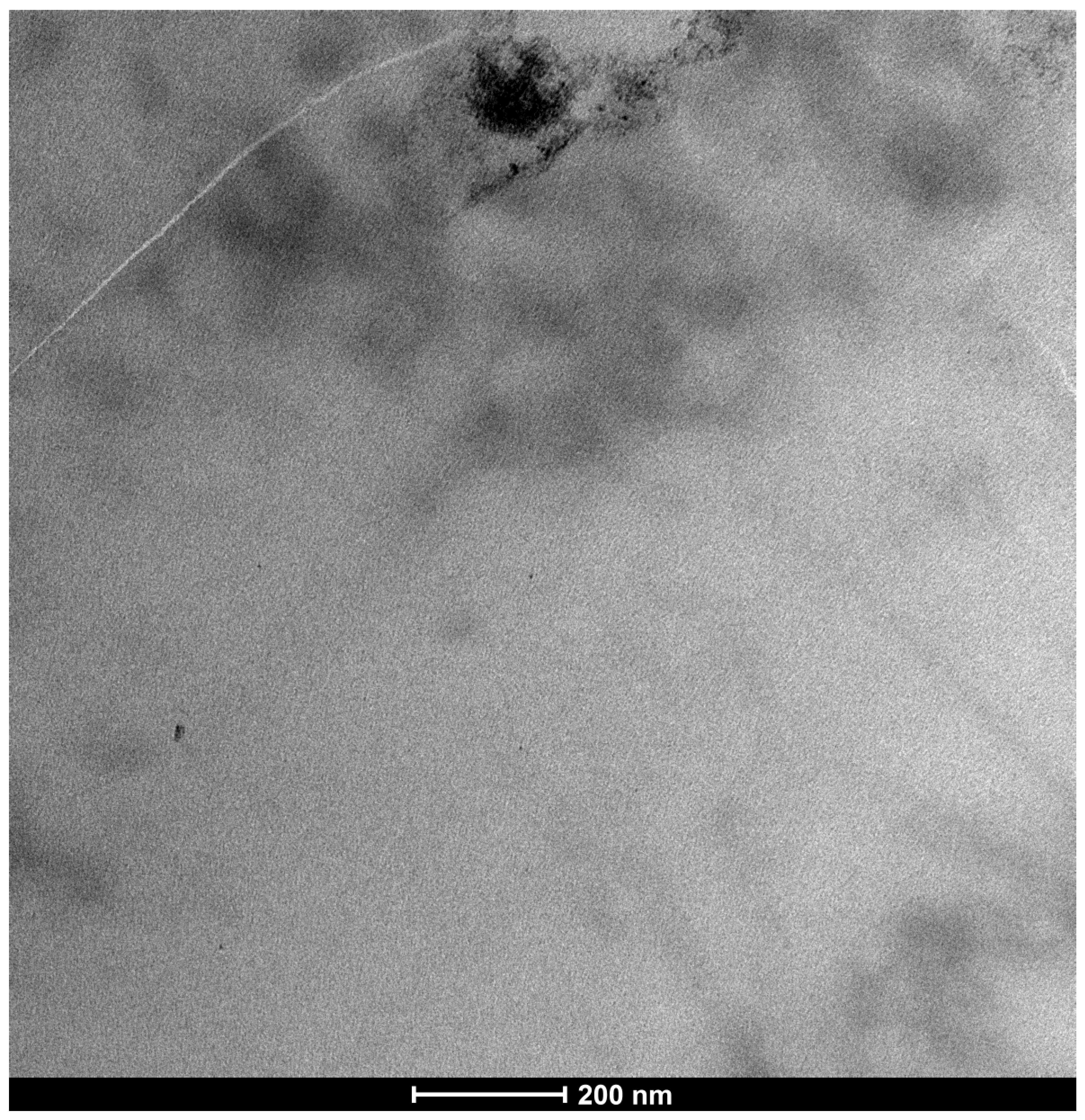
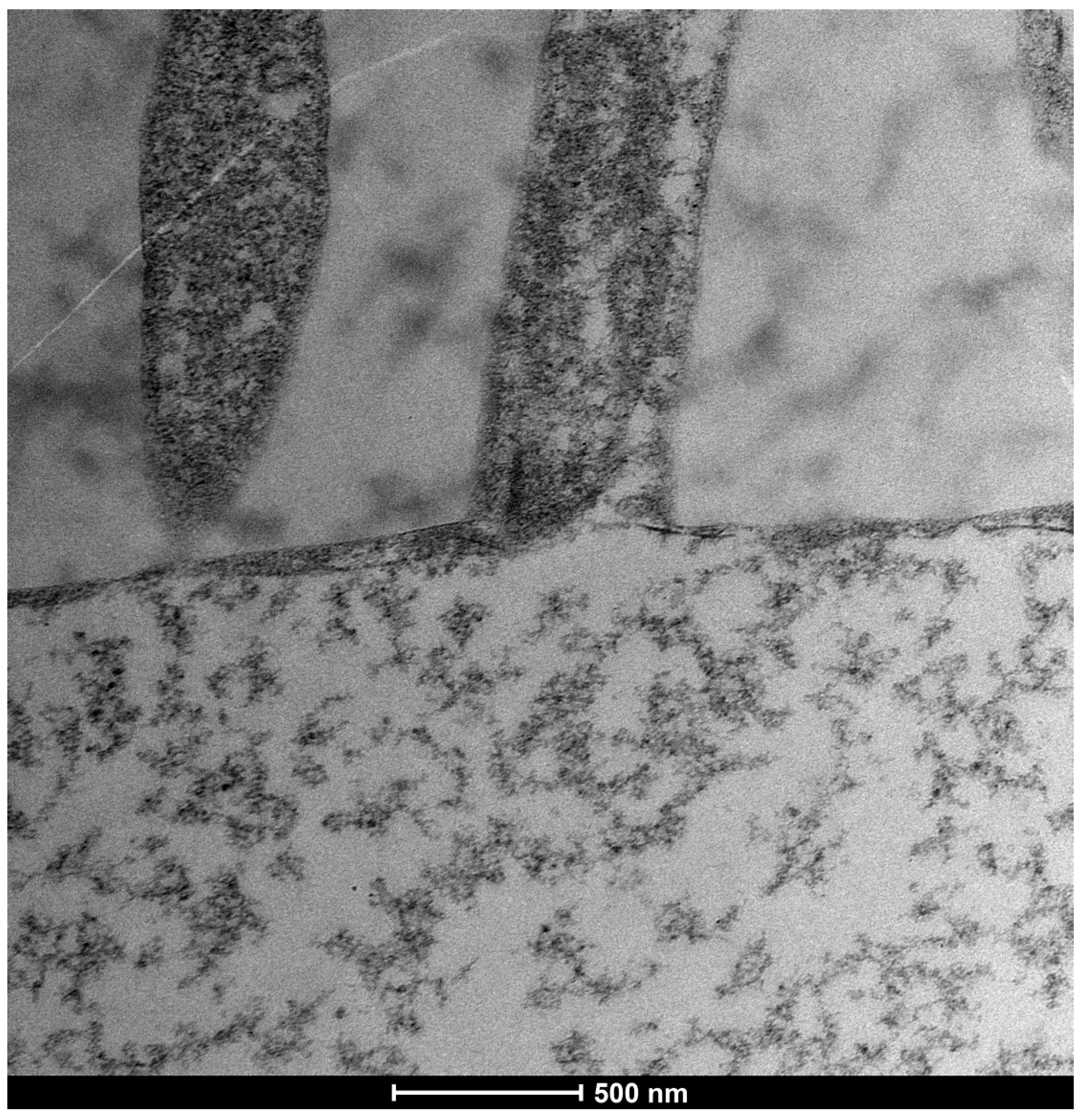

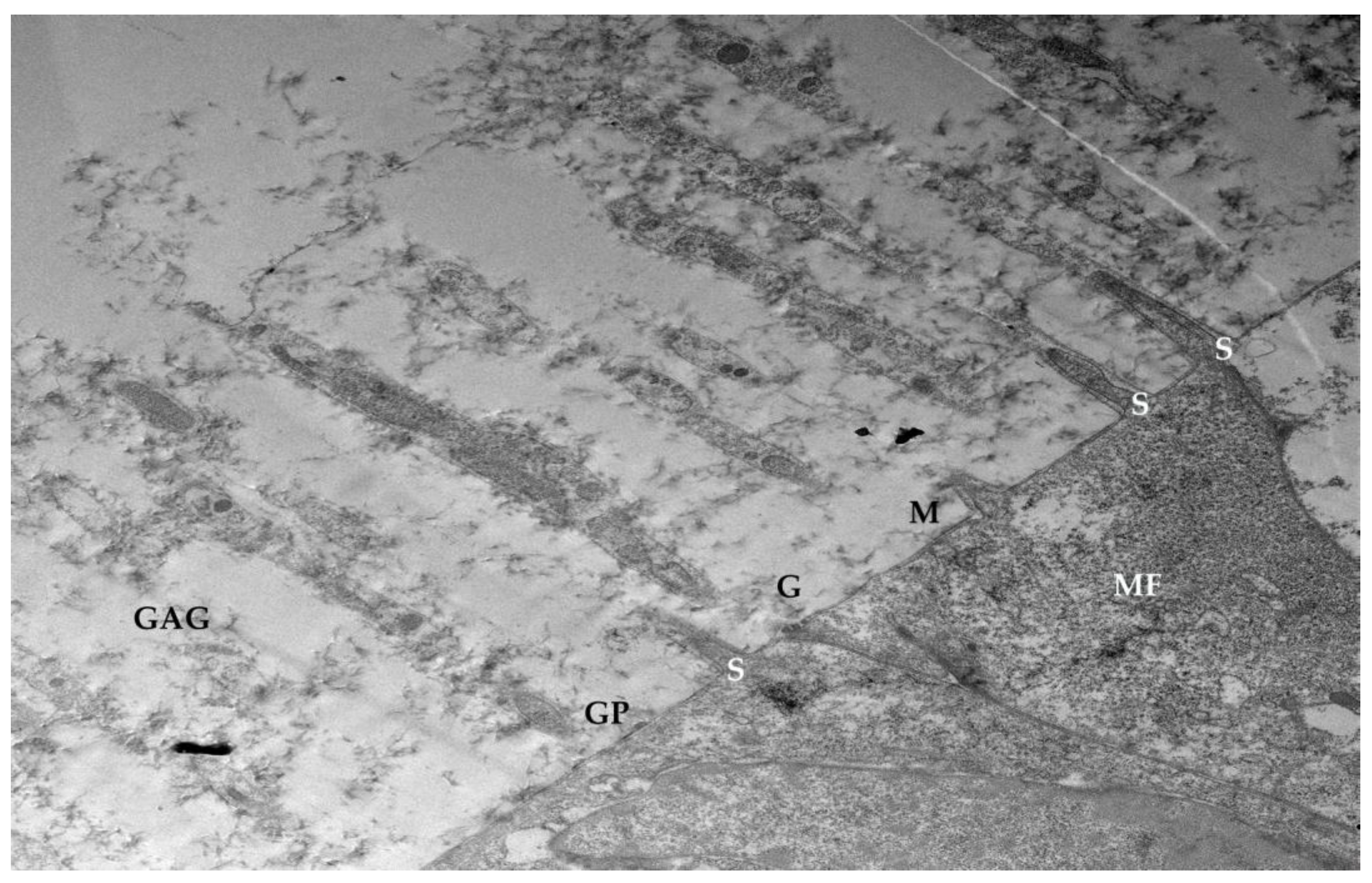
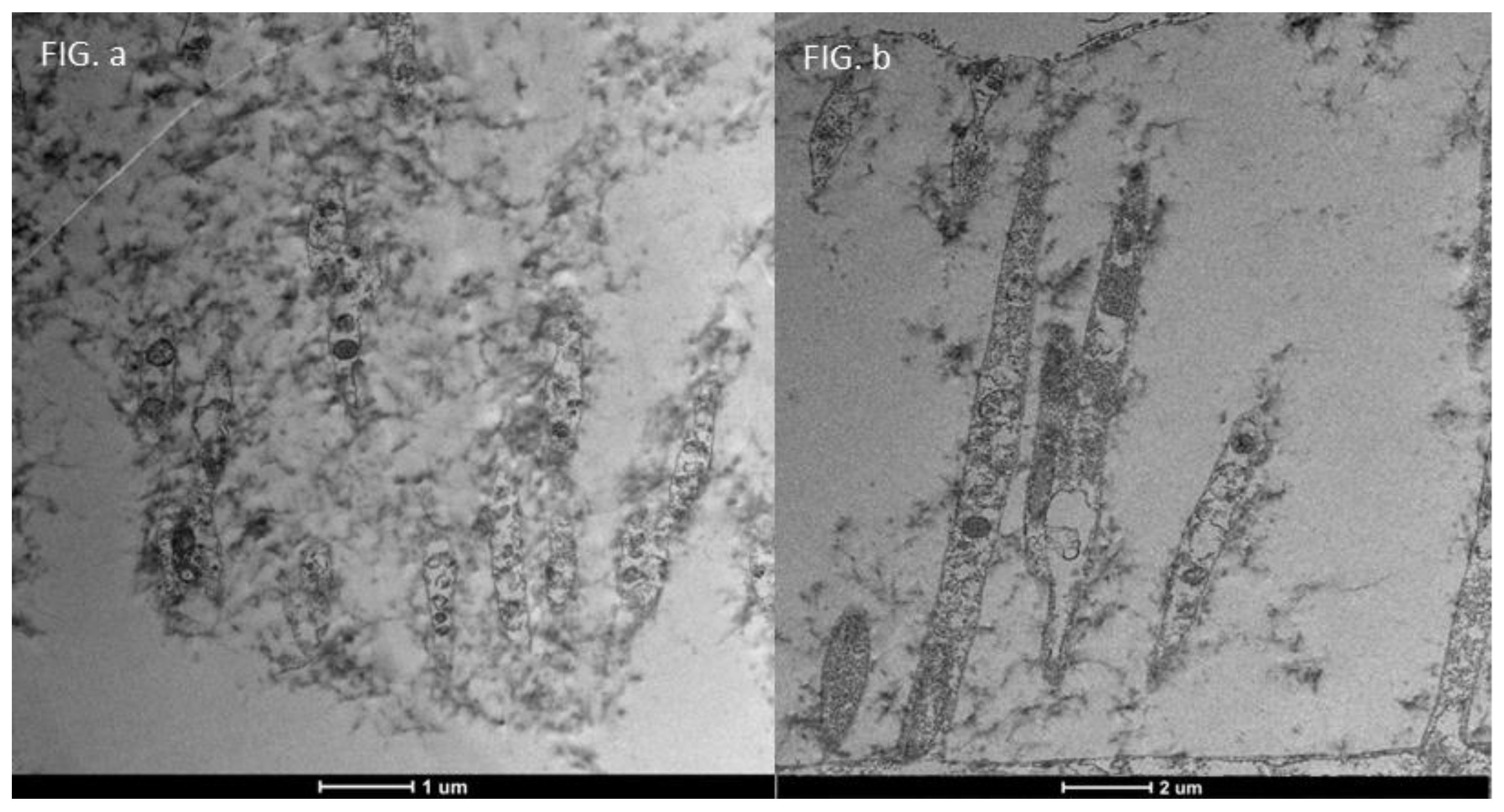


| SEM Evaluation Table | ||||
|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Microvilli on site | Microvilli on site | Microvilli on site | Microvilli on site | Microvilli not on site smooth area |
| Normal Surface | Normal Surface | Low alteration of the Surface | High alteration of the Surface | High alteration of surface |
| High microvilliar distribution | Low microvilliar distribution | microvilliar distribution on spot | microvilliar sensible reduction with spotted smooth areas | No microvillar presence on surface area reading |
| Structure of Microvilli are arborescent | Structure of Microvilli are not totally arborescent | Pseudo-microvilli structure | Pseudo-microvilli structure | Smooth surface, Moon-surface |
| TEM Evaluation Table | ||||
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Presence of glicocalyx and presence vescicular transport | Reduction of Glicocalyx and presence of vescicular transport | Absence of glicocalyx and reduction of vescicular transport | No glicocalyx, No vescicular transport, Reduction of lenght of microvilli | flattening of the plasma membrane, absence of exchange with the outside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Prete, S.; Marasco, D.; Troisi, S.; Troisi, M.; Del Prete, A. Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes, and Trehalose with Transmission Electron Microscopy: A Pilot Study. Life 2025, 15, 1611. https://doi.org/10.3390/life15101611
Del Prete S, Marasco D, Troisi S, Troisi M, Del Prete A. Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes, and Trehalose with Transmission Electron Microscopy: A Pilot Study. Life. 2025; 15(10):1611. https://doi.org/10.3390/life15101611
Chicago/Turabian StyleDel Prete, Salvatore, Daniela Marasco, Salvatore Troisi, Mario Troisi, and Antonio Del Prete. 2025. "Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes, and Trehalose with Transmission Electron Microscopy: A Pilot Study" Life 15, no. 10: 1611. https://doi.org/10.3390/life15101611
APA StyleDel Prete, S., Marasco, D., Troisi, S., Troisi, M., & Del Prete, A. (2025). Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes, and Trehalose with Transmission Electron Microscopy: A Pilot Study. Life, 15(10), 1611. https://doi.org/10.3390/life15101611






