PDE4-Selective Inhibition in Chronic Obstructive Pulmonary Disease and Pulmonary Fibrosis: Different Agents or Different Targets?
Abstract
1. Introduction
2. COPD and Pulmonary Fibrosis: Similarities and Differences in Clinical Presentations and Therapy
2.1. COPD—Definition and Sub-Classes
2.2. Pulmonary Fibrosis—Definition and Sub-Classes
3. Molecular Pharmacology of the PDE4 Family: Genes, mRNAs, and Proteins
3.1. cAMP Signaling
3.2. The PDE Superfamily
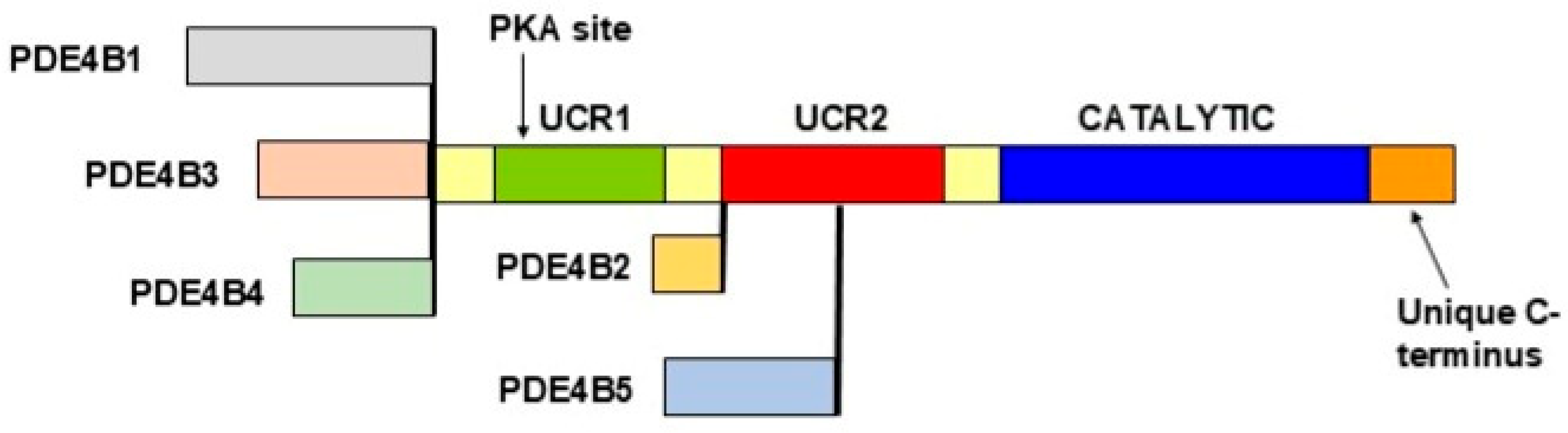
3.3. PDE4 Multiplicity: Genes, mRNAs and Proteins
3.4. Regulation of the PDE4 Proteins
3.5. Structure and Regulation of the PDE4 Proteins: Relevance to Roflumilast and Nerandomilast
4. Defining the Cellular Targets of PDE4 Inhibitors: Insights from Single-Cell Sequencing
5. Pre-Clinical Models of COPD and Fibrotic Lung Disease: Clues to the Mechanisms of PDE4 Action in Lung Diseases
5.1. Pre-Clinical Models of COPD Demonstrate Multiple Beneficial Effects of PDE4 Inhibition in COPD
5.2. Role of PDE4 in Pulmonary Inflammation
5.3. Other Actions of Roflumilast in the Lung
5.4. Actions of Roflumilast in Animal Models of COPD
5.5. Pre-Clinical Models of Fibrotic Lung Disease and the Role of PDE4 Inhibition
5.6. PDE4s and Fibroblast Proliferation in the Human Lung
5.7. Actions of Roflumilast and Nerandomilast in Animal Models of Fibrotic Lung Disease
5.8. PDE4 Expression and Function in Key Pulmonary Cell Types: Relationship to COPD and Pulmonary Fibrosis
6. Insights from Clinical Investigation, Clinical Trials, and Translational Research
6.1. Lessons from Clinical Trials of Roflumilast in COPD; Role of Inflammation
6.2. Lessons from Human Inflammatory Disorders That Lead to Fibrotic Lung Disease
6.3. PDE4 Inhibitors in COPD and Pulmonary Fibrosis: Different Diseases, Common Mechanisms of Action
6.4. The Comparative “Druggability” of Roflumilast and Nerandomilast
7. Ongoing and Possible Future Clinical Trials of PDE4 Inhibitors and Other Agents in COPD and Pulmonary Fibrosis
7.1. Currently Recruiting Clinical Trials of Roflumilast, Nerandomilast, Pirfenidone and Nintedanib
7.2. Considerations for the Design of Future Clinical Trials
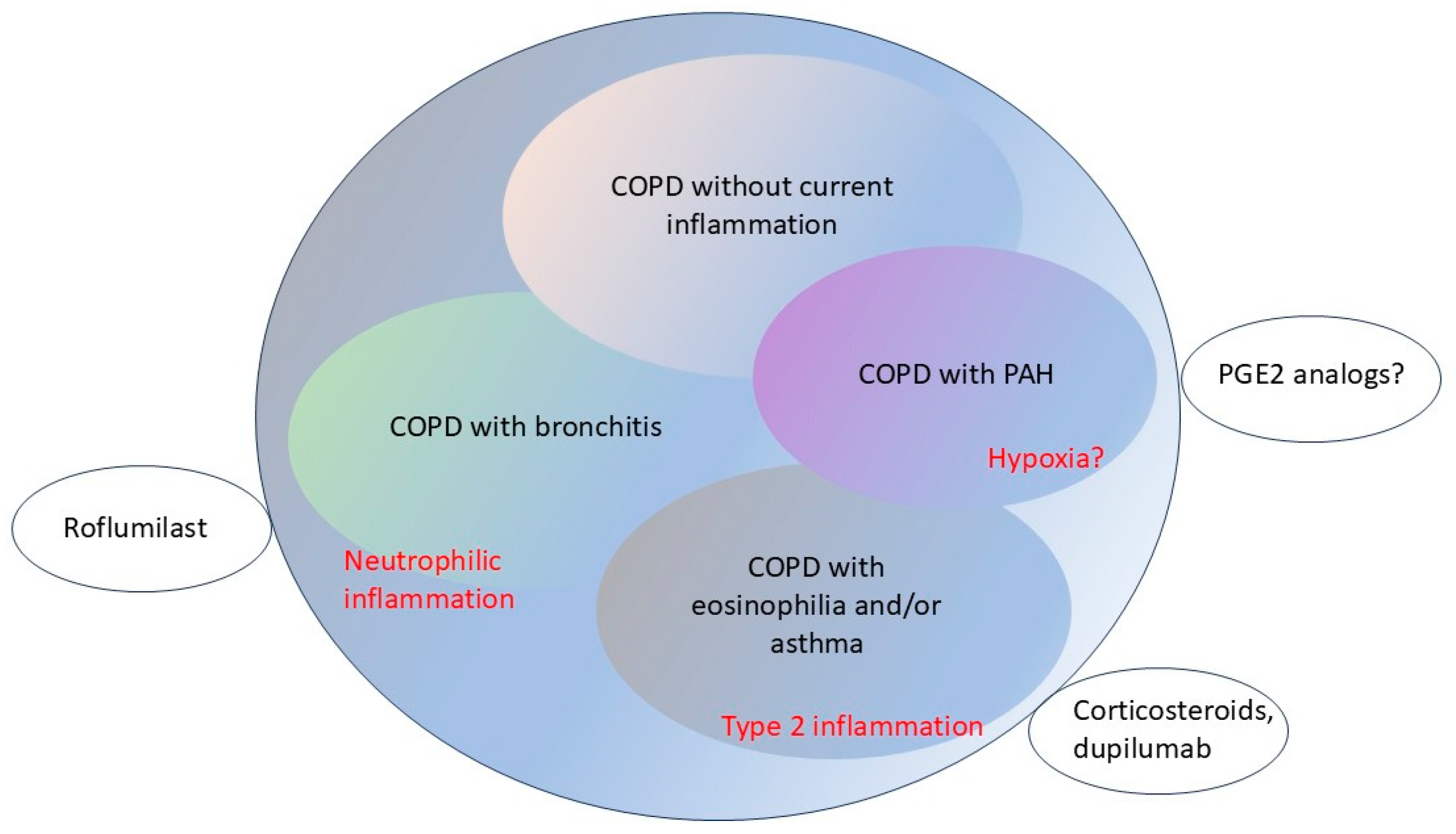
8. Future Directions: Subclassifications, Biomarkers, and Pathways
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, M.P.; Nikolaev, V.O.; Gobejishvili, L.; Lugnier, C.; Hesslinger, C.; Nickolaus, P.; Kass, D.A.; Pereira de Vasconcelos, W.; Fischmeister, R.; Brocke, S.; et al. Cyclic nucleotide phosphodiesterases as drug targets. Pharmacol. Rev. 2025, 77, 100042. [Google Scholar] [CrossRef]
- Hanania, N.A.; Celli, B.R. Phosphodiesterase Inhibition as a Therapeutic Strategy for Chronic Obstructive Pulmonary Disease: Where We Have Been and What Lies Ahead. Chronic Obstr. Pulm. Dis. 2025, 12, 82–92. [Google Scholar] [CrossRef]
- Conti, M.; Beavo, J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef]
- Janjua, S.; Fortescue, R.; Poole, P. Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2020, 5, CD002309. [Google Scholar] [CrossRef]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef]
- Hatzelmann, A.; Morcillo, E.J.; Lungarella, G.; Adnot, S.; Sanjar, S.; Beume, R.; Schudt, C.; Tenor, H. The preclinical pharmacology of roflumilast—A selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2010, 23, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Bolger, G.B. Therapeutic Targets and Precision Medicine in COPD: Inflammation, Ion Channels, Both, or Neither? Int. J. Mol. Sci. 2023, 24, 17363. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.; Rabe, K.F.; Goehring, U.M.; Kristiansen, S.; Fabbri, L.M.; Martinez, F.J. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009, 374, 685–694. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Calverley, P.M.; Izquierdo-Alonso, J.L.; Bundschuh, D.S.; Brose, M.; Martinez, F.J.; Rabe, K.F. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: Two randomised clinical trials. Lancet 2009, 374, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Calverley, P.M.; Goehring, U.M.; Brose, M.; Fabbri, L.M.; Rabe, K.F. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicentre randomised controlled trial. Lancet 2015, 385, 857–866. [Google Scholar] [CrossRef]
- Roflumilast foam (Zoryve) for seborrheic dermatitis. Med. Lett. Drugs Ther. 2024, 66, 57–59. [CrossRef] [PubMed]
- Roflumilast cream (Zoryve) for atopic dermatitis. Med. Lett. Drugs Ther. 2024, 66, 150–151. [CrossRef]
- Crisaborole (Eucrisa) for atopic dermatitis. Med. Lett. Drugs Ther. 2017, 59, 34–35.
- Drugs for COPD. Med. Lett. Drugs Ther. 2024, 66, 137–144. [CrossRef]
- Ensifentrine (Ohtuvayre) for COPD. Med. Lett. Drugs Ther. 2024, 66, 131–133. [CrossRef]
- Maher, T.M.; Assassi, S.; Azuma, A.; Cottin, V.; Hoffmann-Vold, A.M.; Kreuter, M.; Oldham, J.M.; Richeldi, L.; Valenzuela, C.; Wijsenbeek, M.S.; et al. Nerandomilast in Patients with Progressive Pulmonary Fibrosis. N. Engl. J. Med. 2025, 392, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Azuma, A.; Cottin, V.; Kreuter, M.; Maher, T.M.; Martinez, F.J.; Oldham, J.M.; Valenzuela, C.; Clerisme-Beaty, E.; Gordat, M.; et al. Nerandomilast in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2025, 392, 2193–2202. [Google Scholar] [CrossRef]
- Drugs for plaque psoriasis. Med. Lett. Drugs Ther. 2024, 66, 153–160. [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease: 2025 Report. 2025. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 26 August 2025).
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Harris, C.H. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet 1965, 1, 775–779. [Google Scholar]
- Pizzichini, E.; Pizzichini, M.M.; Gibson, P.; Parameswaran, K.; Gleich, G.J.; Berman, L.; Dolovich, J.; Hargreave, F.E. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am. J. Respir. Crit. Care Med. 1998, 158, 1511–1517. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Brightling, C.E.; Monteiro, W.; Ward, R.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, I.D. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2000, 356, 1480–1485. [Google Scholar] [CrossRef]
- Siva, R.; Green, R.H.; Brightling, C.E.; Shelley, M.; Hargadon, B.; McKenna, S.; Monteiro, W.; Berry, M.; Parker, D.; Wardlaw, A.J.; et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur. Respir. J. 2007, 29, 906–913. [Google Scholar] [CrossRef]
- Pascoe, S.; Locantore, N.; Dransfield, M.T.; Barnes, N.C.; Pavord, I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 2015, 3, 435–442. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Martinez, F.J.; Papi, A.; Pavord, I.D.; Wedzicha, J.A.; Vogelmeier, C.F.; Halpin, D.M.G. Blood Eosinophils and Chronic Obstructive Pulmonary Disease: A Global Initiative for Chronic Obstructive Lung Disease Science Committee 2022 Review. Am. J. Respir. Crit. Care Med. 2022, 206, 17–24. [Google Scholar] [CrossRef]
- Drugs for COPD. Med. Lett. Drugs Ther. 2020, 62, 137–144.
- Agusti, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Calverley, P.M.; Goehring, U.M.; Bredenbroker, D.; Martinez, F.J. Reduction of exacerbations by the PDE4 inhibitor roflumilast—The importance of defining different subsets of patients with COPD. Respir. Res. 2011, 12, 18. [Google Scholar] [CrossRef]
- Anzueto, A.; Barjaktarevic, I.Z.; Siler, T.M.; Rheault, T.; Bengtsson, T.; Rickard, K.; Sciurba, F. Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials). Am. J. Respir. Crit. Care Med. 2023, 208, 406–416. [Google Scholar] [CrossRef]
- Sciurba, F.C.; Christenson, S.A.; Rheault, T.; Bengtsson, T.; Rickard, K.; Barjaktarevic, I.Z. Effect of Dual Phosphodiesterase 3 and 4 Inhibitor Ensifentrine on Exacerbation Rate and Risk in Patients with Moderate to Severe COPD. Chest 2025, 167, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Yappalparvi, A.; Balaraman, A.K.; Padmapriya, G.; Gaidhane, S.; Kaur, I.; Lal, M.; Iqbal, S.; Prasad, G.V.S.; Pramanik, A.; Vishwakarma, T.; et al. Safety and efficacy of ensifentrine in COPD: A systemic review and meta-analysis. Respir. Med. 2025, 236, 107863. [Google Scholar] [CrossRef]
- Hammadeh, B.M.; Younis, O.M.; Alsufi, M.I.; Idrees, M.; Hussein, A.M.; Aldalati, A.Y.; Qtaishat, F.A.; Qatawneh, B.; Bugazia, A.; Hamed, R.A. Efficacy and safety of ensifentrine in treatment of COPD: A systematic review and meta-analysis of clinical trials. Ther. Adv. Respir. Dis. 2025, 19, 17534666251347775. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Cazzola, M.; Gholamalishahi, S.; Rogliani, P. The novel inhaled dual PDE3 and PDE4 inhibitor ensifentrine for the treatment of COPD: A systematic review and meta-analysis protocol on trough FEV(1) and exacerbation according to PRISMA statement. Curr. Res. Pharmacol. Drug Discov. 2024, 7, 100195. [Google Scholar] [CrossRef] [PubMed]
- Boswell-Smith, V.; Spina, D.; Oxford, A.W.; Comer, M.B.; Seeds, E.A.; Page, C.P. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6, 7-tetrahydro-2H-pyrimido [6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquino lin-4-one]. J. Pharmacol. Exp. Ther. 2006, 318, 840–848. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Ralpha antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; Patel, N.; et al. Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N. Engl. J. Med. 2024, 390, 2274–2283. [Google Scholar] [CrossRef]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.M.; Korn, S.; Lugogo, N.; Martinot, J.B.; Sagara, H.; Albers, F.C.; Bradford, E.S.; et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef]
- Sciurba, F.C.; Criner, G.J.; Christenson, S.A.; Martinez, F.J.; Papi, A.; Roche, N.; Bourbeau, J.; Korn, S.; Bafadhel, M.; Han, M.K.; et al. Mepolizumab to Prevent Exacerbations of COPD with an Eosinophilic Phenotype. N. Engl. J. Med. 2025, 392, 1710–1720. [Google Scholar] [CrossRef]
- Li, S.; Yi, B.; Wang, H.; Xu, X.; Yu, L. Efficacy and Safety of Biologics Targeting Type 2 Inflammation in COPD: A Systematic Review and Network Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2025, 20, 2143–2159. [Google Scholar] [CrossRef]
- Mohamed, M.M.G.; Kamel, G.; Charbek, E. Role of Monoclonal Antibodies in the Management of Eosinophilic Chronic Obstructive Pulmonary Disease: A Meta-analysis of Randomized Controlled Trials. Ann. Am. Thorac. Soc. 2025, 22, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef] [PubMed]
- Hurdayal, R.; Brombacher, F. Interleukin-4 Receptor Alpha: From Innate to Adaptive Immunity in Murine Models of Cutaneous Leishmaniasis. Front. Immunol. 2017, 8, 1354. [Google Scholar] [CrossRef] [PubMed]
- Mepolizumab (Nucala) for COPD. Med. Lett. Drugs Ther. 2025, 67, 131–132. [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma–present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Maher, T.M. Interstitial Lung Disease: A Review. JAMA 2024, 331, 1655–1665. [Google Scholar] [CrossRef]
- Baas, J.D.; Varga, J.; Feghali-Bostwick, C.; Peters-Golden, M.; Fortier, S.M. Distinct cAMP Regulation in Scleroderma Lung and Skin Myofibroblasts Governs Their Dedifferentiation via p38alpha Inhibition. FASEB J. 2025, 39, e70762. [Google Scholar] [CrossRef]
- Conte, P.; Ascierto, P.A.; Patelli, G.; Danesi, R.; Vanzulli, A.; Sandomenico, F.; Tarsia, P.; Cattelan, A.; Comes, A.; De Laurentiis, M.; et al. Drug-induced interstitial lung disease during cancer therapies: Expert opinion on diagnosis and treatment. ESMO Open 2022, 7, 100404. [Google Scholar] [CrossRef] [PubMed]
- Mushiroda, T.; Wattanapokayakit, S.; Takahashi, A.; Nukiwa, T.; Kudoh, S.; Ogura, T.; Taniguchi, H.; Kubo, M.; Kamatani, N.; Nakamura, Y.; et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J. Med. Genet. 2008, 45, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Borie, R.; Tabeze, L.; Thabut, G.; Nunes, H.; Cottin, V.; Marchand-Adam, S.; Prevot, G.; Tazi, A.; Cadranel, J.; Mal, H.; et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur. Respir. J. 2016, 48, 1721–1731. [Google Scholar] [CrossRef]
- Dressen, A.; Abbas, A.R.; Cabanski, C.; Reeder, J.; Ramalingam, T.R.; Neighbors, M.; Bhangale, T.R.; Brauer, M.J.; Hunkapiller, J.; Reeder, J.; et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: A candidate gene sequencing study. Lancet Respir. Med. 2018, 6, 603–614. [Google Scholar] [CrossRef]
- Peljto, A.L.; Blumhagen, R.Z.; Walts, A.D.; Cardwell, J.; Powers, J.; Corte, T.J.; Dickinson, J.L.; Glaspole, I.; Moodley, Y.P.; Vasakova, M.K.; et al. Idiopathic Pulmonary Fibrosis Is Associated with Common Genetic Variants and Limited Rare Variants. Am. J. Respir. Crit. Care Med. 2023, 207, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Miedema, J.R.; Moor, C.C.; Veltkamp, M.; Baart, S.; Lie, N.S.L.; Grutters, J.C.; Wijsenbeek, M.S.; Mostard, R.L.M. Safety and tolerability of pirfenidone in asbestosis: A prospective multicenter study. Respir. Res. 2022, 23, 139. [Google Scholar] [CrossRef]
- Chang, S.; Wen, S.; Zhang, W.; Zhang, H.; Guo, Y.; Wang, Q.; Hu, X.; Liu, Z.; Sun, Y.; Yang, A. The updated evidence of pirfenidone treated silicosis based on network pharmacology, molecular docking and experimental validation. Front. Med. 2025, 12, 1573241. [Google Scholar] [CrossRef]
- Behr, J. The diagnosis and treatment of idiopathic pulmonary fibrosis. Dtsch. Arztebl. Int. 2013, 110, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Costabel, U.; Selman, M.; Kim, D.S.; Hansell, D.M.; Nicholson, A.G.; Brown, K.K.; Flaherty, K.R.; Noble, P.W.; Raghu, G.; et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2011, 365, 1079–1087. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Richeldi, L.; Azuma, A.; Cottin, V.; Hesslinger, C.; Stowasser, S.; Valenzuela, C.; Wijsenbeek, M.S.; Zoz, D.F.; Voss, F.; Maher, T.M.; et al. Trial of a Preferential Phosphodiesterase 4B Inhibitor for Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2022, 386, 2178–2187. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Behr, J.; Bendstrup, E.; Crestani, B.; Gunther, A.; Olschewski, H.; Skold, C.M.; Wells, A.; Wuyts, W.; Koschel, D.; Kreuter, M.; et al. Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2016, 4, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; du Bois, R.M.; Fagan, E.A.; Fishman, R.S.; Glaspole, I.; Glassberg, M.K.; Lancaster, L.; et al. Pirfenidone for idiopathic pulmonary fibrosis: Analysis of pooled data from three multinational phase 3 trials. Eur. Respir. J. 2016, 47, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Corte, T.J.; Fischer, A.; Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Axmann, J.; Kirchgaessler, K.U.; Samara, K.; Gilberg, F.; et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Vancheri, C.; Kreuter, M.; Richeldi, L.; Ryerson, C.J.; Valeyre, D.; Grutters, J.C.; Wiebe, S.; Stansen, W.; Quaresma, M.; Stowasser, S.; et al. Nintedanib with Add-on Pirfenidone in Idiopathic Pulmonary Fibrosis. Results of the INJOURNEY Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 356–363. [Google Scholar] [CrossRef]
- Ikeda, S.; Sekine, A.; Baba, T.; Kato, T.; Katano, T.; Tabata, E.; Shintani, R.; Yamakawa, H.; Oda, T.; Okuda, R.; et al. Randomized phase II study of nintedanib with or without pirfenidone in patients with idiopathic pulmonary fibrosis who experienced disease progression during prior pirfenidone administration. Medicine 2022, 101, e29232. [Google Scholar] [CrossRef]
- Qiu, Y.; Ye, W. Therapeutic efficacy of pirfenidone and nintedanib in pulmonary fibrosis; a systematic review and meta-analysis. Ann. Thorac. Med. 2025, 20, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, E.; Provenzani, A.; Vitulo, P.; Polidori, P. Systematic Review and Meta-analysis of Pirfenidone, Nintedanib, and Pamrevlumab for the Treatment of Idiopathic Pulmonary Fibrosis. Ann. Pharmacother. 2021, 55, 723–731. [Google Scholar] [CrossRef]
- Lee, J.S. Progress through Persistence-Turning the Page in Pulmonary Fibrosis Clinical Trials. N. Engl. J. Med. 2025, 392, 2267–2269. [Google Scholar] [CrossRef]
- Sgalla, G.; Simonetti, J.; Cortese, S.; Richeldi, L. BI 1015550: An investigational phosphodiesterase 4B (PDE4B) inhibitor for lung function decline in idiopathic pulmonary fibrosis (IPF). Expert Opin. Investig. Drugs 2023, 32, 17–23. [Google Scholar] [CrossRef]
- Nathan, S.D.; Behr, J.; Cottin, V.; Lancaster, L.; Smith, P.; Deng, C.Q.; Pearce, N.; Bell, H.; Peterson, L.; Flaherty, K.R. Study design and rationale for the TETON phase 3, randomised, controlled clinical trials of inhaled treprostinil in the treatment of idiopathic pulmonary fibrosis. BMJ Open Respir. Res. 2022, 9, e001310. [Google Scholar] [CrossRef]
- Ismat, F.A.; Usansky, H.H.; Villa, R.; Zou, J.; Teper, A. Safety, Tolerability, and Pharmacokinetics of Treprostinil Palmitil Inhalation Powder for Pulmonary Hypertension: A Phase 1, Randomized, Double-Blind, Single- and Multiple-Dose Study. Adv. Ther. 2022, 39, 5144–5157. [Google Scholar] [CrossRef] [PubMed]
- Cullivan, S.; Genecand, L.; El-Merhie, N.; MacKenzie, A.; Lichtblau, M. Inhaled treprostinil in group 3 pulmonary hypertension associated with lung disease: Results of the INCREASE and PERFECT studies. Breathe 2025, 21, 240242. [Google Scholar] [CrossRef]
- Nathan, S.D.; Johri, S.; Joly, J.M.; King, C.S.; Raina, A.; McEvoy, C.A.; Lee, D.; Shen, E.; Smith, P.; Deng, C.; et al. Survival analysis from the INCREASE study in PH-ILD: Evaluating the impact of treatment crossover on overall mortality. Thorax 2024, 79, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.; Palevsky, H.; El-Kersh, K.; Restrepo-Jaramillo, R.; Bajwa, A.A.; Desai, S.; Joly, J.M.; Spikes, L.A.; Eggert, M.S.; Johri, S.; et al. BREEZE Optional Extension Phase: Long-term safety and efficacy of treprostinil dry powder inhaler (Tyvaso DPI) in pulmonary arterial hypertension. Respir. Med. 2025, 248, 108318. [Google Scholar] [CrossRef]
- Bolger, G.B. The PDE-Opathies: Diverse Phenotypes Produced by a Functionally Related Multigene Family. Trends Genet. 2021, 37, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Gloerich, M.; Bos, J.L. Epac: Defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Dekker, F.J.; Maarsingh, H. Exchange protein directly activated by cAMP (epac): A multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 2013, 65, 670–709. [Google Scholar] [CrossRef] [PubMed]
- Santoro, B.; Shah, M.M. Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels as Drug Targets for Neurological Disorders. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 109–131. [Google Scholar] [CrossRef]
- Tucker, S.J.; Zorn, A.J. The role of Popeye domain-containing protein 1 (POPDC1) in the progression of the malignant phenotype. Br. J. Pharmacol. 2022, 179, 2829–2843. [Google Scholar] [CrossRef]
- Tibbo, A.J.; Mika, D.; Dobi, S.; Ling, J.; McFall, A.; Tejeda, G.S.; Blair, C.; MacLeod, R.; MacQuaide, N.; Gok, C.; et al. Phosphodiesterase type 4 anchoring regulates cAMP signaling to Popeye domain-containing proteins. J. Mol. Cell Cardiol. 2022, 165, 86–102. [Google Scholar] [CrossRef]
- Smith, F.D.; Esseltine, J.L.; Nygren, P.J.; Veesler, D.; Byrne, D.P.; Vonderach, M.; Strashnov, I.; Eyers, C.E.; Eyers, P.A.; Langeberg, L.K.; et al. Local protein kinase A action proceeds through intact holoenzymes. Science 2017, 356, 1288–1293. [Google Scholar] [CrossRef]
- Omar, M.H.; Scott, J.D. AKAP Signaling Islands: Venues for Precision Pharmacology. Trends Pharmacol. Sci. 2020, 41, 933–946. [Google Scholar] [CrossRef]
- Bucko, P.J.; Scott, J.D. Drugs That Regulate Local Cell Signaling: AKAP Targeting as a Therapeutic Option. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 361–379. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Card, G.L.; Suzuki, Y.; Artis, D.R.; Fong, D.; Gillette, S.; Hsieh, D.; Neiman, J.; West, B.L.; Zhang, C.; et al. A glutamine switch mechanism for nucleotide selectivity by phosphodiesterases. Mol. Cell 2004, 15, 279–286. [Google Scholar] [CrossRef]
- Salter, E.A.; Wierzbicki, A. The mechanism of cyclic nucleotide hydrolysis in the phosphodiesterase catalytic site. J. Phys. Chem. B 2007, 111, 4547–4552. [Google Scholar] [CrossRef] [PubMed]
- Bolger, G.; Michaeli, T.; Martins, T.; St, J.T.; Steiner, B.; Rodgers, L.; Riggs, M.; Wigler, M.; Ferguson, K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol. Cell. Biol. 1993, 13, 6558–6571. [Google Scholar] [CrossRef]
- Houslay, M.D.; Sullivan, M.; Bolger, G.B. The multienzyme PDE4 cyclic AMP-specific phosphodiesterase family: Intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and anti-depressant actions. Adv. Pharmacol. 1998, 44, 225–342. [Google Scholar] [PubMed]
- Campbell, S.L.; van Groen, T.; Kadish, I.; Smoot, L.H.M.; Bolger, G.B. Altered phosphorylation, electrophysiology, and behavior on attenuation of PDE4B action in hippocampus. BMC Neurosci. 2017, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Bolger, G.B.; Smoot, L.H.M.; van Groen, T. Dominant-Negative Attenuation of cAMP-Selective Phosphodiesterase PDE4D Action Affects Learning and Behavior. Int. J. Mol. Sci. 2020, 21, 5704. [Google Scholar] [CrossRef]
- Cedervall, P.; Aulabaugh, A.; Geoghegan, K.F.; McLellan, T.J.; Pandit, J. Engineered stabilization and structural analysis of the autoinhibited conformation of PDE4. Proc. Natl. Acad. Sci. USA 2015, 112, E1414–E1422. [Google Scholar] [CrossRef]
- Millar, J.K.; Pickard, B.S.; Mackie, S.; James, R.; Christie, S.; Buchanan, S.R.; Malloy, M.P.; Chubb, J.E.; Huston, E.; Baillie, G.S.; et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 2005, 310, 1187–1191. [Google Scholar] [CrossRef]
- Yarwood, S.J.; Steele, M.R.; Scotland, G.; Houslay, M.D.; Bolger, G.B. The RACK1 signaling scaffold protein selectively interacts with the cAMP-specific phosphodiesterase PDE4D5 isoform. J. Biol. Chem. 1999, 274, 14909–14917. [Google Scholar] [CrossRef]
- Perry, S.J.; Baillie, G.S.; Kohout, T.A.; McPhee, I.; Magiera, M.M.; Ang, K.L.; Miller, W.E.; McLean, A.J.; Conti, M.; Houslay, M.D.; et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 2002, 298, 834–836. [Google Scholar] [CrossRef]
- Dodge-Kafka, K.L.; Soughayer, J.; Pare, G.C.; Carlisle Michel, J.J.; Langeberg, L.K.; Kapiloff, M.S.; Scott, J.D. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 2005, 437, 574–578. [Google Scholar] [CrossRef]
- Sette, C.; Conti, M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J. Biol. Chem. 1996, 271, 16526–16534. [Google Scholar] [CrossRef]
- Hoffmann, R.; Wilkinson, I.R.; McCallum, J.F.; Engels, P.; Houslay, M.D. cAMP-specific phosphodiesterase HSPDE4D3 mutants which mimic activation and changes in rolipram inhibition triggered by protein kinase A phosphorylation of Ser-54: Generation of a molecular model. Biochem. J. 1998, 333 Pt 1, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Linglart, A.; Fryssira, H.; Hiort, O.; Holterhus, P.M.; de Perez, N.G.; Argente, J.; Heinrichs, C.; Kuechler, A.; Mantovani, G.; Leheup, B.; et al. PRKAR1A and PDE4D Mutations Cause Acrodysostosis but Two Distinct Syndromes with or without GPCR-Signaling Hormone Resistance. J. Clin. Endocrinol. Metab. 2012, 97, E2328–E2338. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Baillie, G.S.; MacKenzie, S.J.; Yarwood, S.J.; Houslay, M.D. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J. 1999, 18, 893–903. [Google Scholar] [CrossRef]
- Hussain, M.; Tejeda, G.S.; Baillie, G.S. Posttranslational modifications of phosphodiesterase type 4 enzymes represent novel points for therapeutic targeting. FEBS J. 2025. [Google Scholar] [CrossRef]
- Herrmann, F.E.; Hesslinger, C.; Wollin, L.; Nickolaus, P. BI 1015550 is a PDE4B Inhibitor and a Clinical Drug Candidate for the Oral Treatment of Idiopathic Pulmonary Fibrosis. Front. Pharmacol. 2022, 13, 838449. [Google Scholar] [CrossRef]
- Bolger, G.B.; Erdogan, S.; Jones, R.E.; Loughney, K.; Scotland, G.; Hoffmann, R.; Wilkinson, I.; Farrell, C.; Houslay, M.D. Characterization of five different proteins produced by alternatively spliced mRNAs from the human cAMP-specific phosphodiesterase PDE4D gene. Biochem. J. 1997, 328 Pt 2, 539–548. [Google Scholar] [CrossRef]
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, L.W.; Zilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Bundschuh, D.S.; Wohlsen, A.; Marx, D.; Beume, R. Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm. Pharmacol. Ther. 2006, 19, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Bozyk, P.D.; Moore, B.B. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 445–452. [Google Scholar] [CrossRef]
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49. [Google Scholar] [CrossRef]
- Cebulla, D.; van Geffen, C.; Kolahian, S. The role of PGE2 and EP receptors on lung’s immune and structural cells; possibilities for future asthma therapy. Pharmacol. Ther. 2023, 241, 108313. [Google Scholar] [CrossRef]
- Fang, Q.; Ma, Y.; Wang, J.; Michalski, J.; Rennard, S.I.; Liu, X. PGE 2 desensitizes beta -agonist effect on human lung fibroblast-mediated collagen gel contraction through upregulating PDE4. Mediat. Inflamm. 2013, 2013, 145197. [Google Scholar] [CrossRef]
- Kohyama, T.; Liu, X.; Wen, F.Q.; Zhu, Y.K.; Wang, H.; Kim, H.J.; Takizawa, H.; Cieslinski, L.B.; Barnette, M.S.; Rennard, S.I. PDE4 inhibitors attenuate fibroblast chemotaxis and contraction of native collagen gels. Am. J. Respir. Cell Mol. Biol. 2002, 26, 694–701. [Google Scholar] [CrossRef]
- Ikari, J.; Michalski, J.M.; Iwasawa, S.; Gunji, Y.; Nogel, S.; Park, J.H.; Nelson, A.J.; Farid, M.; Wang, X.; Schulte, N.; et al. Phosphodiesterase 4 Inhibition Augments Human Lung Fibroblast VEGF Production Induced by PGE2. Am. J. Respir. Cell Mol. Biol. 2013, 49, 571–581. [Google Scholar] [CrossRef]
- Moshkovitz, N.; Epstein Shochet, G.; Shitrit, D. Prostaglandin E2 (PGE2) and Roflumilast Involvement in IPF Progression. Int. J. Mol. Sci. 2023, 24, 12393. [Google Scholar] [CrossRef]
- Kolosionek, E.; Savai, R.; Ghofrani, H.A.; Weissmann, N.; Guenther, A.; Grimminger, F.; Seeger, W.; Banat, G.A.; Schermuly, R.T.; Pullamsetti, S.S. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: The role of phosphodiesterase 4. Mol. Biol. Cell 2009, 20, 4751–4765. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Kadono, T.; Ihn, H.; Sato, S.; Igarashi, A.; Nakagawa, H.; Tamaki, K.; Takehara, K. Growth regulation in scleroderma fibroblasts: Increased response to transforming growth factor-beta 1. J. Investig. Dermatol. 1995, 105, 128–132. [Google Scholar] [CrossRef]
- Togo, S.; Liu, X.; Wang, X.; Sugiura, H.; Kamio, K.; Kawasaki, S.; Kobayashi, T.; Ertl, R.F.; Ahn, Y.; Holz, O.; et al. PDE4 inhibitors roflumilast and rolipram augment PGE2 inhibition of TGF-beta1-stimulated fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L959–L969. [Google Scholar] [CrossRef]
- Wojcik-Pszczola, K.; Chlon-Rzepa, G.; Jankowska, A.; Slusarczyk, M.; Ferdek, P.E.; Kusiak, A.A.; Swierczek, A.; Pociecha, K.; Koczurkiewicz-Adamczyk, P.; Wyska, E.; et al. A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-beta Signaling and Activation of cAMP/PKA Signaling. Int. J. Mol. Sci. 2020, 21, 4008. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.D.; Baillie, G.S.; McCall, J.R.; Passino, M.A.; Schachtrup, C.; Wallace, D.A.; Dunlop, A.J.; MacKenzie, K.F.; Klussmann, E.; Lynch, M.J.; et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J. Cell Biol. 2007, 177, 1119–1132. [Google Scholar] [CrossRef]
- Sabatini, F.; Petecchia, L.; Boero, S.; Silvestri, M.; Klar, J.; Tenor, H.; Beume, R.; Hatzelmann, A.; Rossi, G.A. A phosphodiesterase 4 inhibitor, roflumilast N-oxide, inhibits human lung fibroblast functions in vitro. Pulm. Pharmacol. Ther. 2010, 23, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tannheimer, S.L.; Wright, C.D.; Salmon, M. Combination of roflumilast with a beta-2 adrenergic receptor agonist inhibits proinflammatory and profibrotic mediator release from human lung fibroblasts. Respir. Res. 2012, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Cortijo, J.; Iranzo, A.; Milara, X.; Mata, M.; Cerda-Nicolas, M.; Ruiz-Sauri, A.; Tenor, H.; Hatzelmann, A.; Morcillo, E.J. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br. J. Pharmacol. 2009, 156, 534–544. [Google Scholar] [CrossRef]
- Iyer, S.N.; Gurujeyalakshmi, G.; Giri, S.N. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J. Pharmacol. Exp. Ther. 1999, 291, 367–373. [Google Scholar] [CrossRef]
- Iyer, S.N.; Gurujeyalakshmi, G.; Giri, S.N. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J. Pharmacol. Exp. Ther. 1999, 289, 211–218. [Google Scholar] [CrossRef]
- Chen, W.C.; Chen, N.J.; Chen, H.P.; Yu, W.K.; Su, V.Y.; Chen, H.; Wu, H.H.; Yang, K.Y. Nintedanib Reduces Neutrophil Chemotaxis via Activating GRK2 in Bleomycin-Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 4735. [Google Scholar] [CrossRef]
- Yu, W.K.; Chen, W.C.; Su, V.Y.; Shen, H.C.; Wu, H.H.; Chen, H.; Yang, K.Y. Nintedanib Inhibits Endothelial Mesenchymal Transition in Bleomycin-Induced Pulmonary Fibrosis via Focal Adhesion Kinase Activity Reduction. Int. J. Mol. Sci. 2022, 23, 8193. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Cheng, Y.; Yang, W.; Wu, X.; Zhu, H.; Hu, M.; Zhang, Y.; Zhang, M. Nintedanib Ameliorates Bleomycin-Induced Pulmonary Fibrosis, Inflammation, Apoptosis, and Oxidative Stress by Modulating PI3K/Akt/mTOR Pathway in Mice. Inflammation 2023, 46, 1531–1542. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Zhang, J. Pirfenidone and nintedanib attenuate pulmonary fibrosis in mice by inhibiting the expression of JAK2. J. Thorac. Dis. 2024, 16, 1128–1140. [Google Scholar] [CrossRef]
- Zhong, Z.; Gao, Y.; He, C.; Li, W.; Sang, L.; Huang, Y.; Chen, X.; Xie, M.; Zhang, C.; Yu, Y.; et al. Nintedanib improves bleomycin-induced pulmonary fibrosis by inhibiting the Clec7a/SPP1 pathway in interstitial macrophages. Cell. Signal. 2025, 128, 111635. [Google Scholar] [CrossRef] [PubMed]
- Udalov, S.; Dumitrascu, R.; Pullamsetti, S.S.; Al-tamari, H.M.; Weissmann, N.; Ghofrani, H.A.; Guenther, A.; Voswinckel, R.; Seeger, W.; Grimminger, F.; et al. Effects of phosphodiesterase 4 inhibition on bleomycin-induced pulmonary fibrosis in mice. BMC Pulm. Med. 2010, 10, 26, Erratum in BMC Pulm. Med. 2022, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Morcillo, E.; Monleon, D.; Tenor, H.; Cortijo, J. Roflumilast Prevents the Metabolic Effects of Bleomycin-Induced Fibrosis in a Murine Model. PLoS ONE 2015, 10, e0133453. [Google Scholar] [CrossRef]
- Lambert, J.A.; Raju, S.V.; Tang, L.P.; McNicholas, C.M.; Li, Y.; Courville, C.A.; Farris, R.F.; Coricor, G.E.; Smoot, L.H.; Mazur, M.M.; et al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am. J. Respir. Cell Mol. Biol. 2014, 50, 549–558. [Google Scholar] [CrossRef]
- Blanchard, E.; Zlock, L.; Lao, A.; Mika, D.; Namkung, W.; Xie, M.; Scheitrum, C.; Gruenert, D.C.; Verkman, A.S.; Finkbeiner, W.E.; et al. Anchored PDE4 regulates chloride conductance in wild-type and DeltaF508-CFTR human airway epithelia. FASEB J. 2014, 28, 791–801. [Google Scholar] [CrossRef]
- Mosnaim, G. Asthma in Adults. N. Engl. J. Med. 2023, 389, 1023–1031. [Google Scholar] [CrossRef]
- Comparison table: Inhaled drugs for treatment of COPD. Med. Lett. Drugs Ther. 2024, 66, e143–e147. [CrossRef]
- Albert, R.K.; Schwartz, D.A. Revealing the Secrets of Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2019, 380, 94–96. [Google Scholar] [CrossRef]
- Huang, J.; Beyer, C.; Palumbo-Zerr, K.; Zhang, Y.; Ramming, A.; Distler, A.; Gelse, K.; Distler, O.; Schett, G.; Wollin, L.; et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 883–890. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Solomon, J.J.; Danoff, S.K.; Woodhead, F.A.; Hurwitz, S.; Maurer, R.; Glaspole, I.; Dellaripa, P.F.; Gooptu, B.; Vassallo, R.; Cox, P.G.; et al. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir. Med. 2023, 11, 87–96. [Google Scholar] [CrossRef]
- Juge, P.A.; Lee, J.S.; Ebstein, E.; Furukawa, H.; Dobrinskikh, E.; Gazal, S.; Kannengiesser, C.; Ottaviani, S.; Oka, S.; Tohma, S.; et al. MUC5B Promoter Variant and Rheumatoid Arthritis with Interstitial Lung Disease. N. Engl. J. Med. 2018, 379, 2209–2219. [Google Scholar] [CrossRef]
- Adegunsoye, A.; Kropski, J.A.; Behr, J.; Blackwell, T.S.; Corte, T.J.; Cottin, V.; Glanville, A.R.; Glassberg, M.K.; Griese, M.; Hunninghake, G.M.; et al. Genetics and Genomics of Pulmonary Fibrosis: Charting the Molecular Landscape and Shaping Precision Medicine. Am. J. Respir. Crit. Care Med. 2024, 210, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Schlecker, C.; Luedtke, D.; Bossert, S.; Zoz, D.F.; Schultz, A. Phase I studies of BI 1015550, a preferential phosphodiesterase 4B inhibitor, in healthy males and patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2022, 8, 00240-2022. [Google Scholar] [CrossRef] [PubMed]
- Pugashetti, J.V.; Adegunsoye, A.; Wu, Z.; Lee, C.T.; Srikrishnan, A.; Ghodrati, S.; Vo, V.; Renzoni, E.A.; Wells, A.U.; Garcia, C.K.; et al. Validation of Proposed Criteria for Progressive Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2023, 207, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Stowasser, S.; Voss, F.; Bendstrup, E.; Kreuter, M.; Martinez, F.J.; Sime, P.J.; Stock, C. Decline in forced vital capacity as a surrogate for mortality in patients with pulmonary fibrosis. Respirology 2023, 28, 1147–1153. [Google Scholar] [CrossRef]
- Buschulte, K.; Kabitz, H.J.; Hagmeyer, L.; Hammerl, P.; Esselmann, A.; Wiederhold, C.; Skowasch, D.; Stolpe, C.; Joest, M.; Veitshans, S.; et al. Disease trajectories in interstitial lung diseases-data from the EXCITING-ILD registry. Respir. Res. 2024, 25, 113. [Google Scholar] [CrossRef]
- Buschulte, K.; Kabitz, H.J.; Hagmeyer, L.; Hammerl, P.; Esselmann, A.; Wiederhold, C.; Skowasch, D.; Stolpe, C.; Joest, M.; Veitshans, S.; et al. Hospitalisation patterns in interstitial lung diseases: Data from the EXCITING-ILD registry. Respir. Res. 2024, 25, 5. [Google Scholar] [CrossRef]
- Blanco, I.; Tura-Ceide, O.; Peinado, V.I.; Barbera, J.A. Updated Perspectives on Pulmonary Hypertension in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Atchley, W.T.; Kakkera, T.K. Pulmonary hypertension in chronic obstructive pulmonary disease: Current understanding, knowledge gaps and future directions. Curr. Opin. Pulm. Med. 2024, 30, 150–155. [Google Scholar] [CrossRef]
- Suresh, V.; Mih, J.D.; George, S.C. Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Matsunaga, K.; Sugiura, H.; Minakata, Y.; Koarai, A.; Akamatsu, K.; Ichikawa, T.; Furukawa, K.; Ichinose, M. Relationship between alveolar nitric oxide concentration in exhaled air and small airway function in COPD. J. Breath. Res. 2013, 7, 046002. [Google Scholar] [CrossRef]
- Malli, F.; Gouvani, A.; Dimeas, I.; Ladias, S.; Papathanasiou, I.V.; Gourgoulianis, K.I.; Daniil, Z. Exhaled Nitric Oxide (FeNO) in Patients Hospitalized for an Exacerbation of Bronchiectasis and/or COPD: FeNO Levels in COPD and Bronchiectasis. Adv. Exp. Med. Biol. 2021, 1337, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Fuschillo, S.; Mormile, I.; Detoraki, A.; Sarnelli, G.; Paulis, A.; Spadaro, G.; Cantone, E. Exhaled Nitric Oxide as Biomarker of Type 2 Diseases. Cells 2023, 12, 2518. [Google Scholar] [CrossRef]
- Zeng, G.; Xu, J.; Zeng, H.; Wang, C.; Chen, L.; Yu, H. Differential Clinical Significance of FENO(200) and CANO in Asthma, Chronic Obstructive Pulmonary Disease (COPD), and Asthma-COPD Overlap (ACO). J. Asthma Allergy 2024, 17, 1151–1161. [Google Scholar] [CrossRef]
- Hogman, M.; Pham-Ngoc, H.; Nguyen-Duy, B.; Ellingsen, J.; Hua-Huy, T.; Van Nguyen, D.; Dinh-Xuan, A.T. Measuring exhaled nitric oxide in COPD: From theoretical consideration to practical views. Expert. Rev. Respir. Med. 2024, 18, 1013–1024. [Google Scholar] [CrossRef]
- Christenson, S.A.; Hanania, N.A.; Bhatt, S.P.; Bafadhel, M.; Rabe, K.F.; Vogelmeier, C.F.; Papi, A.; Singh, D.; Laws, E.; Dakin, P.; et al. Type 2 inflammation biomarkers and their association with response to dupilumab in COPD (BOREAS): An analysis of a randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2025, 13, 687–697. [Google Scholar] [CrossRef]
- Rabe, K.F.; Celli, B.R.; Wechsler, M.E.; Abdulai, R.M.; Luo, X.; Boomsma, M.M.; Staudinger, H.; Horowitz, J.E.; Baras, A.; Ferreira, M.A.; et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: A genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir. Med. 2021, 9, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Fainberg, H.P.; Moodley, Y.; Triguero, I.; Corte, T.J.; Sand, J.M.B.; Leeming, D.J.; Karsdal, M.A.; Wells, A.U.; Renzoni, E.; Mackintosh, J.; et al. Cluster analysis of blood biomarkers to identify molecular patterns in pulmonary fibrosis: Assessment of a multicentre, prospective, observational cohort with independent validation. Lancet Respir. Med. 2024, 12, 681–692. [Google Scholar] [CrossRef]
- Brownstein, A.J.; Mura, M.; Ruffenach, G.; Channick, R.N.; Saggar, R.; Kim, A.; Umar, S.; Eghbali, M.; Yang, X.; Hong, J. Dissecting the lung transcriptome of pulmonary fibrosis-associated pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 327, L520–L534. [Google Scholar] [CrossRef]
- Liadaki, K.; Zafiriou, E.; Giannoulis, T.; Alexouda, S.; Chaidaki, K.; Gidarokosta, P.; Roussaki-Schulze, A.V.; Tsiogkas, S.G.; Daponte, A.; Mamuris, Z.; et al. PDE4 Gene Family Variants Are Associated with Response to Apremilast Treatment in Psoriasis. Genes 2024, 15, 369. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Jones, I.; Dransfield, M.T.; Haque, N.; Gleason, S.; Hayes, K.A.; Kulmatycki, K.; Yates, D.P.; Danahay, H.; Gosling, M.; et al. Efficacy and Safety of the CFTR Potentiator Icenticaftor (QBW251) in COPD: Results from a Phase 2 Randomized Trial. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2399–2409. [Google Scholar] [CrossRef] [PubMed]

| PDE4-Selective Inhibitor | Roflumilast | Roflumilast | Crisaborole | Ensifentrine | Nerandomilast | Apremilast |
|---|---|---|---|---|---|---|
| Route | Oral | Topical | Topical | Inhaled | Oral | Oral |
| Selectivity | Pan-PDE4 | Pan-PDE4 | Pan-PDE4 | PDE3/4 | PDE4B, PDE4D | Pan-PDE4 |
| Clinical indications | COPD | Eczema, atopic dermatitis | Eczema, atopic dermatitis | COPD | Pulmonary fibrosis | Psoriasis, psoriatic arthritis |
| References | [10,11,12] | [13,14] | [15] | [16,17] | [18,19] | [20] |
| Drug | Pirfenidone | Nintedanib | Nerandomilast | Treprostinil | Treprostinil |
|---|---|---|---|---|---|
| Targets | (see text) | Receptor tyrosine-kinases for VEGF, PDGF, FGF | PDE4B/D | Prostaglandin receptor | Prostaglandin receptor |
| Route | Oral | Oral | Oral | Inhaler | Oral |
| Other diseases | Scleroderma | ||||
| References | (see text) | [63,64,65,66] | [19,67] | (see text) |
| Cell/Tissue Type | Respiratory Epithelium | Ionocytes | Neutrophils | Macrophages | Fibroblasts | Eosinophils |
|---|---|---|---|---|---|---|
| Major pathways and mediators | Mucociliary clearance | CFTR Cl- activity | ROS, IL-8 | TNFα | TGF-β | |
| Roflumilast target(s) in COPD | Pan-PDE4 | Pan-PDE4 | Pan-PDE4 | Pan-PDE4 | Pan-PDE4 | Pan-PDE4 |
| Nerandomilast Target(s) in IPF | (uncertain) | (uncertain) | PDE4B/D | PDE4B/D | PDE4B/D | (uncertain) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolger, G.B. PDE4-Selective Inhibition in Chronic Obstructive Pulmonary Disease and Pulmonary Fibrosis: Different Agents or Different Targets? Life 2025, 15, 1600. https://doi.org/10.3390/life15101600
Bolger GB. PDE4-Selective Inhibition in Chronic Obstructive Pulmonary Disease and Pulmonary Fibrosis: Different Agents or Different Targets? Life. 2025; 15(10):1600. https://doi.org/10.3390/life15101600
Chicago/Turabian StyleBolger, Graeme B. 2025. "PDE4-Selective Inhibition in Chronic Obstructive Pulmonary Disease and Pulmonary Fibrosis: Different Agents or Different Targets?" Life 15, no. 10: 1600. https://doi.org/10.3390/life15101600
APA StyleBolger, G. B. (2025). PDE4-Selective Inhibition in Chronic Obstructive Pulmonary Disease and Pulmonary Fibrosis: Different Agents or Different Targets? Life, 15(10), 1600. https://doi.org/10.3390/life15101600






