Influence of Surgical Pleth Index-Guided Versus Conventional Analgesia on Opioid Consumption During Gastric Sleeve Surgery: A Pilot Study
Abstract
1. Introduction
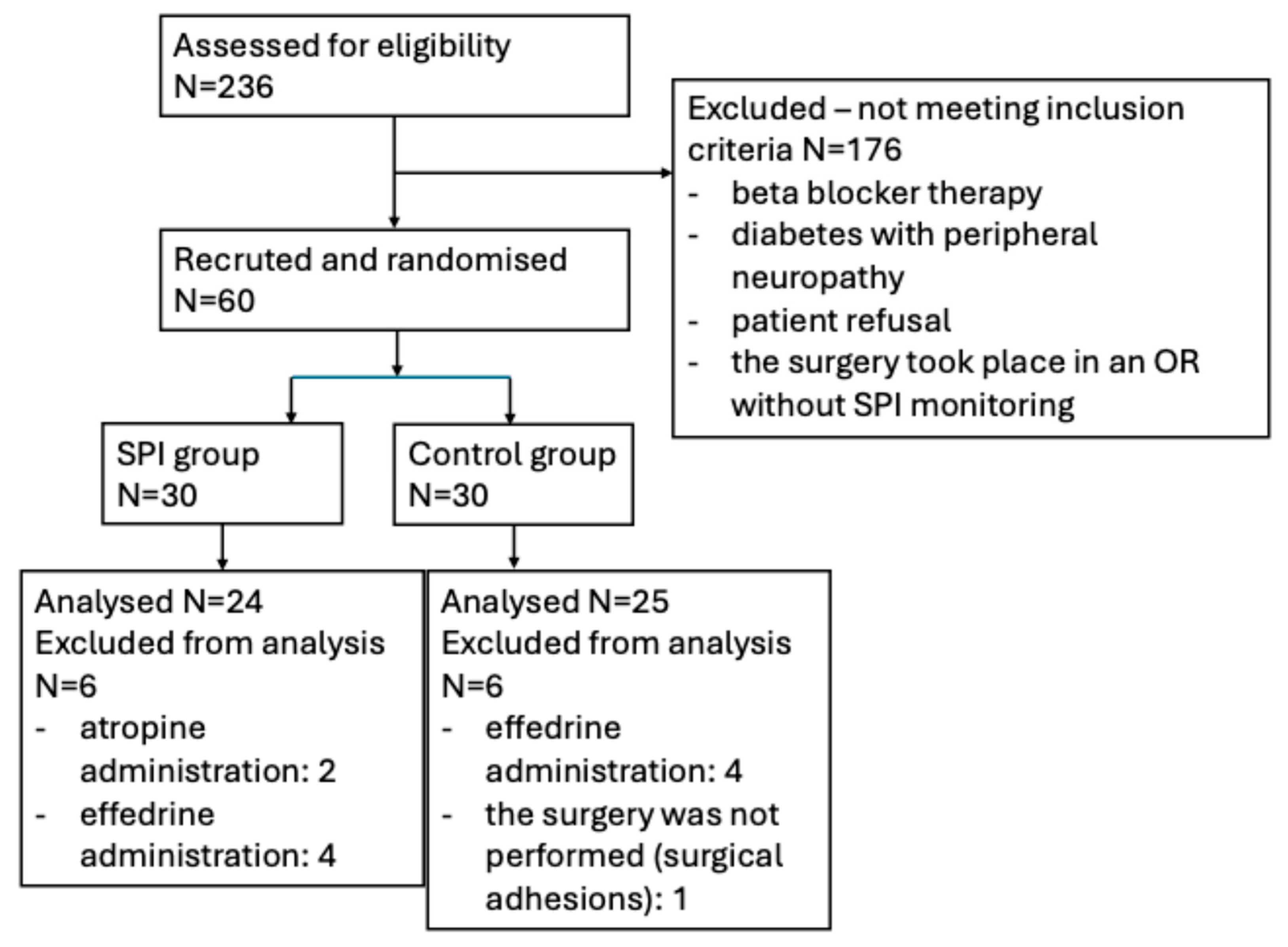
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Anesthesia
2.4. Data Collection
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPI | Surgical pleth index |
| ECG | Electrocardiography |
| NIBP | Non-invasive blood pressure |
| EEG | Electroencephalography |
| ANI | Analgesia nociception index |
| ASA | American society of anesthesiologists physical status score |
| HR | Heart rate |
| MAP | Mean arterial pressure |
| TOF | Train-of-four |
| BIS | Bispectral index |
| PACU | Post anesthesia care unit |
| LBW | Lean body weight |
| PBW | Predicted body weight |
| PONV | Postoperative nausea and vomiting |
References
- Klein, A.A.; Meek, T.; Allcock, E.; Cook, T.M.; Mincher, N.; Morris, C.; Nimmo, A.F.; Pandit, J.J.; Pawa, A.; Rodney, G.; et al. Recommendations for Standards of Monitoring during Anaesthesia and Recovery 2021. Anaesthesia 2021, 76, 1212–1223. [Google Scholar] [CrossRef]
- European Board of Anaesthesiology (EBA) Recommendations for Minimal Monitoring during Anaesthesia and Recovery. Available online: https://www.eba-uems.eu/resources/PDFS/safety-guidelines/EBA-Minimal-monitor.pdf (accessed on 25 August 2025).
- Ryalino, C.; Sahinovic, M.M.; Drost, G.; Absalom, A.R. Intraoperative Monitoring of the Central and Peripheral Nervous Systems: A Narrative Review. Br. J. Anaesth. 2024, 132, 285–299. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Ledowski, T. Objective Monitoring of Nociception: A Review of Current Commercial Solutions. Br. J. Anaesth. 2019, 123, e312–e321. [Google Scholar] [CrossRef]
- Martinez-Vazquez, P.; Jensen, E.W. Different Perspectives for Monitoring Nociception during General Anesthesia. Korean J. Anesth. 2022, 75, 112–123. [Google Scholar] [CrossRef]
- Snoek, M.A.J.; van den Berg, V.J.; Dahan, A.; Boon, M. Comparison of Different Monitors for Measurement of Nociception during General Anaesthesia: A Network Meta-Analysis of Randomised Controlled Trials. Br. J. Anaesth. 2025, 134, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Huiku, M.; Uutela, K.; van Gils, M.; Korhonen, I.; Kymäläinen, M.; Meriläinen, P.; Paloheimo, M.; Rantanen, M.; Takala, P.; Viertiö-Oja, H.; et al. Assessment of Surgical Stress during General Anaesthesia. Br. J. Anaesth. 2007, 98, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, V.; Uutela, K.; Hans, G.; Maquoi, I.; Born, J.D.; Brichant, J.F.; Lamy, M.; Hans, P. Comparison of the Surgical Pleth IndexTM with Haemodynamic Variables to Assess Nociception–Anti-Nociception Balance during General Anaesthesia. Br. J. Anaesth. 2011, 106, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Won, Y.J.; Lim, B.G. Surgical Pleth Index Monitoring in Perioperative Pain Management: Usefulness and Limitations. Korean J. Anesth. 2024, 77, 31–45. [Google Scholar] [CrossRef]
- Bergmann, I.; Göhner, A.; Crozier, T.A.; Hesjedal, B.; Wiese, C.H.; Popov, A.F.; Bauer, M.; Hinz, J.M. Surgical Pleth Index-Guided Remifentanil Administration Reduces Remifentanil and Propofol Consumption and Shortens Recovery Times in Outpatient Anaesthesia. Br. J. Anaesth. 2013, 110, 622–628. [Google Scholar] [CrossRef]
- Gruenewald, M.; Willms, S.; Broch, O.; Kott, M.; Steinfath, M.; Bein, B. Sufentanil Administration Guided by Surgical Pleth Index vs Standard Practice during Sevoflurane Anaesthesia: A Randomized Controlled Pilot Study. Br. J. Anaesth. 2014, 112, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, V.; Schreiberova, J.; Bartos, M.; Kukralova, L.; Dostal, P. Surgical Pleth Index and Analgesia Nociception Index for Intraoperative Analgesia in Patients Undergoing Neurosurgical Spinal Procedures: A Comparative Randomized Study. Minerva Anestesiol. 2019, 85, 1265–1272. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, W.; Shi, Q.; Bao, F.; Xu, J. Effect of Surgical Pleth Index-Guided Analgesia versus Conventional Analgesia Techniques on Fentanyl Consumption under Multimodal Analgesia in Laparoscopic Cholecystectomy: A Prospective, Randomized and Controlled Study. BMC Anesth. 2021, 21, 167. [Google Scholar] [CrossRef]
- Bapteste, L.; Szostek, A.S.; Chassard, D.; Desgranges, F.P.; Bouvet, L. Can Intraoperative Surgical Pleth Index Values Be Predictive of Acute Postoperative Pain? Anaesth. Crit. Care Pain Med. 2019, 38, 391–392. [Google Scholar] [CrossRef]
- Ledowski, T.; Schneider, M.; Gruenewald, M.; Goyal, R.K.; Teo, S.R.; Hruby, J. Surgical Pleth Index: Prospective Validation of the Score to Predict Moderate-to-Severe Postoperative Pain. Br. J. Anaesth. 2019, 123, e328–e332. [Google Scholar] [CrossRef]
- Chen, X.; Thee, C.; Gruenewald, M.; Ilies, C.; Höcker, J.; Hanss, R.; Steinfath, M.; Bein, B. Correlation of Surgical Pleth Index with Stress Hormones during Propofol-Remifentanil Anaesthesia. Sci. World J. 2012, 2012, 879158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; He, Q.; Li, S.; Zhao, Y.; Zuo, Y. Current Perioperative Nociception Monitoring and Potential Directions. Asian J. Surg. 2024, 47, 2558–2565. [Google Scholar] [CrossRef]
- Ilies, C.; Ludwigs, J.; Gruenewald, M.; Thee, C.; Hanf, J.; Hanss, R.; Steinfath, M.; Bein, B. The Effect of Posture and Anaesthetic Technique on the Surgical Pleth Index. Anaesthesia 2012, 67, 508–513. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. Publisher Correction: 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2023, 33, 15–16. [Google Scholar] [CrossRef]
- Wynn-Hebden, A.; Bouch, D.C. Anaesthesia for the Obese Patient. BJA Educ. 2020, 20, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Eipe, N.; Jones, S.B.; Nancy, A. Nussmeier Anesthesia for the Patient with Obesity. Available online: https://www.uptodate.com/contents/anesthesia-for-the-patient-with-obesity (accessed on 4 September 2025).
- Ingrande, J.; Lemmens, H.J.M. Dose Adjustment of Anaesthetics in the Morbidly Obese. Br. J. Anaesth. 2010, 105, i16–i23. [Google Scholar] [CrossRef]
- Seyni-Boureima, R.; Zhang, Z.; Antoine, M.M.L.K.; Antoine-Frank, C.D. A Review on the Anesthetic Management of Obese Patients Undergoing Surgery. BMC Anesth. 2022, 22, 98. [Google Scholar] [CrossRef]
- Stenberg, E.; dos Reis Falcão, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and Mortality Associated with Obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, C.E.; Margarson, M.P.; Shearer, E.; Redman, J.W.; Lucas, D.N.; Cousins, J.M.; Fox, W.T.A.; Kennedy, N.J.; Venn, P.J.; Skues, M.; et al. Peri-Operative Management of the Obese Surgical Patient 2015. Anaesthesia 2015, 70, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Dagher, C.; Mattar, R.; Aoun, M.; Tohme, J.; Naccache, N.; Jabbour, H. Opioid-Free Anesthesia in Bariatric Surgery: A Prospective Randomized Controlled Trial. Eur. J. Med. Res. 2025, 30, 320. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Garg, K.; Devgan, S. Comparison of Opioid-Based and Opioid-Free TIVA for Laparoscopic Urological Procedures in Obese Patients. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 481. [Google Scholar] [CrossRef] [PubMed]
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A Decade Analysis of Trends and Outcomes of Male vs Female Patients Who Underwent Bariatric Surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef]
- Téoule, P.; Pozek, E.; Hielscher, T.; Reißfelder, C.; Stier, C.; Otto, M.; Schölch, S. Geschlechtsspezifische Unterschiede in Der Adipositaschirurgie: Epidemiologie, Therapie Und Ergebnisse. Die Chir. 2024, 95, 721–729. [Google Scholar] [CrossRef]
- Chen, I.-W.; Hung, K.-C. Low Body Mass Index May Impact the Accuracy of Surgical Pleth Index to Predict Postoperative Major Pain. Anaesth. Crit. Care Pain Med. 2020, 39, 543–544. [Google Scholar] [CrossRef]
- Chen, X.; Thee, C.; Gruenewald, M.; Wnent, J.; Illies, C.; Hoecker, J.; Hanss, R.; Steinfath, M.; Bein, B. Comparison of Surgical Stress Index-Guided Analgesia with Standard Clinical Practice during Routine General Anesthesia. Anesthesiology 2010, 112, 1175–1183. [Google Scholar] [CrossRef]
- Colombo, R.; Raimondi, F.; Rech, R.; Castelli, A.; Fossali, T.; Marchi, A.; Borghi, B.; Corona, A.; Guzzetti, S. Surgical Pleth Index Guided Analgesia Blunts the Intraoperative Sympathetic Response to Laparoscopic Cholecystectomy. Minerva Anestesiol. 2015, 81, 837–845. [Google Scholar]
- Jiao, Y.; He, B.; Tong, X.; Xia, R.; Zhang, C.; Shi, X. Intraoperative Monitoring of Nociception for Opioid Administration: A Meta-Analysis of Randomized Controlled Trials. Minerva Anestesiol. 2019, 85, 522–530. [Google Scholar] [CrossRef]
- Jain, N.; Gera, A.; Sharma, B.; Sood, J.; Chugh, P. Comparison of Surgical Pleth Index-Guided Analgesia Using Fentanyl versus Conventional Analgesia Technique in Laparoscopic Cholecystectomy. Minerva Anestesiol. 2019, 85, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.J.; Lim, B.G.; Kim, Y.S.; Lee, M.; Kim, H. Usefulness of Surgical Pleth Index-Guided Analgesia during General Anesthesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Int. Med. Res. 2018, 46, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, L.; David, A.; Carles, P.; Leuillet, S.; Chastel, B.; Fleureau, C.; Dewitte, A.; Ouattara, A. Benefits of Intraoperative Analgesia Guided by the Analgesia Nociception Index (ANI) in Bariatric Surgery: An Unmatched Case-Control Study. Anaesth. Crit. Care Pain Med. 2019, 38, 35–39. [Google Scholar] [CrossRef] [PubMed]
| Standard Analgesia | SPI-Guided Analgesia | p | |
|---|---|---|---|
| Age, years (mean ± std deviation) | 40 (±11.1) | 38.6 (±12) | 0.692 |
| Height, cm (mean ± std deviation) | 168 (±0.08) | 167 (±0.08) | 0.639 |
| Weight, kg (mean ± std deviation) | 122 (±21) | 114 (±22.1) | 0.158 |
| Lean Body Weight, kg (mean) | 58.5 (±7.66) | 60.3 (±7.34) | 0.802 |
| BMI (mean ± std deviation) | 43.9 (±7.07) | 41.6 (±6.74) | 0.410 |
| ASA score (ASA II/ASA III/Total) | 6/19/25 | 8/16/24 | 0.538 |
| Gender (Female/Total) | 20/25 | 16/24 | 0.29 |
| Standard Analgesia | SPI-Guided Analgesia | p | ||
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | Preoperatively | 93.60 ± 13.40 | 93.60 ± 13.54 | 0.99 |
| OR-admission | 96.20 ± 15.37 | 95.60 ± 13.71 | 0.87 | |
| 1 min after induction | 83.00 ± 9.36 | 88.20 ± 10.12 | 0.07 | |
| 1 min after incision | 85.90 ± 7.72 | 88.40 ± 8.81 | 0.304 | |
| 1 min after pneumoperitoneum | 86.40 ± 9.59 | 88.30 ± 9.85 | 0.481 | |
| After exsuflation | 86.30 ± 11.33 | 88.50 ± 10.55 | 0.474 | |
| Heart rate (beats/min) | Preoperatively | 75 ± 10.55 | 79 ± 12.51 | 0.302 |
| OR-admission | 78 ± 12.60 | 81 ± 12.38 | 0.596 | |
| 1 min after induction | 76 ± 7.48 | 77.5 ± 8.41 | 0.489 | |
| 1 min after incision | 74 ± 7.55 | 75.5 ± 8.83 | 0.077 | |
| 1 min after pneumoperitoneum | 75 ± 6.76 | 75 ± 7.27 | 0.462 | |
| After exsuflation | 72 ± 8.8 | 77 ± 10.04 | 0.055 | |
| SPI | OR-admission | 40.2 ± 13.49 | 38.5 ± 12.2 | 0.681 |
| 1 min after induction | 45 ± 10.74 | 40.8 ± 13.22 | 0.154 | |
| 1 min after incision | 48.4 ± 7.63 | 43.8 ± 8.57 | 0.066 | |
| 1 min after pneumoperitoneum | 52.7 ± 8.58 | 48.5 ± 6.61 | 0.069 | |
| After exsuflation | 51.7 ± 8.5 | 52 ± 5.49 | 0.809 | |
| Standard Analgesia | SPI-Guided Analgesia | p | |
|---|---|---|---|
| Fentanyl dose (mcg) | 450 ± 55.9 | 400 ± 101.06 | 0.100 |
| Fentanyl/LBW (mcg/kg) | 7.54 ± 1.09 | 6.93 ± 1.52 | 0.111 |
| Time to wake-up (min) | 9.61 ± 1.34 | 8.1 ± 1.62 | <0.001 |
| Hemodinamic events | 7/25 (28%) | 2/24 (8.33%) | 0.07 |
| Rescue analgesia | 9/25 (36%) | 4/24 (16.66%) | 0.12 |
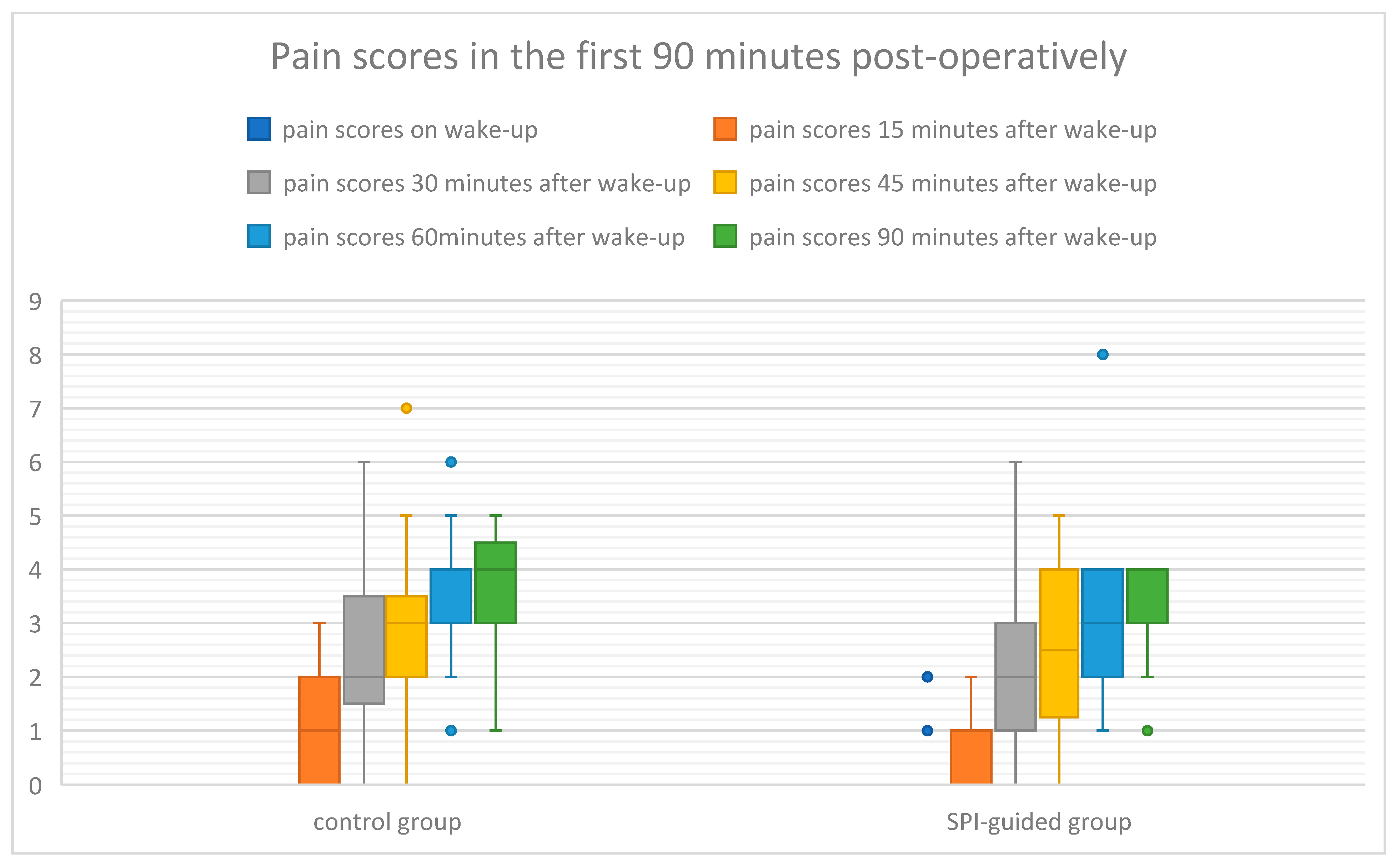
| Standard Analgesia | SPI-Guided Analgesia | p | |
|---|---|---|---|
| Wake-up | 0 ± 0 | 0 ± 0.448 | 0.153 |
| at 15 min | 1 ± 0.92 | 1 ± 0.72 | 0.889 |
| at 30 min | 2 ± 1.65 | 2 ± 1.5 | 0.682 |
| at 45 min | 3 ± 1.52 | 2.5 ± 1.38 | 0.344 |
| at 60 min | 4 ± 1.22 | 3 ± 1.35 | 0.248 |
| at 90 min | 4 ± 1.06 | 4 ± 0.86 | 0.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leahu, C.-E.; Petrisor, C.; Cocu, S.; Boldis, A.M.; Dindelegan, G.C. Influence of Surgical Pleth Index-Guided Versus Conventional Analgesia on Opioid Consumption During Gastric Sleeve Surgery: A Pilot Study. Life 2025, 15, 1570. https://doi.org/10.3390/life15101570
Leahu C-E, Petrisor C, Cocu S, Boldis AM, Dindelegan GC. Influence of Surgical Pleth Index-Guided Versus Conventional Analgesia on Opioid Consumption During Gastric Sleeve Surgery: A Pilot Study. Life. 2025; 15(10):1570. https://doi.org/10.3390/life15101570
Chicago/Turabian StyleLeahu, Crina-Elena, Cristina Petrisor, Simona Cocu, Alexandra Maria Boldis, and George Calin Dindelegan. 2025. "Influence of Surgical Pleth Index-Guided Versus Conventional Analgesia on Opioid Consumption During Gastric Sleeve Surgery: A Pilot Study" Life 15, no. 10: 1570. https://doi.org/10.3390/life15101570
APA StyleLeahu, C.-E., Petrisor, C., Cocu, S., Boldis, A. M., & Dindelegan, G. C. (2025). Influence of Surgical Pleth Index-Guided Versus Conventional Analgesia on Opioid Consumption During Gastric Sleeve Surgery: A Pilot Study. Life, 15(10), 1570. https://doi.org/10.3390/life15101570





