Abstract
Hepatic cancer is a world health concern due to its high lethality. The main risk factor worldwide is having hepatic cirrhosis. The etiology of hepatic cirrhosis has changed in recent years, with metabolic-associated steatotic liver disease (MASLD) becoming the leading cause, displacing hepatitis C and B viruses and alcoholic liver disease. It is of the utmost importance to develop screening programs in at-risk populations for early detection. The survival rate of HCC, as determined by a group of specialists or an interdisciplinary committee, is a challenge we have taken on in a public health hospital in Ecuador. This retrospective study identified 71 patients diagnosed with hepatocellular carcinoma, mostly middle-aged men with a history of liver cirrhosis. No significant association was found between the presence of cirrhosis, laboratory abnormalities, and survival. However, the identification by imaging vascular invasion and extrahepatic extension were associated. This study highlights that patients with liver lesions identified through HCC screening have a higher survival rate over a one-year follow-up period.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, characterized by its high mortality rate. It is the sixth most common cancer worldwide and the third leading cause of cancer death globally [1].
In Ecuador, the incidence rate published in the national tumor registry is 4.6 cases per 100,000 inhabitants, which is lower than the rest of South America and much higher than the rates in East Asia and North Africa. Regarding mortality, Ecuador has a rate of 4.4 cases per 100,000 inhabitants for both sexes [2]. This lower incidence of HCC in Ecuador is unfortunately underestimated because there is no national or regional policy for the management of liver cancer; all records go into a large database coordinated by INEC (National Institute of Statistics and Census); however, many sub-records are kept in third-level hospitals, for this, the overall statistics are not accurate. Due to this inconvenience, our liver and biliary tract tumor committee upholds a more real and measurable quantification of cases with diagnosis of liver cancer since 2018, when it was created.
Most cases arise in developing countries, especially in Asia and Africa, where high rates of chronic hepatitis B and C infections, as well as exposure to aflatoxins, contribute significantly to the burden of this disease. In developed countries, risk factors such as alcohol consumption and obesity, which lead to metabolically impaired steatosis liver disease (MASLD), contribute to the increased incidence of HCC [3].
In the Americas, HCC represents a significant challenge for both public and private health systems. Although incidence rates are comparatively lower than in other regions, such as Asia and Africa, HCC still represents a major issue in public health. The prevalence of risk factors varies among countries in the region: some have higher rates of viral hepatitis and alcohol-related liver disease, while in recent years, MASLD has become the main risk factor for chronic liver disease and its complications [3,4].
Various treatments have been proposed for the management of HCH, based primarily on the patient’s characteristics, focusing on whether or not they have cirrhosis, portal hypertension, their functional stage determined by the Child–Pugh scale, one or more lesions, and if they have vascular involvement. The staging system of The Barcelona Liver Cancer Clinic (BCLC) is the most common and popular in America, it is considered a great guide that should be adapted to the needs and realities of each country, which leads us to a better management of curative and palliative therapies [5]. The objective of this study is to evaluate the factors related to the survival of patients with hepatocellular carcinoma evaluated by the interdisciplinary committee formed in a tertiary hospital in Quito, Ecuador. The findings will provide a valuable contribution to the global body of literature on liver cancer.
2. Methods
This is a retrospective cohort study of patients seen in the outpatient clinic and/or hospitalized at a national public health hospital of significant importance (Eugenio Espejo Hospital) in Quito, Ecuador, between 2016 and 2023. All cases were evaluated by a liver and biliary tract tumor committee.
This study included patients with hepatocellular carcinoma (HCC) who were diagnosed according to the guidelines of the American Association for the Study of Liver Diseases (AASLD) [6] and the European Association for the Study of the Liver (EASL) [7]. Patients with cirrhosis, regardless of the cause, as well as those who developed HCC without underlying chronic liver disease, were also included. In patients with cirrhosis and suspected HCC, contrast studies such as magnetic resonance imaging or computed tomography were used and LI-RADS (Liver Imaging Reporting Data System) criteria were applied. For patients without cirrhosis, the diagnosis was established through biopsy. All patients were evaluated through a multidisciplinary approach by a dedicated committee.
Pregnant patients and minors were excluded from the study. We recorded demographic data, the cause of underlying liver disease, comorbidities, family history of HCC, method of diagnosis, treatment, clinical progression, and one-, two-, and three-year survival rates. For imaging, we focused on variables such as tumor count, the diameter of the largest tumor, vascular invasion, and extrahepatic lesions.
Data were extracted from medical records by the hospital’s statistics department and, to preserve anonymity, all analyses were performed using a coded database. Descriptive and inferential statistics were computed using R Pack and Microsoft Excel. The patients were part of the ESCALON Project, a collaborative network between Latin America and Europe focused on hepatobiliary cancer, and provided written consent for their participation in this and related studies.
3. Results
Seventy-one patients were included in this study for demographic and laboratory variables, and seventy patients were considered for the survival analysis. The sample consisted predominantly of males, with a male-to-female ratio of 1.15, and the median age of patients was 67.85 years, ranging from 21 to 89 years. Table 1 presents information on ethnic groups, age, level of education, habits, and comorbidities. Finally, the most affected ethnic group was mestizos or Hispanics in 70 cases (98.6%).

Table 1.
Demographic characteristics of patients with hepatocellular carcinoma.
In patients with cirrhosis, most cases were classified as Child–Pugh A and B, with a smaller proportion classified as Child–Pugh C. Additionally, almost two-thirds of the patients were classified as BCLC stages A or B. All stages were considered for the study. Table 2 shows the distribution of patients according to Child–Pugh and BCLC classifications.

Table 2.
Classification of patients by Child–Pugh and BCLC Staging.
Patients included in this study were diagnosed with HCC after clinical manifestations in 81.7% (n = 51) of cases and by surveillance in 18.3% (n = 13) of cases. Surveillance protocol follows the standard recommendation of international societies of use of ultrasound in addition to alpha-fetoprotein every six months.
No significant association was found between the presence of cirrhosis or laboratory test results and the survival rate at the end of study. However, serum albumin values were higher in patients that died at one year than in those who survived (3.6 ± 0.947 vs. 3.14 ± 00.581, p = 0.015). Table 3 describes laboratory test results according to the presence of cirrhosis.

Table 3.
Laboratory test results according to the presence of cirrhosis.
Treatments received by the patients are shown in Table 4; these include palliative, pharmacological, or surgical procedures.

Table 4.
Treatments offered for hepatocellular carcinoma.
In this study, the mean survival time since diagnosis was 13.69 months (410.87 days) with a Std. Deviation of 483.15 (minimum 1–maximum 2065) (CI 95%); with 22 out of the 70 patients still alive by the end of the data collection and 2.82% of patients alive at 5 years.
Kaplan–Meyer shows that vascular invasion was significantly related to survival time in days (X2 = 3.98, dL = 1, p = 0.046), as well as the extra-hepatic spread (X2 = 8.7, dL = 1, p = 0.003). No association was found between the Child–Pugh classification and survival at the end of 5 years; there is an association only at the end of the one year and there was also a significant association with BCLC categories (X2 = 11.66, dL = 3, p = 0.009). Comparing survival and treatment, no significant correlations were found (X2 = 1.754, dL = 1, p = 0.185). Nevertheless, a better survival rate at 500 days was observed with TACE (Figure 1). Comparing cases identified by screening with those identified by clinical manifestations, patients identified while on screening had better survival rates (Mantel–Cox log rank X2 = 7.394, dL = 1, p = 0.007).
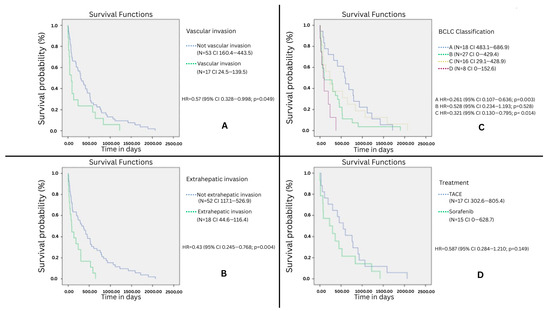
Figure 1.
Kaplan–Meyer test survival plot, x: time in days, and y: percentage of patients alive in function of time. (A) Survival according to the presence of intravascular invasion, (B) survival according to the presence of extravascular spread, (C) survival according to the BCLC classification, (D) comparison between TACE (n = 17) and sorafenib (n = 15).
A binary logistic regression was performed by steps, showing that independent variables (Age, stage Child–Pugh/BCLC scale, cirrhosis and treatment) over the probability of occurrence in four steps of dependent variable (X2 = 33.493, df = 11 p ≤ 0.001) explain the 0.26% of change over the dependent variable (R2 Nagelkerke = 0.51). All the effects of each variable are displayed in the Supplementary Table S1; Child–Pugh category and treatment are the two variables that were kept after the four steps of the model, RFA treatment present an odds ratio of 23.6, associated with surviving the first year. Child–Pugh category presents an accumulated significance; however, by categories, only Child–Pugh C is related with an odds ratio 0.086, suggesting association with death before the first year, but its significance is only in the limit (p = 0.051).
We performed a Cox proportional-hazards model adjusting of BCLC stage (X2 = 11.989, df = 4, p = 0.017), where presence of cirrhosis presents a non-statistically significant value (B/SE = −0.177, 0.292, Wald = 0.366, p = 00.545, CI 0.472–1.486); this confirms that cirrhosis is not associated with survival.
4. Discussion
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, with a high regional and global impact. This study, the first of its kind to be conducted in Ecuador’s public health system with the participation of a multidisciplinary committee, examined the demographic characteristics and survival factors of patients with HCC.
Similarly to what was found in the present study, almost 80% of HCC cases develop in the context of a cirrhotic liver, which, by having a cellular alteration associated with fibrosis, creates a chronic inflammatory state, which leads to DNA alterations and abnormal cell proliferation; this is independent of the underlying etiology of chronic liver disease, with an estimated annual incidence of 2% to 4% [8,9].
In our study, approximately 21% of hepatocellular carcinoma (HCC) patients were non-cirrhotic. The majority of these patients presented diabetes and metabolic dysfunction-associated steatotic liver disease (MASLD). This finding is not surprising, as surveillance is well-established for cirrhotic patients of any etiology, as well as for those with HBV and HCV infections, but not for the other risk factors for chronic liver disease. A similar incidence has been previously reported. A large retrospective study of 1400 HCC patients over a 10-year period identified at least 17% of patients as non-cirrhotic. Recent reports suggest that this population is growing [10,11]. Non-cirrhotic HCC shows a distinct age distribution with a double peak, one peak occurring in the second decade and a second peak in the seventh decade of life. Furthermore, the fibrolamellar subtype is the most common in this population [12]. Hepatocellular carcinoma (HCC) subtypes were not included in our results. This was because histological data were not consistently available for all patients, and the diagnosis was based on clinical and radiological evidence.
In this retrospective study, 71 cases of HCC were identified at a national public health hospital of significant importance. The male-to-female ratio was 1.15, with the average age of 67 years. This male predisposition is a tendency that has already been demonstrated in epidemiological and survival studies, with some studies reporting ratios as high as 2 to 1 [13,14]. At 5 years, HCC has an overall 5-year survival rate of 18%, with reported geographic variations. In Europe, the United Kingdom has one of the highest 5-year survival rates, at 12.1%. In the United States, this rate reaches 8.1% over the same period [14,15]. In Latin America, Argentina reports a 36% survival rate, while Colombia and Chile report average survival times of 90.5 and 6.3 months, respectively.
Although a higher proportion of deaths occurred in patients with cirrhosis, we found no significant association between cirrhosis and 3-year survival. This lack of statistical significance may be due to the small sample size of non-cirrhotic patients in our study.
Among the laboratory tests, levels of albumin, lymphocytes, INR, and total bilirubin were associated with first-year survival. In other studies, various tumor parameters, such as size or vascular invasion, were associated with albumin levels below 30.5 mg/dL, suggesting that lower albumin levels were correlated with more aggressive tumors, thereby indicating a poorer prognosis [16,17]. In this study, alpha-fetoprotein (AFP) levels were not associated with mortality; however, there was a statistically significant difference between the survival and death groups in the first, second, and third years (Supplementary Table S2). This finding aligns with previous reports indicating that levels exceeding 1000 ng/mL are associated with a poorer prognosis and higher recurrence rates [18].
Regarding staging, the majority of our patients (63.4%) were classified as stage A or B, according to the BCLC staging system, making them candidates for curative treatment. This staging significantly correlated with survival time. However, only 7% of patients underwent curative treatments, including liver transplantation or surgical resection. Notably, a high percentage (12.7%) did not receive any treatment, and approximately one-third were diagnosed at an advanced stage, requiring only palliative care.
We did not include an analysis of the factors that could explain delays in accessing treatment. A large retrospective study conducted in the late 1990s and early 2000s showed that low socioeconomic status was associated with reduced survival rates. This study also found that this trend has been increasing over time [19].
A key variable to consider is that our hospital is public and of national reference that offers specialized care at the third level; consequently, the vast majority of patients in this study were referred from various healthcare systems across Ecuador, taking into account that the administrative cooperation process between healthcare levels leads to significant diagnostic delays. Primary care facilities lack the resources for a definitive liver tumor diagnosis and must refer patients to an intermediate level for imaging and laboratory tests. Without having an anatomopathological study of a biopsy, there is a significant time delay for an efficient diagnosis of cancer. This issue is likely prevalent across Latin America, revealing a clear disparity in diagnosis and treatment of HCC. Argentina and Brazil found that 25% and 86% of patients were unaware of their chronic liver disease before HCC diagnosis. It shows that the first step to improve our results in HCC management is to identify the susceptible population who needs to be included in surveillance programs [20]. However, a multicenter cohort study in the United States shows that just 14% of patients received semiannual surveillance and 22.3% received annual surveillance [21]. Therefore, perhaps globally, we need to better identify patients and standardize not only screening methods but also their access. An analysis of the social determinants of health indicates that patients from low-income areas exhibit both a higher incidence of HCC and lower survival rates, compared to the general population. This disparity is partially attributable to limited access to healthcare, as previously mentioned, as well as several contributing factors such as inadequate health education, failure to adhere to liver pathology screening programs, and significant delays in obtaining six-month abdominal ultrasound appointments as per a scarcity of hepatology specialists at the second level of care. Ultimately, these issues have a negative impact on the overall management of liver cancer.
Access to healthcare systems or health insurance has a significant impact on HCC outcomes. This analysis showed a median overall survival of 34 months for patients with private health insurance, compared to 9 months for those without it [22].
Among the patients who received treatment, although a significant correlation was not observed between treatment type and survival, those who underwent transarterial chemoembolization (TACE) showed higher survival rates. The absence of statistical significance could also be attributed to the small sample size. Local-regional treatment is available worldwide and includes transarterial chemotherapy and radioembolization (TARE). Even though TACE is the most widely used treatment, TARE appears to be a promising therapy with a positive impact on overall survival in patients with intermediate-stage HCC and also in patients with advanced-stage HCC, but with limited extension to survival [23,24]. It is worth mentioning that the use of chemotherapeutic agents such as lenvatinib or atezolizumab plus bevacizumab has shown a positive effect on the survival of patients undergoing conversion therapy, especially those with BCLC stage B [25]. Furthermore, no stage reduction was reported among our patients.
Patients in whom liver lesions were identified and diagnosed with HCC following active surveillance protocols had better survival rates than those whose diagnosis was prompted by clinical manifestations (Supplementary Table S1). This finding is consistent with the ones presented by a meta-analysis of 47 studies, where 50.8% of patients undergoing surveillance survived for 3 years, compared to 28.2% of patients who were not monitored [26]. Unfortunately, the lack of a national HCC surveillance guide prevents different health systems from identifying which patients need to be screened for this tumor and how often.
This outcome also reinforces the importance of a multidisciplinary approach to patients with liver injury. Such an approach, which extends beyond a mere protocol, facilitates earlier and more accurate diagnoses, leading to a longer life expectancy [27]. The benefits of this approach are evident even in patients with poor liver function, elevated alpha-fetoprotein (AFP) levels, and advanced tumor stages [28].
The involvement of a multidisciplinary committee has been associated with improved outcomes in other types of gastrointestinal cancer, including lower recurrence rates and enhanced disease-free survival [29]. Thirty years ago, the Calman–Hein report recommended restructuring cancer services to include multidisciplinary management and ensure equitable access to care for the cancer population [30].
5. Conclusions
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide. The epidemiology of chronic liver disease risk factors is changing, particularly due to the growing obesity epidemic and the prevalence of metabolically associated steatosis liver disease (MASLD). This study found that men, with an average age of approximately 67 years, had the highest rates of HCC and the diagnosis was more frequently made after patients presented with symptoms rather than through surveillance protocols for cirrhotic patients. Our median overall survival was 13.86 months. We observed better outcomes in patients who received TACE within the first 500 days of follow-up. The most important limitation of our study is the sample’s size. The sample included just 71 patients, despite the study being conducted in a national reference center. This small sample limits the generalization of the results. Further studies are needed to validate these findings and explore other contributing factors, such as treatment delay.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life15101565/s1, Figure S1: Kaplan-Meier survival analysis by treatment; Table S1: Annual survival and diagnosis by symptomatology; Table S2: Laboratory and survival association; Table S3: Multivariate logistic regression analysis of factors associated with first-year survival in patients with hepatocellular carcinoma.
Author Contributions
Conceptualization, E.C., J.A. (Jaysoom Abarca), J.A. (Johana Acuña), M.A., D.A., C.B., W.C., D.C., M.G., A.M., M.P., D.Q., M.Q., J.F.S., F.T., C.T. and G.V.; Methodology, E.C. and D.G.; Formal Analysis, D.G.; Investigation, J.A. (Johana Acuña); Resources, M.A., D.A., C.B., W.C., D.C., M.G., A.M., D.Q., M.Q., J.F.S., F.T., C.T. and G.V.; Data Curation, E.C. and J.A. (Johana Acuña); Writing—Original Draft Preparation, E.C. and J.A. (Johana Acuña); Writing—Review and Editing, E.C., J.A. (Johana Acuña) and D.G.; Visualization, E.C.; Supervision, E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Guidelines for the Prevention, Diagnosis, Care and Treatment for People with Chronic Hepatitis B Infection; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/376353/9789240090903-eng.pdf?sequence=1 (accessed on 15 March 2025).
- Grupo Editorial Gráficas Amaranta. Sociedad de Lucha contra el Cáncer/Registro Nacional de Tumores. In Epidemiología del Cáncer en Quito 2015–2019, 17th ed.; Grupo Editorial Gráficas Amaranta: Quito, Ecuador, 2024; p. 152. [Google Scholar]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 112, 1301–1314. [Google Scholar] [CrossRef]
- Farah, M.; Díaz-Ferrer, J.; Liza-Baca, E.; Mattos, Z.A.; Carrera, E. Changing epidemiology of hepatocellular carcinoma in South America: A report from the South American liver research network. Ann. Hepatol. 2022, 28, 100876. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fábrega, J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Grandhi, M.S.; Kim, A.K.; Ronnekleiv-Kelly, S.M.; Ghasebeh, M.A.; Pawlik, T.M. Hepatocellular carcinoma: From diagnosis to treatment. Surg. Oncol. 2016, 25, 74–85. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.-P.; Lee, W.J.; Meloni, M.F.; Clevert, D.-A.; Chammas, M.C.; Tannapfel, A.; Forgione, A.; Piscaglia, F.; Dietrich, C.F. Hepatocellular carcinoma in the non-cirrhotic liver. Clin. Hemorheol. Microcirc. 2022, 80, 423–436. [Google Scholar] [CrossRef]
- Altshuler, E.; Richhart, R.; Aryan, M.; King, W.; Pan, K.; Mathavan, A.; Mathavan, A.; Rodriguez, D.; Paudel, B.; Northern, N.; et al. Advanced Hepatocellular Carcinoma in Adults Without Cirrhosis: A Single-Institution Retrospective Review. J. Hepatocell. Carcinoma 2022, 9, 1299–1307. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.M. Primary malignant tumours in the non-cirrhotic liver. Eur. J. Radiol. 2017, 95, 349–361. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Zhang, X.; Thrift, A.P.; El-Serag, H.B. Predictors of five-year survival among patients with hepatocellular carcinoma in the United States: An analysis of SEER-Medicare. Cancer Causes Control 2021, 32, 317–325. [Google Scholar] [CrossRef]
- Beecroft, S.; O’Connell, M.; Nassar, A.; Noon, K.; Pollock, K.G. Major variation in hepatocellular carcinoma treatment and outcomes in England: A retrospective cohort study. Front. Gastroenterol. 2022, 14, 19–24. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Y.; Zhang, D. Molecular mechanism of albumin in suppressing invasion and metastasis of hepatocellular carcinoma. Liver Int. 2022, 42, 696–709. [Google Scholar] [CrossRef]
- Carr, B.I.; Guerra, V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int. J. Biol. Markers 2017, 32, e391–e396. [Google Scholar] [CrossRef]
- Piñero, F.; Dirchwolf, M.; Pessôa, M.G. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020, 9, 1370. [Google Scholar] [CrossRef]
- Guo, A.; Pomenti, S.; Wattacheril, J. Health Disparities in Screening, Diagnosis, and Treatment of Hepatocellular Carcinoma. Clin. Liver Dis. 2021, 17, 353–358. [Google Scholar] [CrossRef]
- Pages, J.; Mendizabal, M.; Castro-Narro, G.; Menéndez, J.M.; Beltran, O.; Da Fonseca, L.G.; Teller, J.P.; Girala, M.; Cano, D.F.A.; Piñero, F. Hepatocellular carcinoma surveillance: Current challenges in Latin America. Ann. Hepatol. 2025, 30, 101935. [Google Scholar] [CrossRef]
- Parikh, N.D.; Tayob, N.; Al-Jarrah, T.; Kramer, J.; Melcher, J.; Smith, D.; Marquardt, P.; Liu, P.-H.; Tang, R.; Kanwal, F.; et al. Barriers to Surveillance for Hepatocellular Carcinoma in a Multicenter Cohort. JAMA Netw. Open 2022, 5, e2223504. [Google Scholar] [CrossRef]
- Kronenfeld, J.P.; Goel, N. An Analysis of Individual and Contextual-Level Disparities in Screening, Treatment, and Outcomes for Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 1209–1219. [Google Scholar] [CrossRef]
- Debes, J.D.; Chan, A.J.; Balderramo, D.; Kikuchi, L.; Gonzalez Ballerga, E.; Prieto, J.E.; Tapias, M.; Idrovo, V.; Davalos, M.B.; Cairo, F.; et al. Hepatocellular carcinoma in South America: Evaluation of risk factors, demographics and therapy. Liver Int. 2018, 38, 136–143. [Google Scholar] [CrossRef]
- Rognoni, C.; Ciani, O.; Sommariva, S.; Facciorusso, A.; Tarricone, R.; Bhoori, S.; Mazzaferro, V. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: Systematic review and meta-analyses. Oncotarget 2016, 7, 72343–72355. [Google Scholar] [CrossRef]
- Tomonari, T.; Tani, J.; Sato, Y.; Tanaka, H.; Tanaka, T.; Taniguchi, T.; Kawano, Y.; Morishita, A.; Okamoto, K.; Sogabe, M.; et al. Clinical Features and Outcomes of Conversion Therapy in Patients with Unresectable Hepatocellular Carcinoma. Cancers 2023, 15, 5221. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Yopp, A.C.; Mansour, J.C.; Beg, M.S.; Arenas, J.; Trimmer, C.; Reddick, M.; Pedrosa, I.; Khatri, G.; Yakoo, T.; Meyer, J.J.; et al. Establishment of a Multidisciplinary Hepatocellular Carcinoma Clinic is Associated with Improved Clinical Outcome. Ann. Surg. Oncol. 2014, 21, 1287–1295. [Google Scholar] [CrossRef]
- Sinn, D.H.; Choi, G.-S.; Park, H.C.; Kim, J.M.; Kim, H.; Song, K.D.; Kang, T.W.; Lee, M.W.; Rhim, H.; Hyun, D.; et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS ONE 2019, 14, e0210730. [Google Scholar] [CrossRef]
- Mangone, L.; Zizzo, M.; Nardecchia, M.; Marinelli, F.; Bisceglia, I.; Braghiroli, M.B.; Banzi, M.C.; Damato, A.; Cerullo, L.; Pellegri, C.; et al. Impact of Multidisciplinary Team Management on Survival and Recurrence in Stage I–III Colorectal Cancer: A Population-Based Study in Northern Italy. Biology 2024, 13, 928. [Google Scholar] [CrossRef]
- Morris, E.; Haward, R.A.; Gilthorpe, M.S.; Craigs, C.; Forman, D. The impact of the Calman-Hine report on the processes and outcomes of care for Yorkshire’s breast cancer patients. Ann. Oncol. 2008, 19, 284–291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).