Pro-Angiogenic and Wound-Healing Potential of Bioactive Polysaccharides Extracted from Moroccan Algae Osmundea pinnatifida
Abstract
1. Introduction
2. Material and Methods
2.1. Algae Collection and Identification
2.2. Extraction of the Polysaccharide-Rich Osmundea Pinnatifida (PSOP)
2.3. Chemical Characterization
2.4. Polysaccharides Spectroscopic Analysis
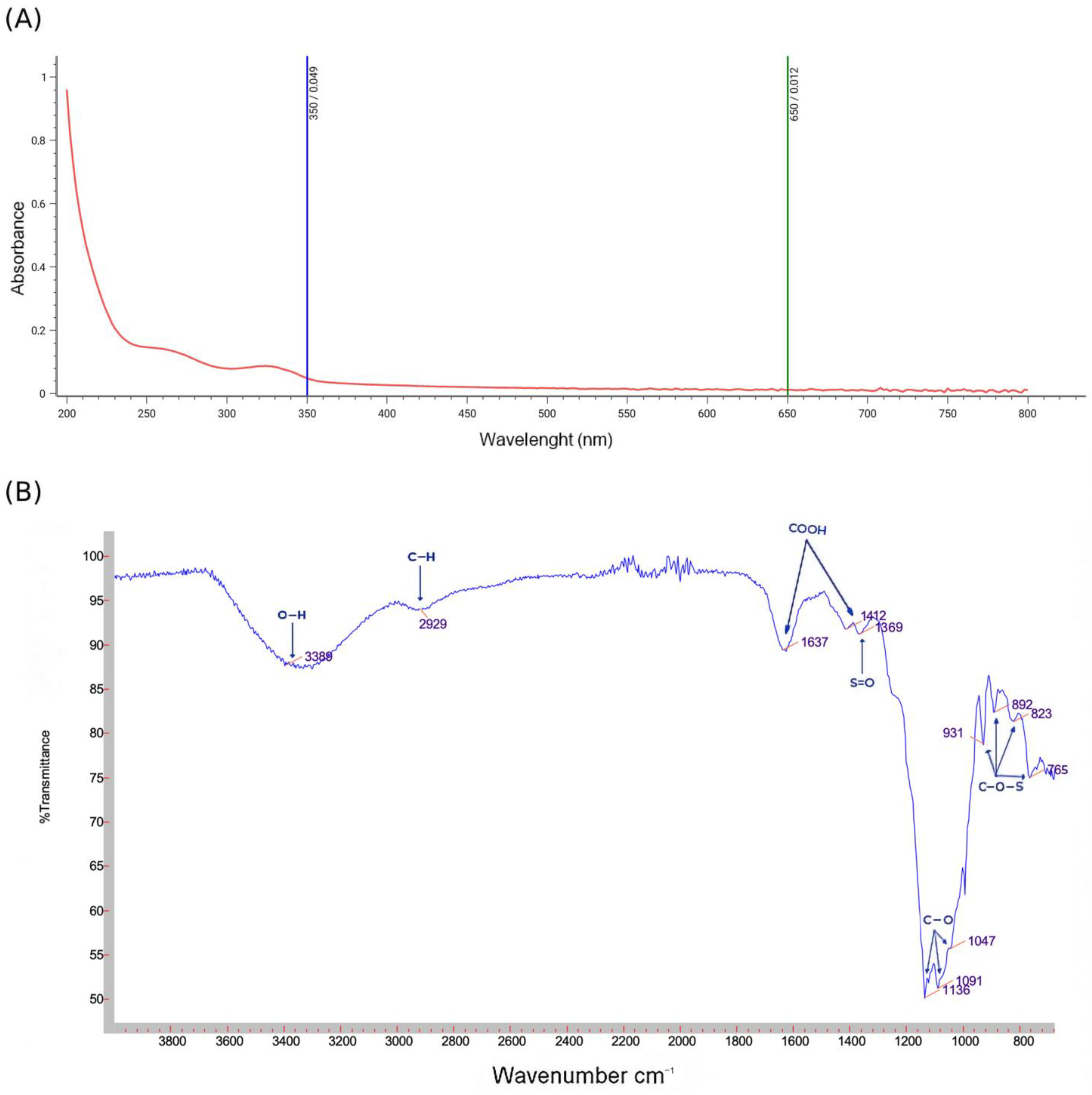
2.4.1. Ultraviolet Absorption Peak Detection
2.4.2. Fourier Transform Infrared Spectrometry Analysis
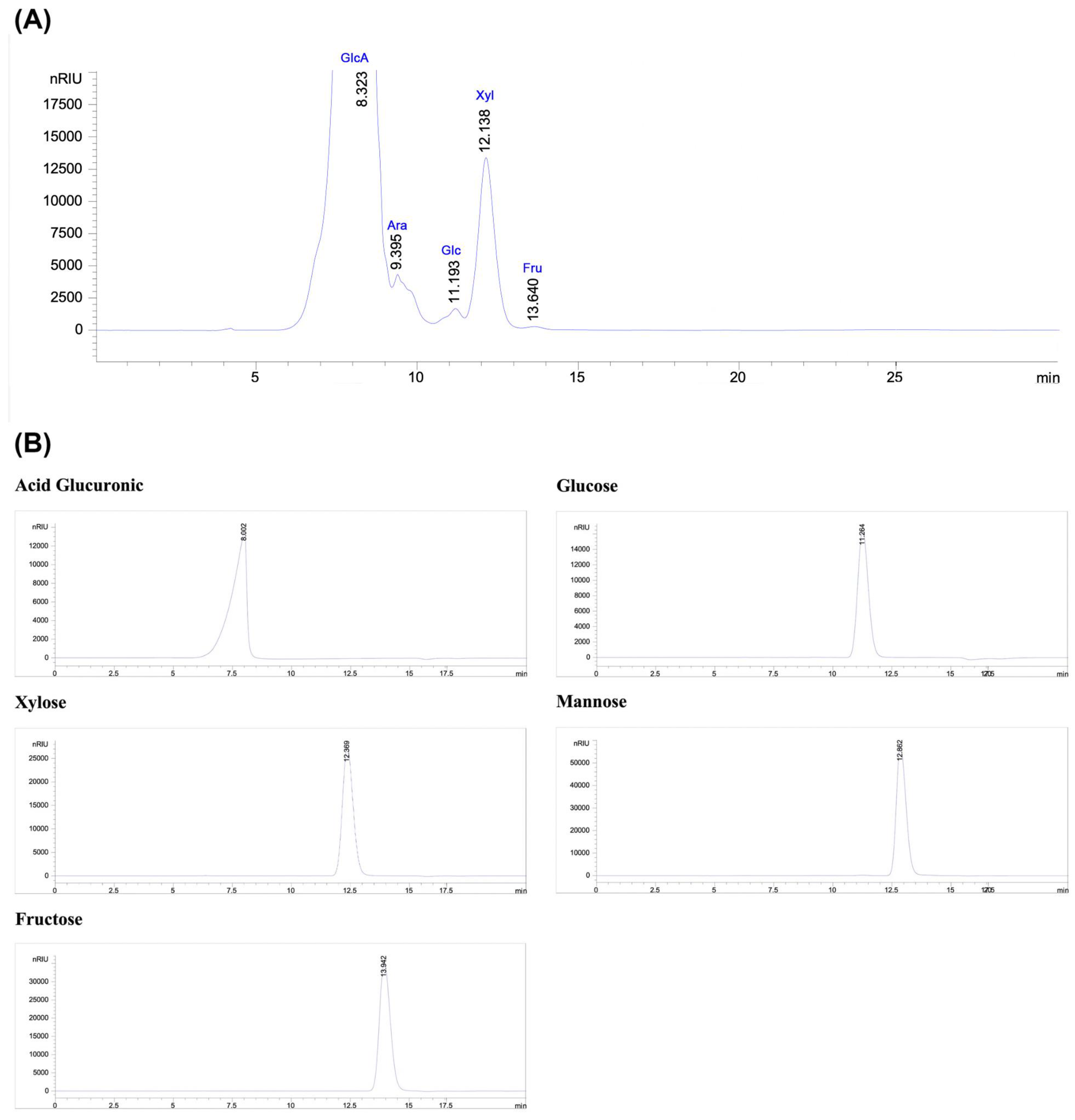
2.4.3. Monosaccharide Analysis by High-Performance Liquid Chromatography (HPLC-RID)
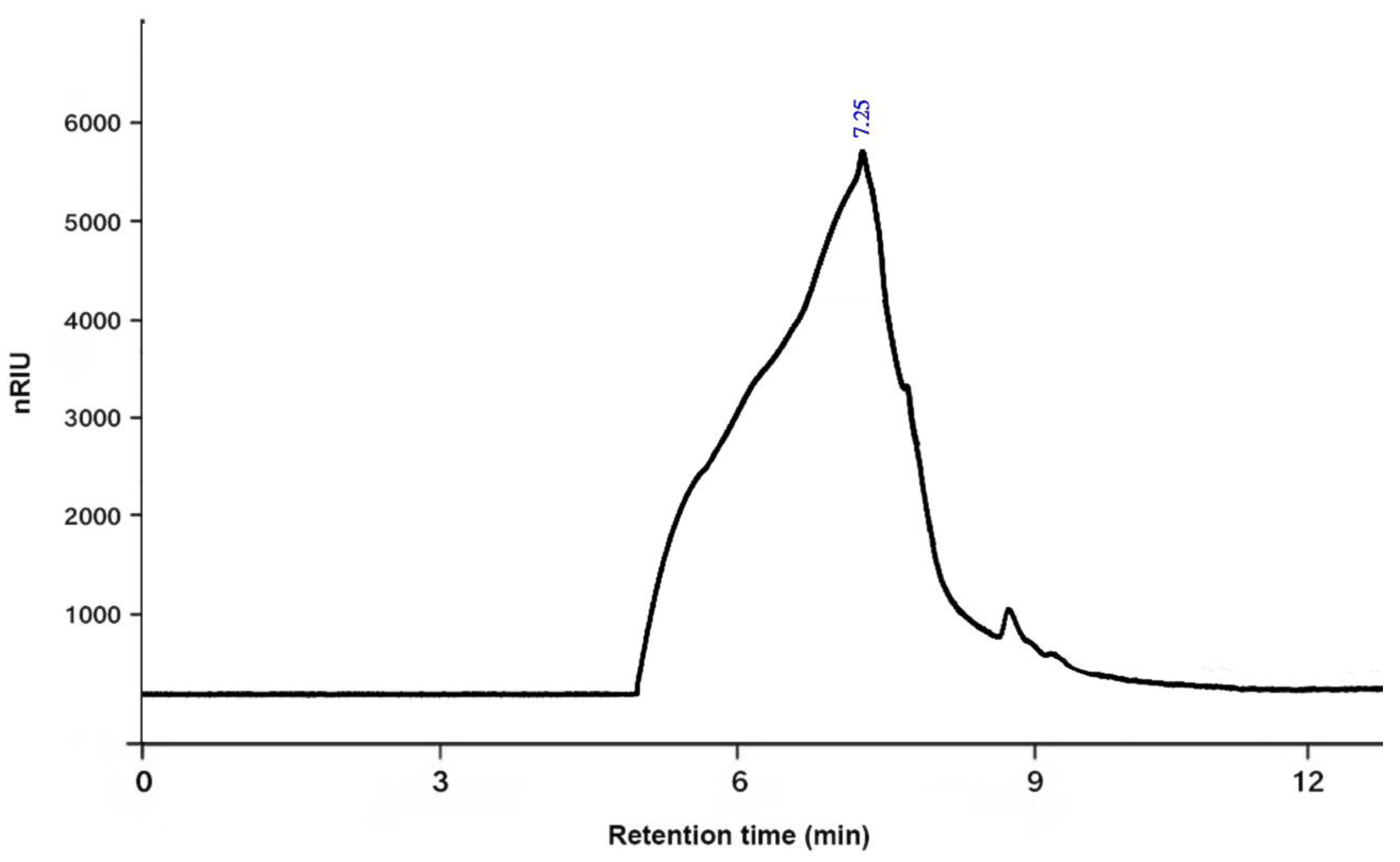
2.4.4. The Average Molecular Weight
2.4.5. Scanning Electron Microscopy
2.4.6. X-Ray Diffraction
2.5. Antioxidant Activity of Polysaccharide
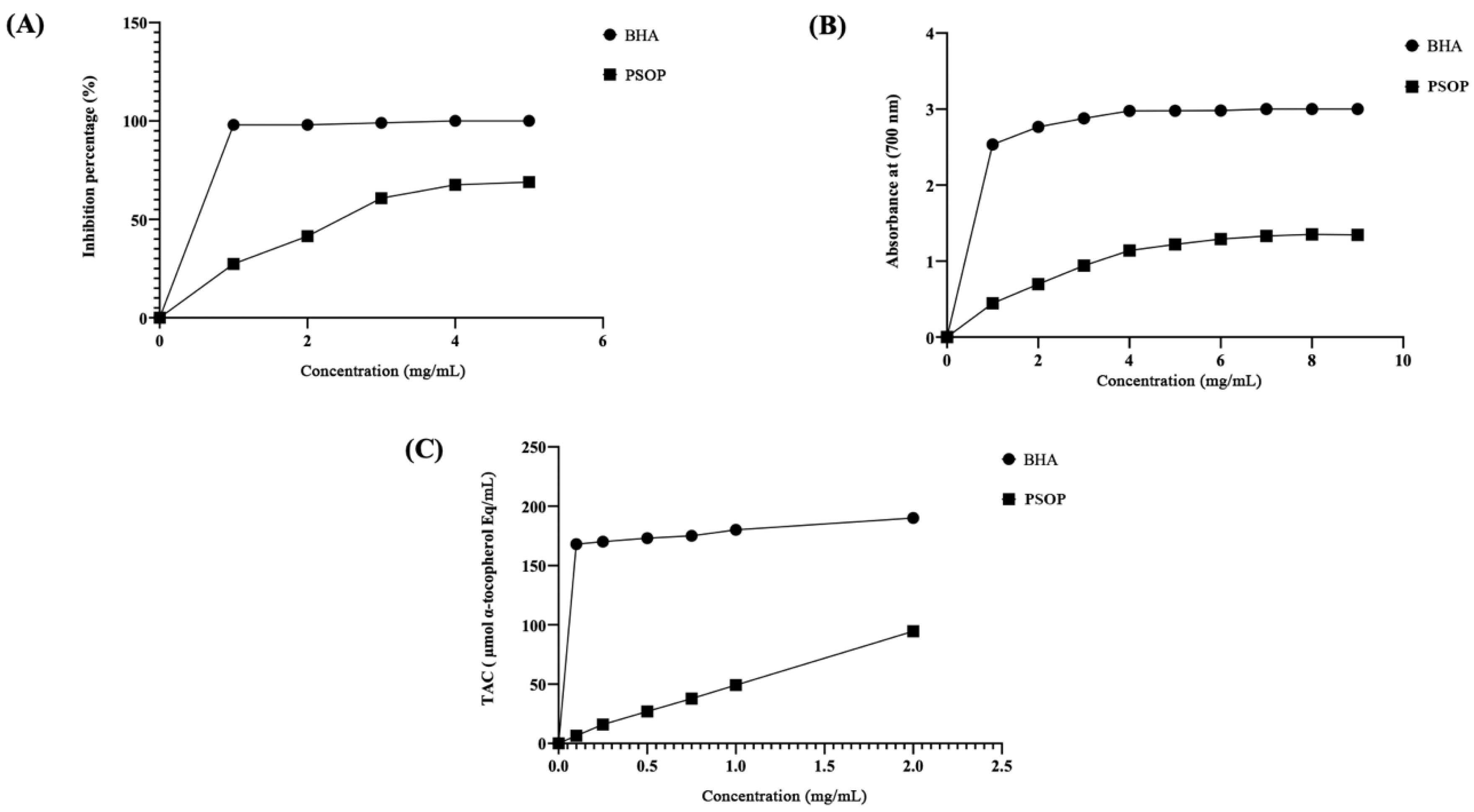
2.5.1. DPPH Free Radical Scavenging Assay
2.5.2. Total Antioxidant Capacity
2.5.3. Reducing Power Assay
2.6. Evaluation of Angiogenic Activity
- Group 1 (Control): Sterile distilled water was applied.
- Group 2 (Negative Control): Diclofenac (DIC) at 50 μg/egg.
- Group 3 (Positive Control): Choriogonadotropin (CG) at 30 μg/egg.
- Group 4: PSOP at 25 μg/egg.
- Group 5: PSOP at 50 μg/egg.
- Group 6: PSOP at 100 μg/egg.
2.7. Quantitative Analysis of Vascular Network
2.8. In Vivo Evaluation of Wound-Healing Activity in Rats
2.8.1. Animals
2.8.2. Experimental Protocol
- Group 1 (Control): Treated with sterile saline (0.9% NaCl).
- Group 2 (Reference Drug): Treated with Cytol Centella, a commercially available medicated cream.
- Group 3 (PSOP Hydrogel): Treated with PSOP hydrogel prepared by mixing lyophilized PSOP in 30% glycerol to achieve a final 15 mg/mL concentration.
- Group 4 (Vehicle): Treated with 30% glycerol.
2.8.3. Measurement of Wound Area and Burn Contraction Rate
2.8.4. Determination of Hydroxyproline Content
2.8.5. Histological Examination
2.9. Computational Modeling and Interactions Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of PSOP
3.2. Spectroscopic Analysis and Monosaccharide Composition
3.3. Antioxidant Activity of PSOP
3.4. Pro-Angiogenic Effect of PSOP
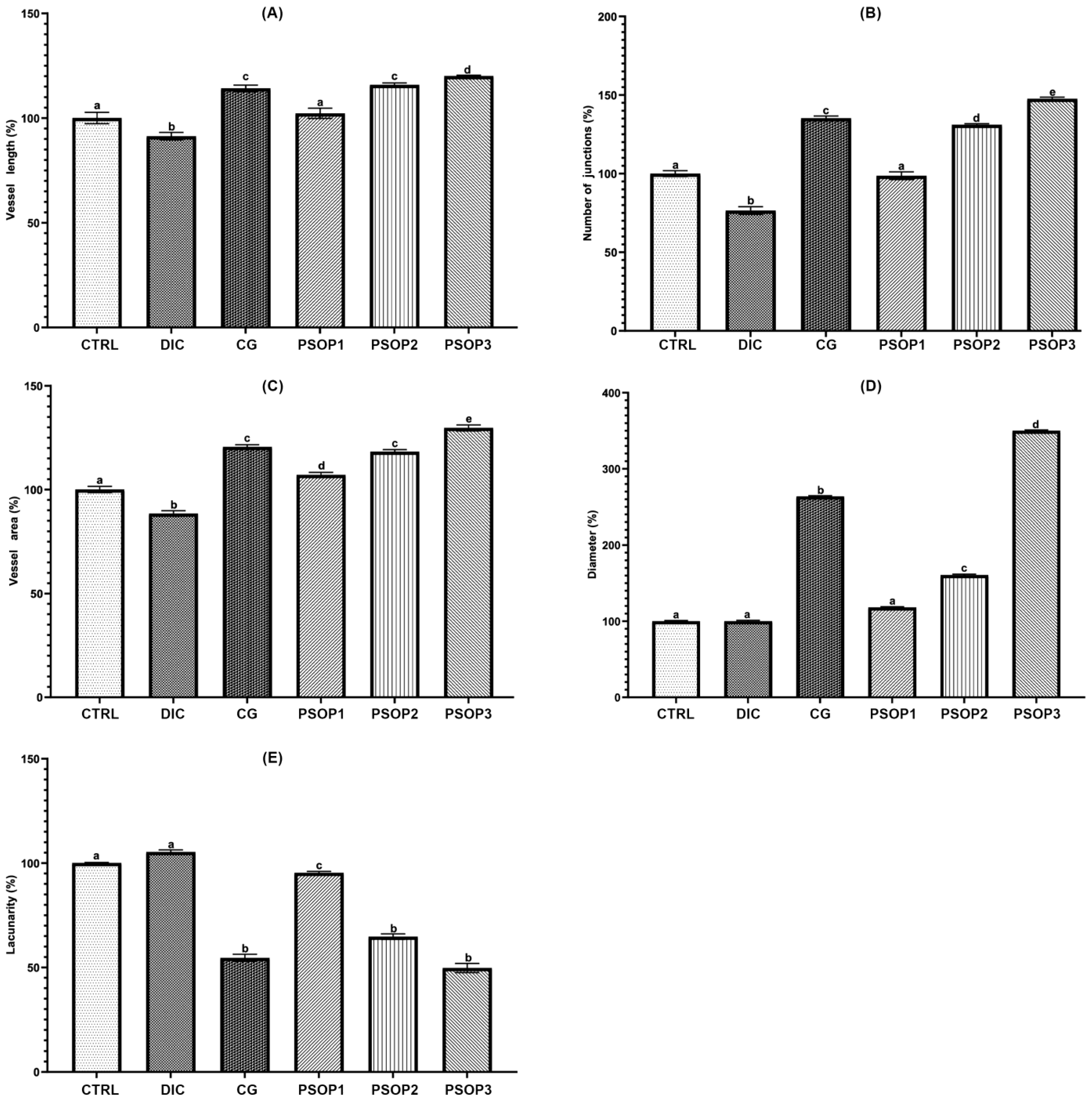
3.5. Quantitative Analysis of Vascular Network
3.6. Evaluation of the Wound-Healing Activities of PSOP In Vivo
3.6.1. Morphological Evaluation
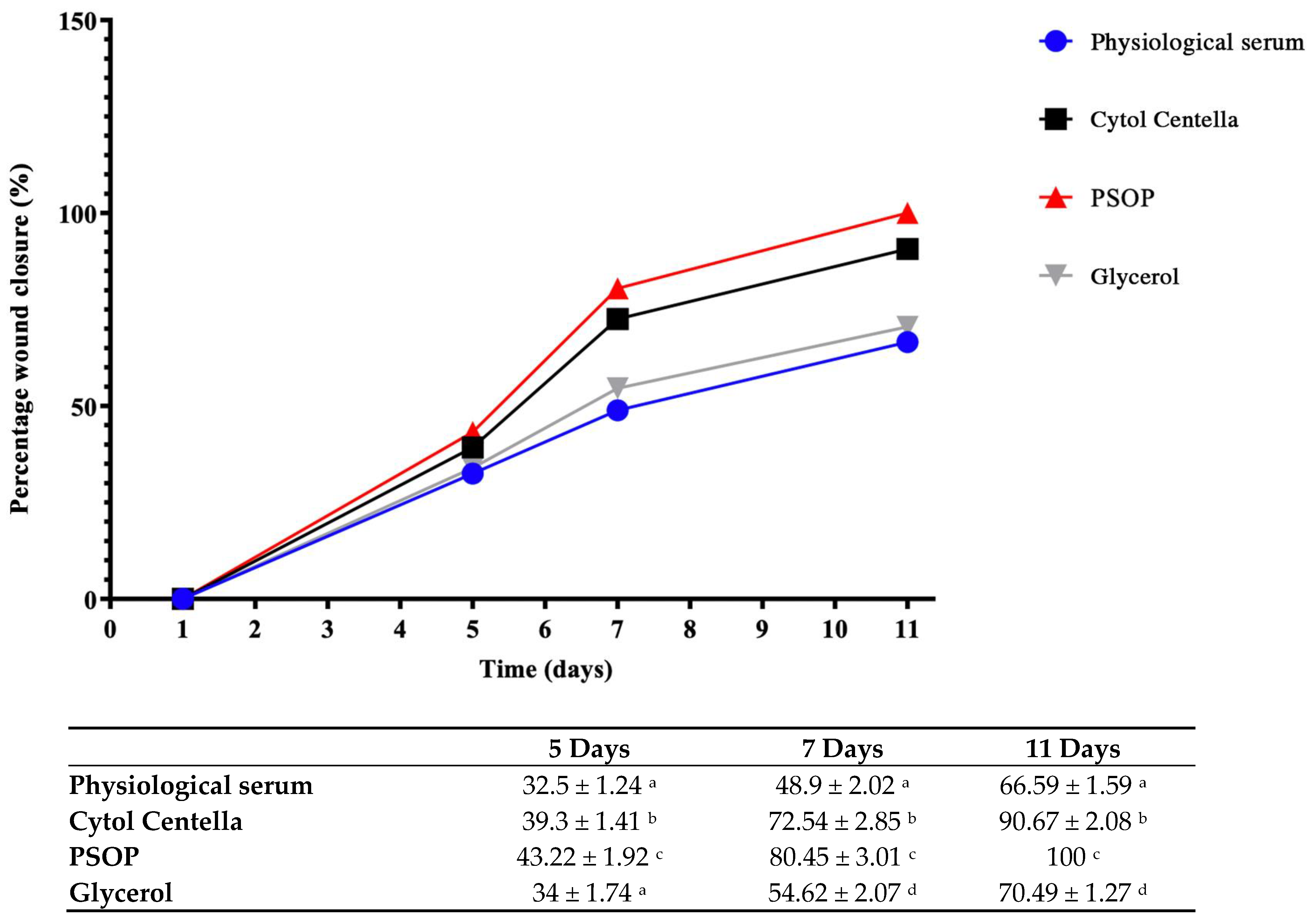
3.6.2. Assessment of Wound Area
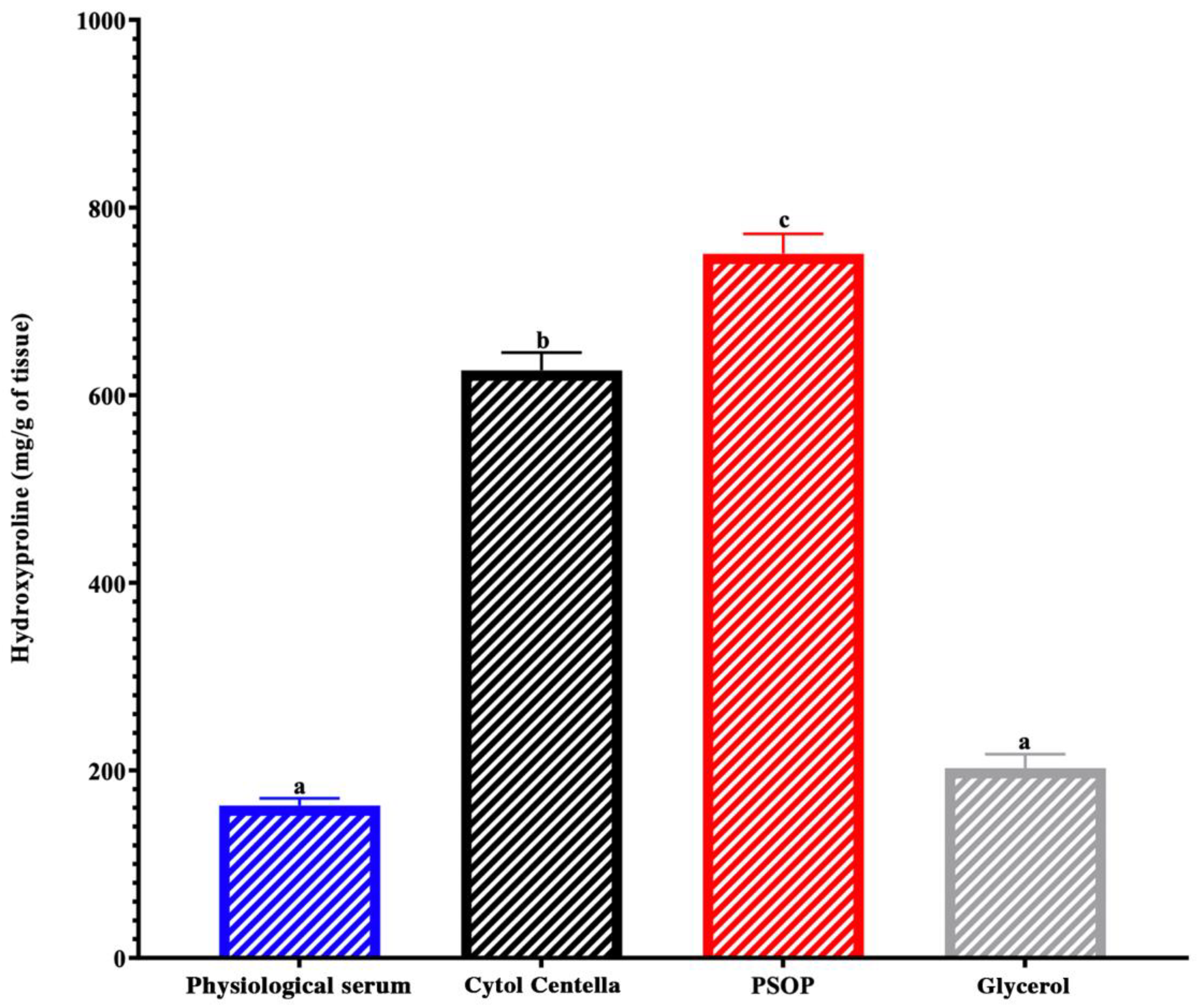
3.6.3. Hydroxyproline and Collagen Regeneration
3.6.4. Histological Evaluation
3.7. Computational Findings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Ju, L.S.; Irudayaraj, J. Oxygenated Wound Dressings for Hypoxia Mitigation and Enhanced Wound Healing. Mol. Pharm. 2023, 20, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Bhat, G.P.; Maurizio, A.; Motta, A.; Podini, P.; Diprima, S.; Malpighi, C.; Brambilla, I.; Martins, L.; Badaloni, A.; Boselli, D.; et al. Structured Wound Angiogenesis Instructs Mesenchymal Barrier Compartments in the Regenerating Nerve. Neuron 2024, 112, 209–229.e11. [Google Scholar] [CrossRef] [PubMed]
- Mota, F.A.R.; Passos, M.L.C.; Santos, J.L.M.; Saraiva, M.L.M.F.S. Comparative Analysis of Electrochemical and Optical Sensors for Detection of Chronic Wounds Biomarkers: A Review. Biosens. Bioelectron. 2024, 251, 116095. [Google Scholar] [CrossRef] [PubMed]
- Rathna, R.P.; Kulandhaivel, M. Advancements in Wound Healing: Integrating Biomolecules, Drug Delivery Carriers, and Targeted Therapeutics for Enhanced Tissue Repair. Arch. Microbiol. 2024, 206, 199. [Google Scholar] [CrossRef]
- Boujhoud, Z.; Feki, A.; Eleroui, M.; Lakhram, M.; Kraiem, M.; Dghim, A.; Zeroual, A.; Youlyouz Marfak, I.; Essayagh, S.; Hilali, S.; et al. The Anti-Angiogenic, Anti-Inflammatory and Anticoagulant Potential of a Polysaccharide Extracted from the Brown Alga Cystoseira humilis. Eur. Polym. J. 2024, 220, 113461. [Google Scholar] [CrossRef]
- Peipei, L.; Qinghong, Z.; Yin, C.; Pengfei, H.; Junjie, Z. Structure and Anticoagulant Activity of a Galactoarabinan Sulfate Polysaccharide and Its Oligosaccharide from the Green Algae, Codium fragile. Int. J. Biol. Macromol. 2024, 279, 135255. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Chondrus Crispus Treated with Ultrasound as a Polysaccharides Source with Improved Antitumoral Potential. Carbohydr. Polym. 2021, 273, 118588. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Yang, M.; Ding, J.; Huang, Y.; Zhu, Y.; Zhou, M.; Yan, B. Antiviral Activity of a Polysaccharide from Sargassum Fusiforme against Respiratory Syncytial Virus. Int. J. Biol. Macromol. 2024, 279, 135267. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.; Wu, J.; Zhao, J. Modifications of Polysaccharide-Based Biomaterials under Structure-Property Relationship for Biomedical Applications. Carbohydr. Polym. 2021, 266, 118097. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-Derived Polysaccharides and Their Therapeutic Potential in Wound Healing Application-A Review. Int. J. Biol. Macromol. 2023, 253, 127331. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, Antibacterial and in Vivo Wound Healing Properties of Laminaran Purified from Cystoseira Barbata Seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, D.W.; Park, C.W.; Kim, B.; Sim, H.; Kim, H.S.; Lee, T.-K.; Lee, J.-C.; Yang, G.E.; Her, Y.; et al. Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice. Mar. Drugs 2020, 18, 345. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Biancacci, C.; Abell, R.; McDougall, G.J.; Day, J.G.; Stanley, M.S. Annual Compositional Variation in Wild Osmundea Pinnatifida (Hudson) Stackhouse from the West Coast of Scotland. J. Appl. Phycol. 2022, 34, 1661–1675. [Google Scholar] [CrossRef]
- Silva, P.; Pereira, L. Concise Review of Osmundea Pinnatifida (Hudson) Stackhouse. J. Appl. Phycol. 2020, 32, 2761–2771. [Google Scholar] [CrossRef]
- Moreira, B.R.; Vega, J.; Sisa, A.D.A.; Bernal, J.S.B.; Abdala-Díaz, R.T.; Maraschin, M.; Figueroa, F.L.; Bonomi-Barufi, J. Antioxidant and Anti-Photoaging Properties of Red Marine Macroalgae: Screening of Bioactive Molecules for Cosmeceutical Applications. Algal Res. 2022, 68, 102893. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of Enzyme- and Ultrasound-Assisted Extraction Methods on Biological Properties of Red, Brown, and Green Seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- GAYRAL, P. Les Algues de La Cote Atlantique Marocaine. Bull. Soc. Sci. Nat. Phys. Maroc. 1958, 42, 1–34. [Google Scholar]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code). In The Nineteenth International Botanical Congress; Koeltz Botanical Books: Shenzhen, China, 2017; ISBN 978-3-946583-16-5. [Google Scholar]
- Fiches FAO d’Identification Des Especes Pour Les Besoins de La Pêche. Méditerranée et Mer Noire (Zone De Pêche 37), Révision 1, Volume 2. Available online: https://openknowledge.fao.org/items/51d216da-0b06-4f3c-9129-d9159f3f50aa (accessed on 21 November 2024).
- Guiry, M.D. AlgaeBase. World-Wide Electronic Publication. Available online: http://www.algaebase.org (accessed on 7 December 2024).
- Liu, G.; Xu, S.; Chen, L. Chemical Composition and Bioactivities of a Water-Soluble Polysaccharide from the Endodermis of Shaddock. Int. J. Biol. Macromol. 2012, 51, 763–766. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Mazuelos, C.; Hermosin, B.; Saiz-Jimenez, C. Chemical Composition ofSpirulina and Eukaryotic Algae Food Products Marketed in Spain. J. Appl. Phycol. 1993, 5, 425–435. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Llyod, A.G.; Tudball, N.; Dodgson, K.S. Infrared Studies on Sulphate Esters III. O-Sulphate Esters of Alcohols, Amino Alcohols and Hydroxylated Amino Acids. Biochim. Biophys. Acta 1961, 52, 413–419. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhao, Y.; Zhao, R.; Sun, P. Antioxidant and Antitumor Activities in Vitro of Polysaccharides from E. Sipunculoides. Int. J. Biol. Macromol. 2015, 78, 56–61. [Google Scholar] [CrossRef]
- Shi, J.-J.; Zhang, J.-G.; Sun, Y.-H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.-J. Physicochemical Properties and Antioxidant Activities of Polysaccharides Sequentially Extracted from Peony Seed Dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef]
- Xie, J.-H.; Xie, M.-Y.; Nie, S.-P.; Shen, M.-Y.; Wang, Y.-X.; Li, C. Isolation, Chemical Composition and Antioxidant Activities of a Water-Soluble Polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction et Caractérisation de Trois Polysaccharides Extraits de Cladodes d’ Opuntia ficus Indica. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a Heated Histidine-Glucose Model System. I: Investigation of the Antioxidant Role of Histidine and Isolation of Antioxidants by High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of Antioxidant and Antimicrobial Activities of Rumex crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, Antiproliferative, and Antiangiogenesis Effects of Polyphenol-Rich Seaweed (Sargassum muticum). BioMed Res. Int. 2013, 2013, 604787. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (US). Institutional Animal Care and Use Committee Guidebook; Office of Laboratory Animal Welfare: Maryland, MD, USA, 2002.
- Edwards, C.A.; O’Brien, W.D. Modified Assay for Determination of Hydroxyproline in a Tissue Hydrolyzate. Clin. Chim. Acta 1980, 104, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Badraoui, R.; Allouche, M.; El Ouaer, D.; Siddiqui, A.J.; Ishak, S.; Hedfi, A.; Beyrem, H.; Pacioglu, O.; Rudayni, H.A.; Boufahja, F. Ecotoxicity of Chrysene and Phenanthrene on Meiobenthic Nematodes with a Case Study of Terschellingia longicaudata: Taxonomics, Toxicokinetics, and Molecular Interactions Modelling. Environ. Pollut. 2023, 316, 120459. [Google Scholar] [CrossRef] [PubMed]
- Chira, A.; Kadmi, Y.; Badraoui, R.; Aouadi, K.; Alhawday, F.; Boudaya, M.; Jamoussi, K.; Kallel, C.; El Feki, A.; Kadri, A.; et al. GC-MS/MS Analysis and Wound Repair Potential of Urtica Dioica Essential Oil: In Silico Modeling and In Vivo Study in Rats. Curr. Pharm. Biotechnol 2024, 26, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.; Allouche, M.; Alotaibi, G.S.; Alwthery, N.S.; Al-Subaie, R.A.; Al-Hoshani, N.; Plavan, O.-A.; Selamoglu, Z.; Özdemir, S.; Plavan, G.; et al. Experimental and Computational Assessment of Antiparkinson Medication Effects on Meiofauna: Case Study of Benserazide and Trihexyphenidyl. Mar. Pollut. Bull. 2024, 205, 116668. [Google Scholar] [CrossRef]
- Arunkumar, K.; Sreena, K.S.; Moosa, M.; Mohan, G.; Raja, R. Cytotoxic Characterization of Optically Negative Codium fragile Polysaccharide against HeLa and MCF Cell Lines. Bioact. Carbohydr. Diet. Fibre 2023, 29, 100341. [Google Scholar] [CrossRef]
- Kammoun, I.; Sellem, I.; Ben Saad, H.; Boudawara, T.; Nasri, M.; Gharsallah, N.; Mallouli, L.; Amara, I.B. Potential Benefits of Polysaccharides Derived from Marine Alga Ulva lactuca against Hepatotoxicity and Nephrotoxicity Induced by Thiacloprid, an Insecticide Pollutant. Environ. Toxicol. 2019, 34, 1165–1176. [Google Scholar] [CrossRef]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-Assisted Hydrothermal Extraction of Sulfated Polysaccharides from Ulva Spp. and Monostroma latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Byankina Barabanova, A.O.; Glazunov, V.P.; Yakovleva, I.M.; Yermak, I.M. Seasonal Variations in a Polysaccharide Composition of Far Eastern Red Seaweed Ahnfeltiopsis flabelliformis (Phyllophoraceae). J. Appl. Phycol. 2018, 30, 535–545. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effet Des Conditions d’extraction Sur Le Rendement et La Pureté de l’ulvane Extrait d’ Ulva lactuca. Food Hydrocoll. 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Siddhanta, A.; Goswami, A.M.; Munisamy, S.; Mody, K.H.; Ramavat, B.K.; Mairh, O.P. Sulphated Galactans of Marine Red Alga Laurencia Spp. (Rhodomelaceae, Rhodophyta) from the West Coast of India. Indian J. Mar. Sci. 2002, 31, 305–309. [Google Scholar]
- Hu, W.; Yu, A.; Bi, H.; Gong, Y.; Wang, H.; Kuang, H.; Wang, M. Recent Advances in Artemisia argyi Levl. et Vant. Polysaccharides: Extractions, Purifications, Structural Characteristics, Pharmacological Activities, and Existing and Potential Applications. Int. J. Biol. Macromol. 2024, 279, 135250. [Google Scholar] [CrossRef]
- Yi, Y.; Xu, W.; Wang, H.-X.; Huang, F.; Wang, L.-M. Natural Polysaccharides Experience Physiochemical and Functional Changes during Preparation: A Review. Carbohydr. Polym. 2020, 234, 115896. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated Modification of Polysaccharides: Synthesis, Characterization and Bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Jose, G.M.; Raghavankutty, M.; Kurup, G.M. Sulfated Polysaccharides from Padina Tetrastromatica Induce Apoptosis in HeLa Cells through ROS Triggered Mitochondrial Pathway. Process Biochem. 2018, 68, 197–204. [Google Scholar] [CrossRef]
- Eleroui, M.; Feki, A.; Kraiem, M.; Hamzaoui, A.; Boujhoud, Z.; Amara, I.B.; Kallel, H. Physicochemical, Structural, and Biological Properties of Novel Water-Soluble Polysaccharide Derived from the Tunisian Hammada Scoparia Plant and Its Application on Beef Meat Preservation. Heliyon 2024, 10, e39562. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Zhang, J.; Kang, W. Isolation, Purification, Structural Analysis and Coagulatory Activity of Water-Soluble Polysaccharides from Ligustrum Lucidum Ait Flowers. Chem. Cent. J. 2017, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Eljoudi, S.; Feki, A.; Bkhairia, I.; Barkia, A.; Ben Amara, I.; Nasri, M.; Hajji, M. New Polysaccharides Extracted from Malcolmia triloba: Structure Characterization, Biological Properties and Application to Beef Meat Preservation. J. Food Compos. Anal. 2022, 107, 104380. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R.; et al. Effects of Extraction Methods on the Physicochemical Characteristics and Biological Activities of Polysaccharides from Okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.S.; Meivelu, M.; Jeganathan, A. Structural Characterization and Anticancer Activity of Extracellular Polysaccharides from Ascidian Symbiotic Bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, S.; Xiao, Z.; Zhou, C.; Hong, P.; Qian, Z.-J. Structural Characterization of Sulfated Polysaccharide Isolated From Red Algae (Gelidium crinale) and Antioxidant and Anti-Inflammatory Effects in Macrophage Cells. Front. Bioeng. Biotechnol. 2021, 9, 794818. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Martera, G.; Goñi, I.; Villanueva-Suárez, M.-J.; Redondo-Cuenca, A. Chemical Structure and Molecular Weight Influence the in Vitro Fermentability of Polysaccharide Extracts from the Edible Seaweeds Himathalia elongata and Gigartina pistillata. Food Hydrocoll. 2018, 83, 348–354. [Google Scholar] [CrossRef]
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian Red Seaweed Chondrus Canaliculatus: Structural Characteristics, Antioxidant Activity and in Vivo Hemato-Nephroprotective Properties on Maneb Induced Toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Costa-Pinto, A.R.; Sousa, S.; Vasconcelos, M.W.; Pintado, M.M.; Pereira, L.; Rocha-Santos, T.; Costa, J.P.D.; Silva, A.; Duarte, A.C.; et al. Sargassum Muticum and Osmundea Pinnatifida Enzymatic Extracts: Chemical, Structural, and Cytotoxic Characterization. Mar. Drugs 2019, 17, 209. [Google Scholar] [CrossRef]
- Ferreira, L.G.; da Silva, A.C.R.; Noseda, M.D.; Fuly, A.L.; de Carvalho, M.M.; Fujii, M.T.; Sanchez, E.F.; Carneiro, J.; Duarte, M.E.R. Chemical Structure and Snake Antivenom Properties of Sulfated Agarans Obtained from Laurencia dendroidea (Ceramiales, Rhodophyta). Carbohydr. Polym. 2019, 218, 136–144. [Google Scholar] [CrossRef]
- Hans, N.; Pattnaik, F.; Malik, A.; Naik, S. Comparison of Different Green Extraction Techniques and Their Influence on Chemical Characteristics of Sulfated Polysaccharide (Fucoidan) from Padina tetrastromatica and Turbinaria conoides. Algal Res. 2023, 74, 103199. [Google Scholar] [CrossRef]
- Konstantin, B.; Anastasia, P.; Nikolay, I.; Daria, P. Seasonal Variations in the Chemical Composition of Arctic Brown Macroalgae. Algal Res. 2023, 72, 103112. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of Monosaccharide Composition and Proportion on the Bioactivity of Polysaccharides: A Review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Lee, Q.; Xue, Z.; Luo, Y.; Lin, Y.; Lai, M.; Xu, H.; Liu, B.; Zheng, M.; Lv, F.; Zeng, F. Low Molecular Weight Polysaccharide of Tremella fuciformis Exhibits Stronger Antioxidant and Immunomodulatory Activities than High Molecular Weight Polysaccharide. Int. J. Biol. Macromol. 2024, 281, 136097. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ma, X.; Ren, H.; Fan, W.; Leng, F.; Yang, M.; Wang, X. Extraction, Purification, and Bioactivities Analyses of Polysaccharides from Glycyrrhiza uralensis. Ind. Crops Prod. 2018, 122, 596–608. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.-M.; Xu, S.-Y.; Liu, Y.; Cheong, K.-L. Physicochemical Characterization and Antioxidant Activity of Sulphated Polysaccharides Derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Teng, C.; Li, S.; Xu, L.; Ma, K.; Lu, Y.; Feng, J.; Chai, Z.; Hu, X.; Zhou, W.; Li, Y. Caractérisation Structurale, Propriétés Physicochimiques et Activité Hypoglycémiante Des Polysaccharides Sulfatés de Porphyra yezoensis. Food Biosci. 2024, 62, 105163. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Tabarsa, M.; Abdollahi, M. Ultrasonic-Assisted Enzymatic Extraction of Sulfated Polysaccharide from Skipjack Tuna by-Products. Ultrason. Sonochem. 2023, 95, 106385. [Google Scholar] [CrossRef]
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Habibzadeh, S.; Munir, M.T.; Vahabi, H.; Bagheri, B.; Rabiee, N.; Zarrintaj, P.; Saeb, M.R. Crystalline Polysaccharides: A Review. Carbohydr. Polym. 2022, 275, 118624. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.-D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant Content and Activity of the Seaweed Saccharina Latissima: A Seasonal Perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Hermund, D. Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; Academic Press: New York, NY, USA, 2018; pp. 201–221. ISBN 978-0-12-813312-5. [Google Scholar]
- Kraiem, M.; Ben Hamouda, S.; Eleroui, M.; Ajala, M.; Feki, A.; Dghim, A.; Boujhoud, Z.; Bouhamed, M.; Badraoui, R.; Pujo, J.M.; et al. Anti-Inflammatory and Immunomodulatory Properties of a Crude Polysaccharide Derived from Green Seaweed Halimeda Tuna: Computational and Experimental Evidences. Mar. Drugs 2024, 22, 85. [Google Scholar] [CrossRef]
- Hamzaoui, A.; Ghariani, M.; Sellem, I.; Hamdi, M.; Feki, A.; Jaballi, I.; Nasri, M.; Amara, I.B. Extraction, Characterization and Biological Properties of Polysaccharide Derived from Green Seaweed “Chaetomorpha Linum” and Its Potential Application in Tunisian Beef Sausages. Int. J. Biol. Macromol. 2020, 148, 1156–1168. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, Antioxidant and Antibacterial Activities of Polysaccharides Extracted from Olive Leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Lakhrem, M.; Eleroui, M.; Boujhoud, Z.; Feki, A.; Dghim, A.; Essayagh, S.; Hilali, S.; Bouhamed, M.; Kallel, C.; Deschamps, N.; et al. Anti-Vasculogenic, Antioxidant, and Anti-Inflammatory Activities of Sulfated Polysaccharide Derived from Codium Tomentosum: Pharmacokinetic Assay. Pharmaceuticals 2024, 17, 672. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2015, 2016, 5692852. [Google Scholar] [CrossRef]
- Feki, A.; Cherif, B.; Sellem, I.; Naifar, M.; Ben Amar, I.; Ben Azaza, Y.; Kallel, R.; Hariz, L.; Zeghal, S.; Makni Ayadi, F.; et al. Biomedical Applications of Polysaccharide Derived from Tetrasporophyte Tufts of Asparagopsis armata (Falkenbergia rufolanosa): Focus on Antioxidant, Anti-Inflammatory, Anti-Coagulant and Hepato-Protective Activities. Algal Res. 2023, 69, 102958. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Najjaa, H.; Zouari, N.; Sebai, H.; Nasri, M. Sulfated Polysaccharides from the Viscera of Mustelus Shark: Characterization and Antioxidant, Anticoagulant and Anti-Proliferative Activities. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100399. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Burri, P.H.; Djonov, V. Chorioallantoic Membrane Capillary Bed: A Useful Target for Studying Angiogenesis and Anti-Angiogenesis in Vivo. Anat. Rec. 2001, 264, 317–324. [Google Scholar] [CrossRef]
- Muhammad, O.; Lama, R. The Effect of Diclofenac Sodium on Blood Vessel Formation. MedBioTech J. 2018, 2, 76–81. [Google Scholar] [CrossRef]
- Berndt, S.; Blacher, S.; Perrier d’Hauterive, S.; Thiry, M.; Tsampalas, M.; Cruz, A.; Péqueux, C.; Lorquet, S.; Munaut, C.; Noël, A.; et al. Chorionic Gonadotropin Stimulation of Angiogenesis and Pericyte Recruitment. J. Clin. Endocrinol. Metab. 2009, 94, 4567–4574. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef]
- Yu, R.; Zhong, J.; Zhou, Q.; Ren, W.; Liu, Z.; Bian, Y. Kaempferol Prevents Angiogenesis of Rat Intestinal Microvascular Endothelial Cells Induced by LPS and TNF-α via Inhibiting VEGF/Akt/P38 Signaling Pathways and Maintaining Gut-Vascular Barrier Integrity. Chem.-Biol. Interact. 2022, 366, 110135. [Google Scholar] [CrossRef]
- Mauroux, A.; Gofflo, S.; Breugnot, J.; Malbouyres, M.; Atlas, Y.; Ardidie-Robouant, C.; Marchand, L.; Monnot, C.; Germain, S.; Bordes, S.; et al. Angiogenesis and Full Thickness Wound Repair in a Cell Sheet-Based Vascularized Skin Substitute. Acta Biomater. 2024, 187, 123–137. [Google Scholar] [CrossRef]
- Jozkowicz, A.; Cooke, J.P.; Guevara, I.; Huk, I.; Funovics, P.; Pachinger, O.; Weidinger, F.; Dulak, J. Genetic Augmentation of Nitric Oxide Synthase Increases the Vascular Generation of VEGF. Cardiovasc. Res. 2001, 51, 773–783. [Google Scholar] [CrossRef]
- Keykhaee, M.; Sorouri, F.; Rahimifard, M.; Baeeri, M.; Forumadi, A.; Firoozpour, L.; Khoobi, M. Polysaccharide-Based Hydrogel Enriched by Epidermal Growth Factor Peptide Fragment for Improving the Wound Healing Process. Heliyon 2023, 9, e22749. [Google Scholar] [CrossRef]
- Malektaj, H.; Nour, S.; Imani, R.; Siadati, M.H. Angiogenesis Induction as a Key Step in Cardiac Tissue Regeneration: From Angiogenic Agents to Biomaterials. Int. J. Pharm. 2023, 643, 123233. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-Scavenging Materials for Skin Wound Healing: Advancements and Applications. Front. Bioeng. Biotechnol. 2023, 11, 1304835. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Zheng, Q.; Lu, H.; Huang, H.; Zhang, J.; Fang, Z.; Lin, L.; Ma, P. Recent Progress in the Efficacy of Algal Saccharides on Skin Repair. Algal Res. 2024, 78, 103403. [Google Scholar] [CrossRef]
- Tan, S.T.; Dosan, R. Lessons From Epithelialization: The Reason Behind Moist Wound Environment. Open Dermatol. J. 2019, 13. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef]
- Sun, L.; Li, L.; Wang, Y.; Li, M.; Xu, S.; Zhang, C. A Collagen-Based Bi-Layered Composite Dressing for Accelerated Wound Healing. J. Tissue Viability 2022, 31, 180–189. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-Dependent Regulation of Collagen Metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of Prolyl Hydroxylation in the Molecular Interactions of Collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Cen, Y. Burn Wound Healing Potential of a Polysaccharide from Sanguisorba officinalis L. in Mice. Int. J. Biol. Macromol. 2018, 112, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Zhang, M.; Xiao, Y.; Jin, J.; Qin, T.; Li, Y. Bovine Collagen Oligopeptides Accelerate Wound Healing by Promoting Fibroblast Migration via PI3K/Akt/mTOR Signaling Pathway. J. Funct. Foods 2022, 90, 104981. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Chen, S.-H.; Chen, C.-H.; Chou, P.-Y.; Yang, C.-C.; Lin, F.-H. Polysaccharide Extracted from Bletilla Striata Promotes Proliferation and Migration of Human Tenocytes. Polymers 2020, 12, 2567. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Li, J.; Cheng, Y.; Rangsinth, P.; Wu, X.; Zheng, C.; Shiu, P.H.-T.; Li, R.; Xu, N.; He, Y.; et al. Characterization of a Polysaccharide from Amauroderma rugosum and Its Proangiogenic Activities in Vitro and in Vivo. Int. J. Biol. Macromol. 2024, 271, 132533. [Google Scholar] [CrossRef]
- Chouikhi, A.; Ktari, N.; Bardaa, S.; Hzami, A.; Ben Slima, S.; Trabelsi, I.; Asehraou, A.; Ben Salah, R. A Novel Probiotic Strain, Lactiplantibacillus Plantarum LC38, Isolated from Tunisian Camel Milk Promoting Wound Healing in Wistar Diabetic Rats. Arch. Microbiol. 2021, 204, 24. [Google Scholar] [CrossRef]
- Ben Saad, H.; Frikha, D.; Bouallegue, A.; Badraoui, R.; Mellouli, M.; Kallel, H.; Pujo, J.M.; Ben Amara, I. Mitigation of Hepatic Impairment with Polysaccharides from Red Alga Albidum Corallinum Supplementation through Promoting the Lipid Profile and Liver Homeostasis in Tebuconazole-Exposed Rats. Pharmaceuticals 2023, 16, 1305. [Google Scholar] [CrossRef]
- Rahmouni, F.; Hamdaoui, L.; Saoudi, M.; Badraoui, R.; Rebai, T. Antioxidant and Antiproliferative Effects of Teucrium Polium Extract: Computational and in Vivo Study in Rats. Toxicol. Mech. Methods 2024, 34, 495–506. [Google Scholar] [CrossRef]
- Bédoui, I.; Nasr, H.B.; Ksouda, K.; Ayadi, W.; Louati, N.; Chamkha, M.; Choura, S.; Gargouri, J.; Hammami, S.; Affes, H.; et al. Phytochemical Composition, Bioavailability and Pharmacokinetics of Scorzonera Undulata Methanolic Extracts: Antioxidant, Anticancer, and Apoptotic Effects on MCF7 Cells. Pharmacogn. Mag. 2024, 20, 218–229. [Google Scholar] [CrossRef]




 ulceration,
ulceration,  inflammatory infiltrate,
inflammatory infiltrate,  and fibers of collagen.
and fibers of collagen.
 ulceration,
ulceration,  inflammatory infiltrate,
inflammatory infiltrate,  and fibers of collagen.
and fibers of collagen.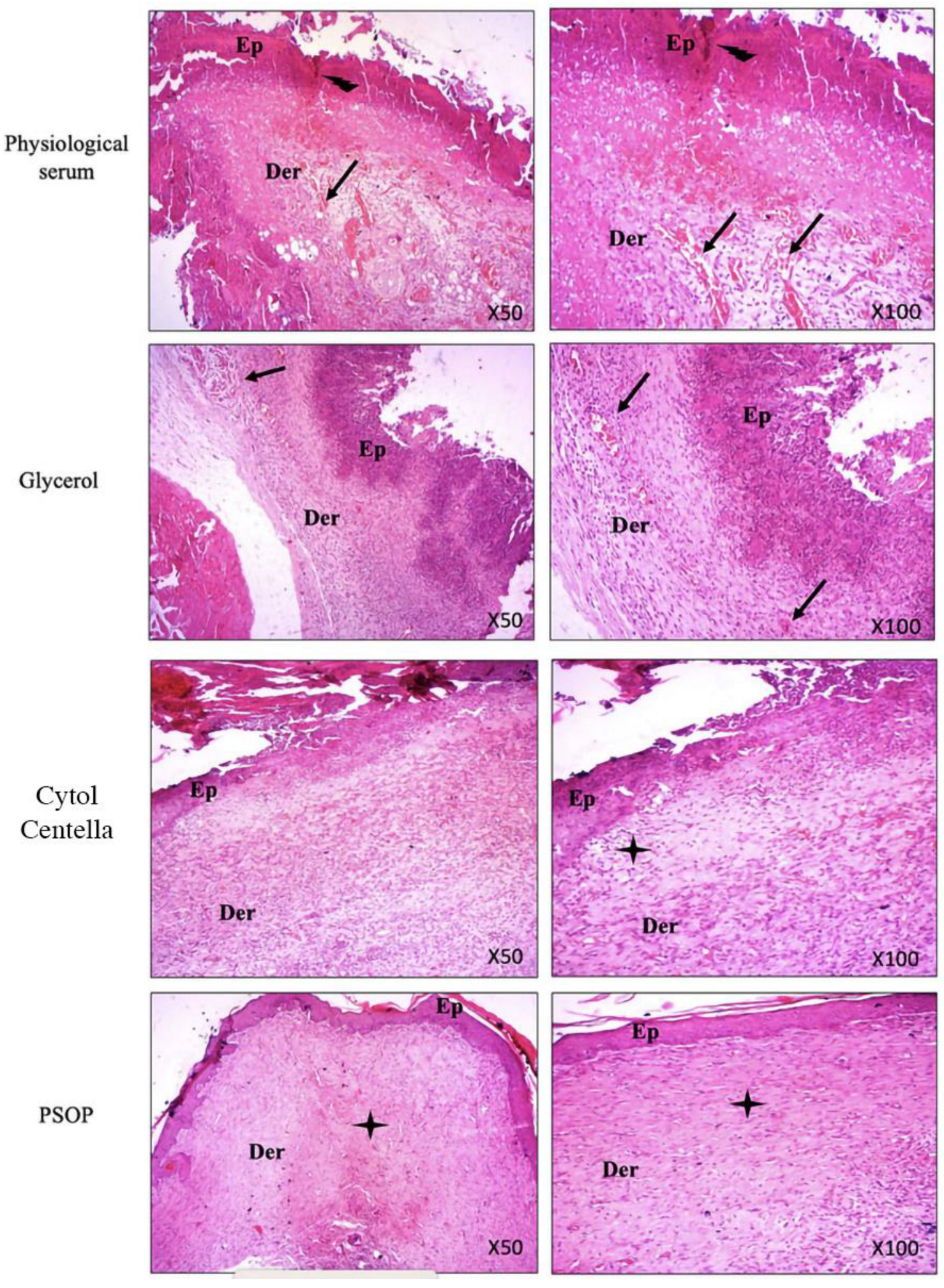

| Groups | Control | Diclofenac (50 μg/egg) | Choriogonadotropin (30 μg/egg) | PSOP (25 μg/egg) | PSOP (50 μg/egg) | PSOP (100 μg/egg) |
|---|---|---|---|---|---|---|
| Vessel Number (%) | 100 a | 71.14 ± 3.97 b | 168.36 ± 2.72 c | 137.22 ± 3.7 d | 200.66 ± 2.73 e | 250.66 ± 3.49 f |
| Compound No. | COX-2 (1cx2) | VEGF (2c7w) |
|---|---|---|
| Binding Affinity (kcal × mol−1) | ||
| Arabinose | −5.7 | −4.5 |
| Fructose | −6.4 | −4.5 |
| Glucose | −6.3 | −4.6 |
| Glucoronic acid | −5.6 | −4.4 |
| Xylose | −6.1 | −4.1 |
| RMSD (lower–upper) | ||
| Arabinose | 0.0–30.13 | 0.0–31.90 |
| Fructose | 0.0–30.82 | 0.0–9.08 |
| Glucose | 0.0–22.70 | 0.0–9.14 |
| Glucoronic acid | 0.0–41.78 | 0.0–14.83 |
| Xylose | 0.0−9.86 | 0.0–30.91 |
| Saccharide | No. of Conventional H-Bonds | Closest Interacting Residues | ||
|---|---|---|---|---|
| Interacting Residues | Closest residue (Distance, Å) | No. of Closest Interacting Residues | ||
| Cyclooxygenase 2 (COX-2) | ||||
| Fructose | 6 | CYS47, ASN39, GLU465, CYS41, GLY45 | GLU465:OE1 (2.072) | 5 |
| Vascular Endothelial growth factor (VEGF) | ||||
| Glucose | 4 | LEU39, THR36, LEU35, LYS45 | LEU39:HN (2.247) | 4 |
| Bold residues: amino acids interacting with conventional H-bond | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boujhoud, Z.; Eleroui, M.; Feki, A.; Saad, H.B.; Kraiem, M.; Youlyouz Marfak, I.; Essayagh, S.; Hilali, S.; Badraoui, R.; Kallel, H.; et al. Pro-Angiogenic and Wound-Healing Potential of Bioactive Polysaccharides Extracted from Moroccan Algae Osmundea pinnatifida. Life 2025, 15, 1564. https://doi.org/10.3390/life15101564
Boujhoud Z, Eleroui M, Feki A, Saad HB, Kraiem M, Youlyouz Marfak I, Essayagh S, Hilali S, Badraoui R, Kallel H, et al. Pro-Angiogenic and Wound-Healing Potential of Bioactive Polysaccharides Extracted from Moroccan Algae Osmundea pinnatifida. Life. 2025; 15(10):1564. https://doi.org/10.3390/life15101564
Chicago/Turabian StyleBoujhoud, Zakaria, Malek Eleroui, Amal Feki, Hajer Ben Saad, Marwa Kraiem, Ibtissam Youlyouz Marfak, Sanah Essayagh, Said Hilali, Riadh Badraoui, Hatem Kallel, and et al. 2025. "Pro-Angiogenic and Wound-Healing Potential of Bioactive Polysaccharides Extracted from Moroccan Algae Osmundea pinnatifida" Life 15, no. 10: 1564. https://doi.org/10.3390/life15101564
APA StyleBoujhoud, Z., Eleroui, M., Feki, A., Saad, H. B., Kraiem, M., Youlyouz Marfak, I., Essayagh, S., Hilali, S., Badraoui, R., Kallel, H., Pujo, J. M., Ben Amara, I., & Hilali, A. (2025). Pro-Angiogenic and Wound-Healing Potential of Bioactive Polysaccharides Extracted from Moroccan Algae Osmundea pinnatifida. Life, 15(10), 1564. https://doi.org/10.3390/life15101564







