Protective Effects of Momordica charantia Fruit Extract on Male Sexual Dysfunction and Testicular Damage in Rats Induced by Chronic Unpredictable Stressors
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. Antioxidant Capacity Assays
2.2.1. Total Phenolic Content Estimation
2.2.2. Flavonoid Content Determination
2.2.3. The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Capacity Assay
Trolox Equivalent
Inhibitory Concentration at 50% (IC50)
2.2.4. Ferric Reducing Antioxidant Power (FRAP) Assay
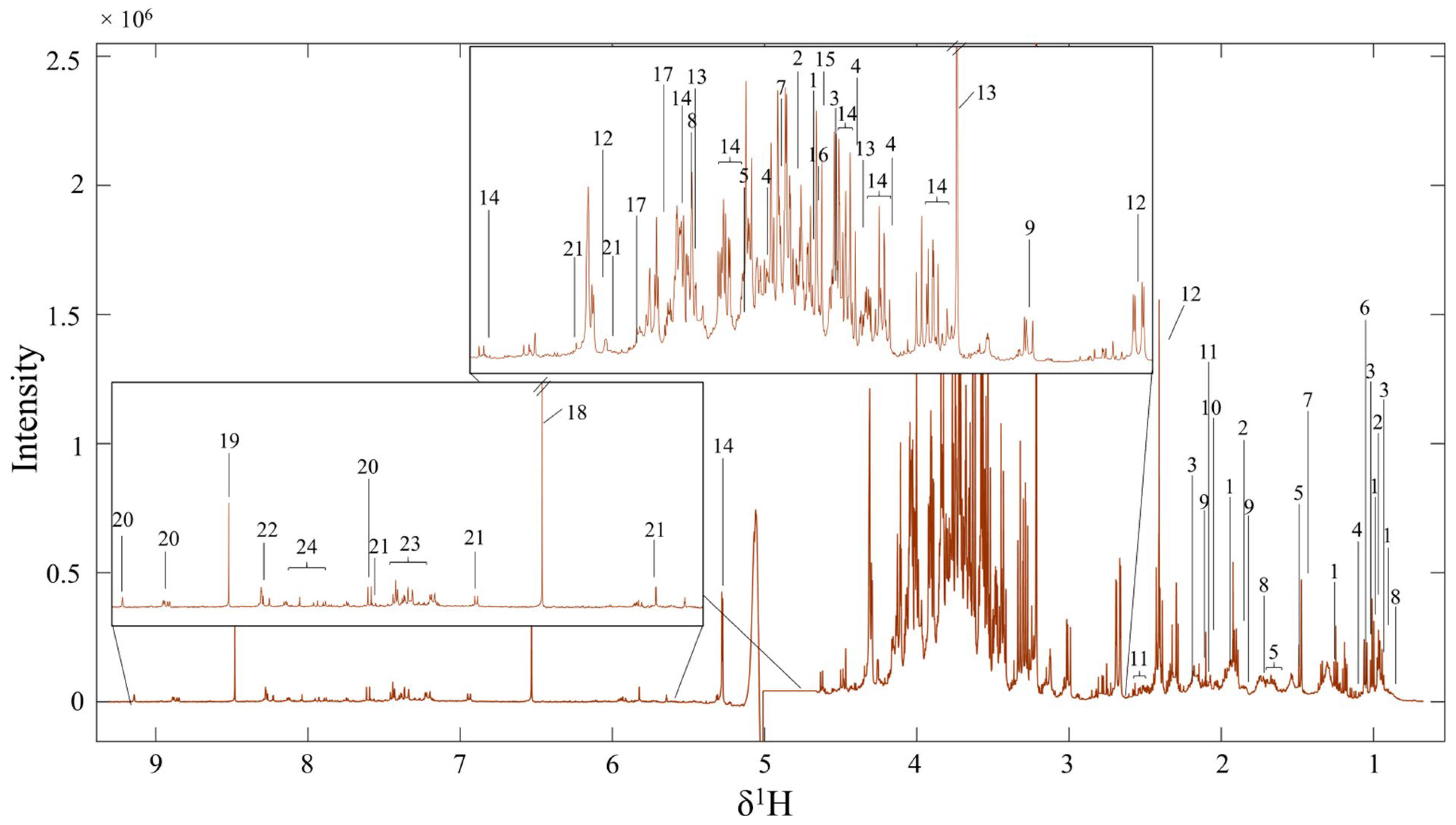
2.3. Nuclear Magnetic Resonance (NMR) Analysis
2.4. Sexual Behavior Test
2.5. Sample Collections
2.6. Serum Hormone Measurement
2.7. Sperm Quality Assays
2.7.1. Sperm Viability
2.7.2. Sperm Concentration
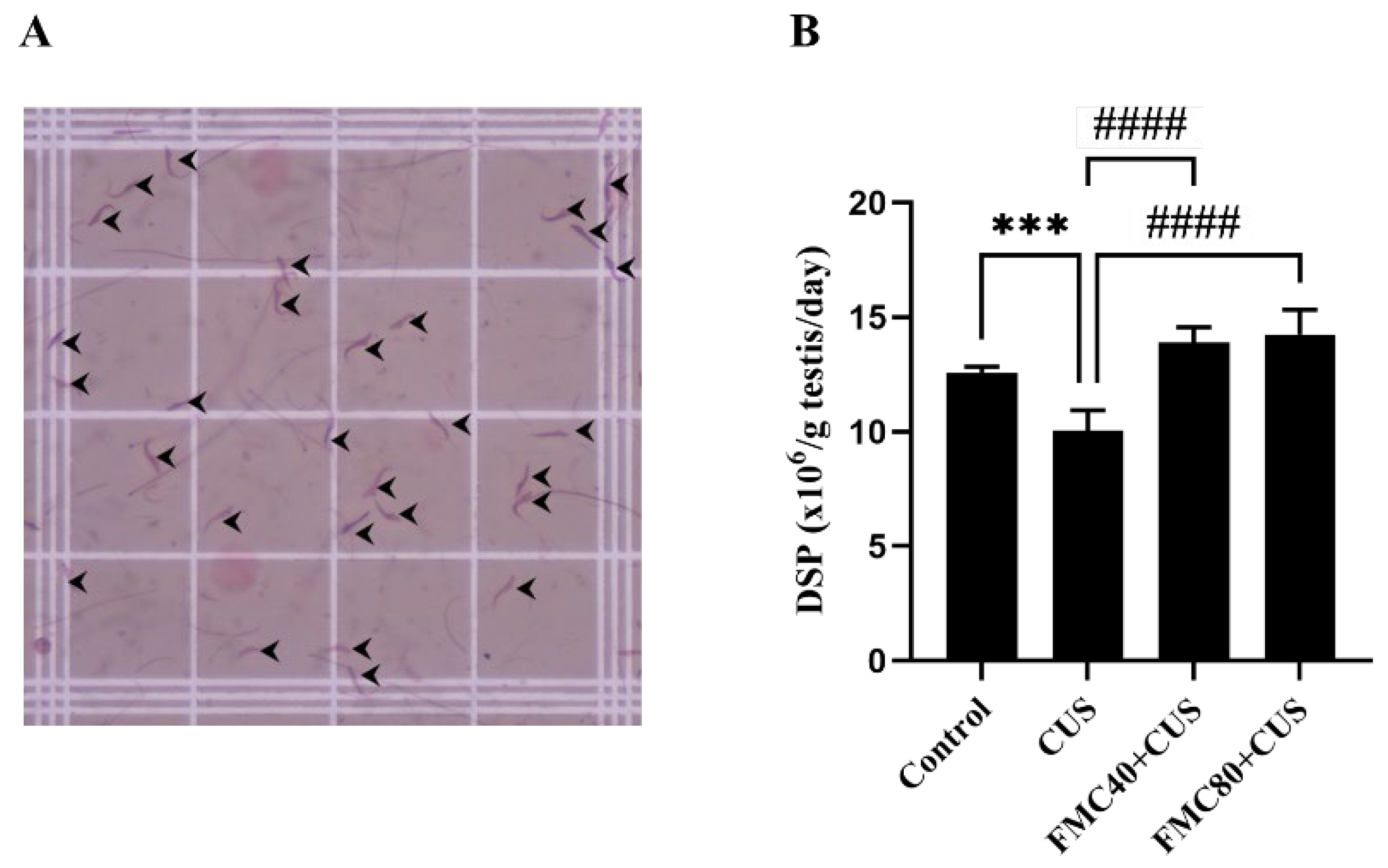
2.8. Daily Sperm Production (DSP)
2.9. Testicular Protein Preparation and Immuno-Western Blotting
2.10. Seminiferous Morphometric Analysis
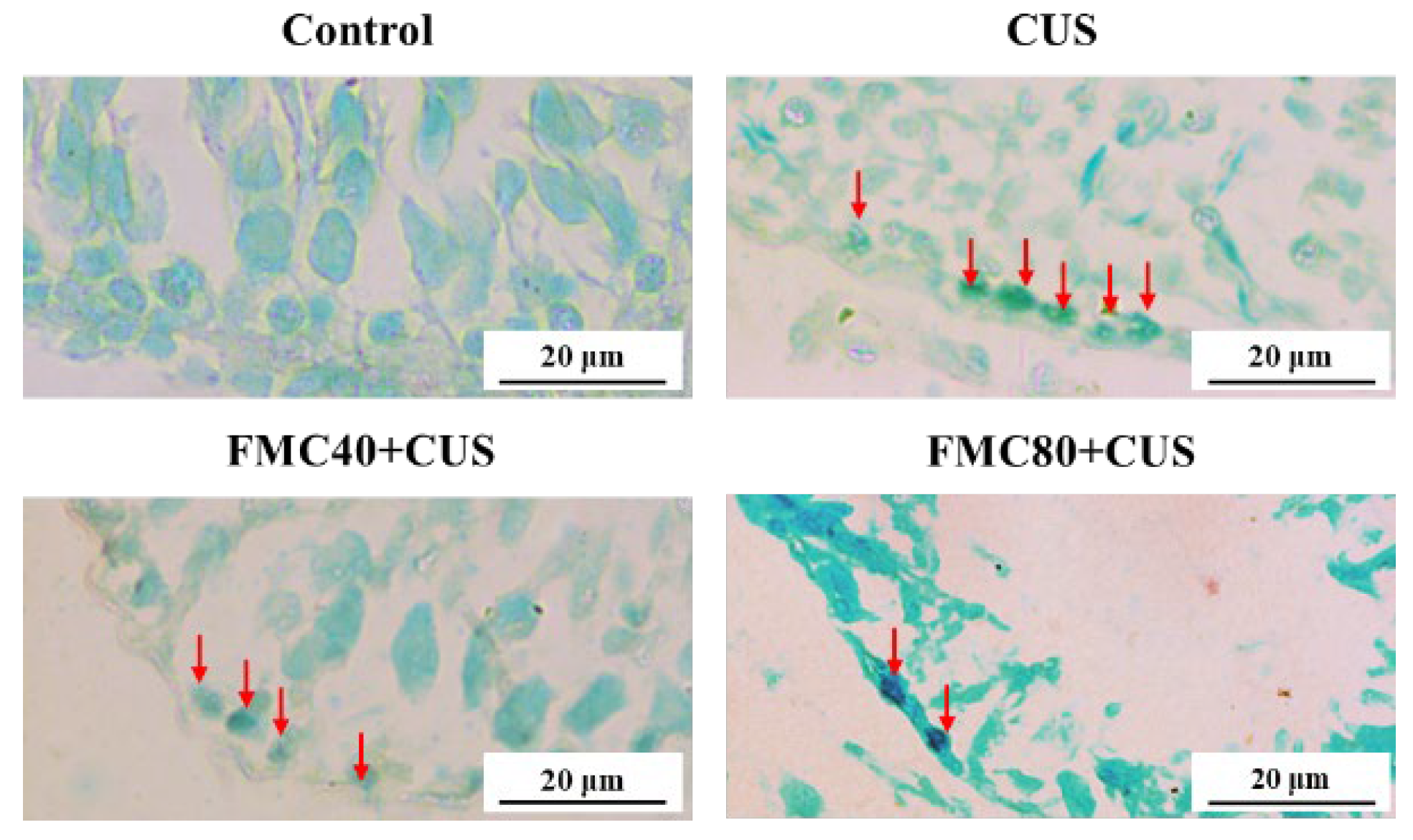
2.11. TUNEL Assay
2.12. Statistical Analysis
3. Results
3.1. Antioxidant Capacity of FMC (Fruit Extract of M. charantia)
3.2. Metabolite Profiles of FMC by Using 1H NMR Spectroscopy
3.3. FMC Extract Increased Body Weight, Testicular Weight, Sperm Count, and Testosterone Level in Stressed Rats
3.4. FMC Extract Increased Male Sexual Behaviors in CUS Rats
3.5. FMC Extract Improves the Daily Sperm Production
3.6. FMC Extract Improved Cleaved Caspase 3 in the CUS Testis
3.7. FMC Extract Decreased Apoptosis in the CUS Testicular Tissue
4. Discussion
5. Conclusions
6. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernberg, E.; Ulleryd, M.A.; Johansson, M.E.; Bergström, G.M.L. Social Disruption Stress Increases IL-6 Levels and Accelerates Atherosclerosis in ApoE−/− Mice. Atherosclerosis 2012, 221, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Nargund, V.H. Effects of Psychological Stress on Male Fertility. Nat. Rev. Urol. 2015, 12, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. The Stress of Life; McGraw-Hill: New York, NY, USA, 1956. [Google Scholar]
- Zardooz, H.; Zahedi Asl, S.; Naseri, M.G. Effect of Chronic Psychological Stress on Insulin Release from Rat Isolated Pancreatic Islets. Life Sci. 2006, 79, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bhongade, M.B.; Prasad, S.; Jiloha, R.C.; Ray, P.C.; Mohapatra, S.; Koner, B.C. Effect of Psychological Stress on Fertility Hormones and Seminal Quality in Male Partners of Infertile Couples. Andrologia 2015, 47, 336–342. [Google Scholar] [CrossRef]
- Choowong-in, P.; Sattayasai, J.; Boonchoong, P.; Poodendaen, C.; Wu, A.T.; Tangsrisakda, N.; Sawatpanich, T.; Arun, S.; Uabundit, N.; Iamsaard, S. Protective Effects of Thai Mucuna pruriens (L.) DC. Var. Pruriens Seeds on Sexual Behaviors and Essential Reproductive Markers in Chronic Unpredictable Mild Stress Mice. J. Tradit. Complement. Med. 2022, 12, 402–413. [Google Scholar] [CrossRef]
- Kelestimur, H.; Bulmus, O.; Serhatlioglu, I.; Ercan, Z.; Ozer Kaya, S.; Yardimci, A.; Ulker, N.; Kacar, E.; Canpolat, S. Effects of Treadmill Exercise on Sexual Behavior and Reproductive Parameters in Chronically Stressed-Male Rats. Physiol. Res. 2021, 70, 765–775. [Google Scholar] [CrossRef]
- Meng, X.; Peng, L.; Xu, J.; Guo, D.; Cao, W.; Xu, Y.; Li, S. Betaine Attenuate Chronic Restraint Stress-Induced Changes in Testicular Damage and Oxidative Stress in Male Mice. Reprod. Biol. Endocrinol. 2022, 20, 80. [Google Scholar] [CrossRef]
- Nordkap, L.; Jensen, T.K.; Hansen, Å.M.; Lassen, T.H.; Bang, A.K.; Joensen, U.N.; Jensen, M.B.; Skakkebæk, N.E.; Jørgensen, N. Psychological Stress and Testicular Function: A Cross-Sectional Study of 1,215 Danish Men. Fertil. Steril. 2016, 105, 174–187.e2. [Google Scholar] [CrossRef]
- Shen, Y.; He, D.; He, L.; Bai, Y.; Wang, B.; Xue, Y.; Hou, G. Chronic Psychological Stress, but Not Chronic Pain Stress, Influences Sexual Motivation and Induces Testicular Autophagy in Male Rats. Front. Psychol. 2020, 11, 826. [Google Scholar] [CrossRef]
- Arun, S.; Burawat, J.; Sukhorum, W.; Sampannang, A.; Maneenin, C.; Iamsaard, S. Chronic Restraint Stress Induces Sperm Acrosome Reaction and Changes in Testicular Tyrosine Phosphorylated Proteins in Rats. Int. J. Reprod. Biomed. 2016, 14, 443–452. [Google Scholar] [CrossRef]
- Lapyuneyong, N.; Tangsrisakda, N.; Choowong-In, P.; Chaisiwamongkol, K.; Uabundit, N.; Sawatpanich, T.; Arun, S.; Wu, A.T.-H.; Iamsaard, S. Seed Extract of Thai Mucuna pruriens Reduced Male Reproductive Damage in Rats Induced by Chronic Stress. Pharm. Biol. 2022, 60, 374–383. [Google Scholar] [CrossRef]
- Juárez-Rojas, A.L.; García-Lorenzana, M.; Aragón-Martínez, A.; Gómez-Quiroz, L.E.; Del Socorro Retana-Márquez, M. Intrinsic and Extrinsic Apoptotic Pathways Are Involved in Rat Testis by Cold Water Immersion-Induced Acute and Chronic Stress. Syst. Biol. Reprod. Med. 2015, 61, 211–221. [Google Scholar] [CrossRef]
- Hari Priya, P.; Reddy, P.S. Effect of Restraint Stress on Lead-Induced Male Reproductive Toxicity in Rats. J. Exp. Zool. 2012, 317, 455–465. [Google Scholar] [CrossRef]
- Zou, P.; Wang, X.; Yang, W.; Liu, C.; Chen, Q.; Yang, H.; Zhou, N.; Zeng, Y.; Chen, H.; Zhang, G.; et al. Mechanisms of Stress-Induced Spermatogenesis Impairment in Male Rats Following Unpredictable Chronic Mild Stress (uCMS). Int. J. Mol. Sci. 2019, 20, 4470. [Google Scholar] [CrossRef]
- Maneenin, C.; Burawat, J.; Maneenin, N.; Nualkaew, S.; Arun, S.; Sampannang, A.; Iamsaard, S. Antioxidant Capacity of Momordica charantia Extract and Its Protective Effect on Testicular Damage in Valproic Acid-Induced Rats. Int. J. Morphol. 2018, 36, 447–453. [Google Scholar] [CrossRef]
- Murakami, T.; Emoto, A.; Matsuda, H.; Yoshikawa, M. Medicinal Foodstuffs. XXI. Structures of New Cucurbitane-Type Triterpene Glycosides, Goyaglycosides-a, -b, -c, -d, -e, -f, -g, and -h, and New Oleanane-Type Triterpene Saponins, Goyasaponins I, II, and III, from the Fresh Fruit of Japanese Momordica charantia L. Chem. Pharm. Bull. 2001, 49, 54–63. [Google Scholar] [CrossRef]
- Tan, S.P.; Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized Aqueous Extraction of Saponins from Bitter Melon for Production of a Saponin-Enriched Bitter Melon Powder. J. Food Sci. 2014, 79, E1372–E1381. [Google Scholar] [CrossRef] [PubMed]
- Gürdal, B.; Kültür, Ş. An Ethnobotanical Study of Medicinal Plants in Marmaris (Muğla, Turkey). J. Ethnopharmacol. 2013, 146, 113–126. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef]
- Abas, R.; Othman, F.; Thent, Z.C. Protective Effect of Momordica charantia Fruit Extract on Hyperglycaemia-Induced Cardiac Fibrosis. Oxid. Med. Cell Longev. 2014, 2014, 429060. [Google Scholar] [CrossRef]
- Soliman, G.A.; Abdel-Rahman, R.F.; Ogaly, H.A.; Althurwi, H.N.; Abd-Elsalam, R.M.; Albaqami, F.F.; Abdel-Kader, M.S. Momordica charantia Extract Protects against Diabetes-Related Spermatogenic Dysfunction in Male Rats: Molecular and Biochemical Study. Molecules 2020, 25, 5255. [Google Scholar] [CrossRef]
- Arun, S.; Kamollerd, T.; Tangsrisakda, N.; Bunsueb, S.; Chaiyamoon, A.; Wu, A.T.-H.; Iamsaard, S. Momordica charantia Fruit Extract with Antioxidant Capacity Improves the Expression of Tyrosine-Phosphorylated Proteins in Epididymal Fluid of Chronic Stress Rats. J. Integr. Med. 2022, 20, 534–542. [Google Scholar] [CrossRef]
- Ågmo, A. Male Rat Sexual Behavior. Brain Res. Protoc. 1997, 1, 203–209. [Google Scholar] [CrossRef]
- Adler, I.-D. Comparison of the Duration of Spermatogenesis between Male Rodents and Humans. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 1996, 352, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and Free Radical-Scavenging Activities of Wheat Germ Protein Hydrolysates (WGPH) Prepared with Alcalase. Process Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic Contents and Antioxidant Activities of Bitter Gourd (Momordica charantia L.) Leaf, Stem and Fruit Fraction Extracts in Vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Robert, H.; Ferguson, L.; Reins, O.; Greco, T.; Prins, M.L.; Folkerts, M. Rodent Estrous Cycle Monitoring Utilizing Vaginal Lavage: No Such Thing As a Normal Cycle. J. Vis. Exp. 2021, 174, e62884. [Google Scholar] [CrossRef]
- Tangsrisakda, N.; Kamollerd, T.; Taoto, C.; Bunsueb, S.; Chaimontri, C.; Choowong-In, P.; Lapyuneyong, N.; Wu, A.T.; Thukhammee, W.; Wattanathorn, J.; et al. Seed Extract of Thai Mucuna pruriens (L.) DC. Var. Pruriens Enhances Sexual Performance and Improves Male Reproductive Damages in Ethanol-Induced Rats. J. Ethnopharmacol. 2022, 292, 115219. [Google Scholar] [CrossRef]
- Vendramini, V.; Cedenho, A.P.; Miraglia, S.M.; Spaine, D.M. Reproductive Function of the Male Obese Zucker Rats: Alteration in Sperm Production and Sperm DNA Damage. Reprod. Sci. 2014, 21, 221–229. [Google Scholar] [CrossRef]
- Johnson, L.; Neaves, W.B. Enhanced Daily Sperm Production in the Remaining Testis of Aged Rats Following Hemicastration. J. Androl. 1983, 4, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Samrid, R.; Taoto, C.; Wu, A.; Sawatpanich, T.; Phunchago, N.; Uabundit, N.; Iamsaard, S. Protective Effect of Mucuna pruriens (L.) DC. Var. Pruriens Seed Extract on Apoptotic Germ Cells in Ethanolic Male Rats. Braz. J. Biol. 2023, 83, e272629. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S.; Hiradate, Y.; Umezu, K.; Hara, K.; Tanemura, K. Capacitation of Mouse Sperm Is Modulated by Gamma-Aminobutyric Acid (GABA) Concentration. J. Reprod. Dev. 2019, 65, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lazaros, L.; Xita, N.; Hatzi, E.; Kaponis, A.; Makrydimas, G.; Takenaka, A.; Sofikitis, N.; Stefos, T.; Zikopoulos, K.; Georgiou, I. Phosphatidylethanolamine N-Methyltransferase and Choline Dehydrogenase Gene Polymorphisms Are Associated with Human Sperm Concentration. Asian J. Androl. 2012, 14, 778–783. [Google Scholar] [CrossRef]
- Sherry, E.B.; Lee, P.; Choi, I.-Y. In Vivo NMR Studies of the Brain with Hereditary or Acquired Metabolic Disorders. Neurochem. Res. 2015, 40, 2647–2685. [Google Scholar] [CrossRef]
- Taoto, C.; Tangsrisakda, N.; Thukhammee, W.; Phetcharaburanin, J.; Iamsaard, S.; Tanphaichitr, N. Rats Orally Administered with Ethyl Alcohol for a Prolonged Time Show Histopathology of the Epididymis and Seminal Vesicle Together with Changes in the Luminal Metabolite Composition. Biomedicines 2024, 12, 1010. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Liu, Y.; Su, Z.; Dawar, F.U.; Dan, H.; He, Y.; Gui, J.-F.; Mei, J. Leucine Mediates Autophagosome-Lysosome Fusion and Improves Sperm Motility by Activating the PI3K/Akt Pathway. Oncotarget 2017, 8, 111807–111818. [Google Scholar] [CrossRef]
- Fouche, G.; Afolayan, A.J.; Wintola, O.A.; Khorombi, T.E.; Senabe, J. Effect of the Aqueous Extract of the Aerial Parts of Monsonia angustifolia E. Mey. Ex A. Rich., on the Sexual Behaviour of Male Wistar Rats. BMC Complement. Altern. Med. 2015, 15, 343. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Akanji, M.A. Effect of Aqueous Extract of Massularia acuminata Stem on Sexual Behaviour of Male Wistar Rats. Evid.-Based Complement. Altern. Med. 2011, 2011, 738103. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, H.P.; Park, H.J.; Yoon, Y.G. Improvement of Testosterone Deficiency by Fermented Momordica charantia Extracts in Aging Male Rats. Food Sci. Biotechnol. 2021, 30, 443–454. [Google Scholar] [CrossRef]
- López López, A.L.; Escobar Villanueva, M.C.; Brianza Padilla, M.; Bonilla Jaime, H.; Alarcón Aguilar, F.J. Chronic Unpredictable Mild Stress Progressively Disturbs Glucose Metabolism and Appetite Hormones in Rats. Acta Endocrinol. 2018, 14, 16–23. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Lee, D.H.; Kang, S.S. Effects of Chronic Restraint Stress on Body Weight, Food Intake, and Hypothalamic Gene Expressions in Mice. Endocrinol. Metab. 2013, 28, 288–296. [Google Scholar] [CrossRef]
- Huang, T.-N.; Lu, K.-N.; Pai, Y.-P.; Hsu, C.; Huang, C.-J. Role of GLP-1 in the Hypoglycemic Effects of Wild Bitter Gourd. Evid.-Based Complement. Altern. Med. 2013, 2013, 625892. [Google Scholar] [CrossRef]
- Blossom, V.; Gokul, M.; Kumar, N.A.; Kini, R.D.; Nayak, S.; Bhagyalakshmi, K. Chronic Unpredictable Stress-Induced Inflammation and Quantitative Analysis of Neurons of Distinct Brain Regions in Wistar Rat Model of Comorbid Depression. Vet. World 2020, 13, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Gokul, M.; Arun Kumar, N.; Durgadas Kini, R.; Blossom, V.; Kodavanji, B.; Noojibail, A.; Murali, N.; Vishwanath Rai, S.P. Evaluation of Biomarkers of Stress in Chronic Stress-Exposed Comorbid Depression Model Wistar Rats. J. Basic. Clin. Physiol. Pharmacol. 2019, 30, 20180215. [Google Scholar] [CrossRef] [PubMed]
- Hidayatik, N.; Purnomo, A.; Fikri, F.; Purnama, M.T.E. Amelioration on Oxidative Stress, Testosterone, and Cortisol Levels after Administration of Vitamins C and E in Albino Rats with Chronic Variable Stress. Vet. World 2021, 14, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kvarta, M.D.; Bradbrook, K.E.; Dantrassy, H.M.; Bailey, A.M.; Thompson, S.M. Corticosterone Mediates the Synaptic and Behavioral Effects of Chronic Stress at Rat Hippocampal Temporoammonic Synapses. J. Neurophysiol. 2015, 114, 1713–1724. [Google Scholar] [CrossRef]
- Mohamed, Z.I.; Sivalingam, M.; Radhakrishnan, A.K.; Jaafar, F.; Zainal Abidin, S.A. Chronic Unpredictable Stress (CUS) Reduced Phoenixin Expression, Induced Abnormal Sperm and Testis Morphology in Male Rats. Neuropeptides 2024, 107, 102447. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.-J.; Starkweather, A.; An, K.; Shim, I. Decreased Interleukin-4 Release from the Neurons of the Locus Coeruleus in Response to Immobilization Stress. Mediat. Inflamm. 2016, 2016, 3501905. [Google Scholar] [CrossRef]
- Sultanov, R.M.; Poleshchuk, T.S.; Ermolenko, E.V.; Kasyanov, S.P. Protective Properties of Marine Alkyl Glycerol Ethers in Chronic Stress. Mar. Drugs 2023, 21, 202. [Google Scholar] [CrossRef]
- Swan, M.P.; Hickman, D.L. Evaluation of the Neutrophil-Lymphocyte Ratio as a Measure of Distress in Rats. Lab. Anim. 2014, 43, 276–282. [Google Scholar] [CrossRef]
- Vélez-Marín, M.; Uribe Velásquez, L.F. Plasma Cortisol Activity in Rats under Conditions of Chronic Stress Supplemented with Resveratrol. Colomb. Med. 2012, 43, 221–225. [Google Scholar] [CrossRef]
- Ribeiro, C.; De Souza, D.; Costa, W.; Sampaio, F.B.; Pereira-Sampaio, M. Immediate and Late Effects of Chronic Stress in the Testes of Prepubertal and Adult Rats. Asian J. Androl. 2018, 20, 385. [Google Scholar] [CrossRef]
- Harding, S.M.; Velotta, J.P. Comparing the Relative Amount of Testosterone Required to Restore Sexual Arousal, Motivation, and Performance in Male Rats. Horm. Behav. 2011, 59, 666–673. [Google Scholar] [CrossRef]
- Kavitha, N.; Babu, S.M.; Rao, M.E.B. Influence of Momordica charantia on Oxidative Stress-Induced Perturbations in Brain Monoamines and Plasma Corticosterone in Albino Rats. Indian J. Pharmacol. 2011, 43, 424–428. [Google Scholar] [CrossRef]
- Yook, J.S.; Kwak, J.-J.; Jeong, W.-M.; Song, Y.H.; Hijioka, Y.; Honda, Y.; Kim, S.E.; Ha, M.-S. Possible Adaptogenic Effects of Momordica charantia on High-Intensity Training-Induced Alteration in the Hypothalamic-Pituitary-Adrenal Axis. J. Clin. Biochem. Nutr. 2020, 67, 290–296. [Google Scholar] [CrossRef] [PubMed]


| Sample | Total Phenolic Content (mg GAE/g Sample) | Flavonoid Content (mg Catechin/g Sample) | DPPH Assay (mg Trolox Equivalents/g Sample) | DPPH Assay (IC50 mg/mL) | FRAP Value (µmol of Fe (II)/g Sample) |
|---|---|---|---|---|---|
| FMC | 19.0 ± 0.27 | 0.31 ± 0.01 | 4.99 ± 0.09 | 2.01 ± 0.01 | 23.70 ± 0.82 |
| Ascorbic acid | - | - | 1554.62 ± 20.71 | 0.01 ± 0.00 | 12,390.55 ± 33.91 |
| α-Tocopherol | - | - | 667.12 ± 6.38 | 0.02 ± 0.01 | 4037.75 ± 80.75 |
| BHT | - | - | 183.05 ± 2.09 | 0.12 ± 0.00 | 2575.05 ± 41.42 |
| NO. | Chemical Shift (ppm) | Multiplicity | STOCSY | p-Value | Metabolites |
|---|---|---|---|---|---|
| 1 | 0.93281 | t | 0.93281 (t), 1.01(d), 1.259 (m), 1.4562 (m), 1.985 (m), 3.662 (d) | 1 × 10−12 | Isoleucine |
| 2 | 0.97536 | t | 0.97536 (t), 1.887 (m), 3.705 (dd) | 1 × 10−14 | 2-aminobutyric acid |
| 3 | 1.0425 | d | 0.980 (d), 1.042 (d), 2.233 (m), 3.59 (d) | 1 × 10−13 | Valine |
| 4 | 1.1427 | d | 1.1427(d), 3.415 (dd), 3.546 (dd), 3.791 (m) | 1 × 10−13 | Propylene glycol |
| 5 | 1.1666 | m | 1.1666 (m), 1.3199 (m), 1.703 (m), 3.873 (d) | 1 × 10−13 | Hydroxy-3-methylvaleric acid |
| 6 | 1.203 | s | 1.203 (s) | 1 × 10−11 | Methylmalonic acid |
| 7 | 1.4745 | d | 1.4745 (d), 3.759 (q) | 1 × 10−13 | Alanine |
| 8 | 1.6995 | m | 0.94 (t), 1.6995 (m), 4.026 (dd) | 1 × 10−13 | Alpha-hydroxy |
| 9 | 1.8903 | m | 1.8903 (m), 2.274 (t), 2.973 (t) | 1 × 10−13 | Gamma-aminobutyric acid |
| 10 | 2.1012 | s | 2.1012 (s) | 1 × 10−10 | Acetic acid |
| 11 | 2.1793 | m | 1.338 (m), 1.52 (m), 1.61 (m), 2.1793 (m), 2.509 (m) | 1 × 10−12 | Saccharopine |
| 12 | 2.3862 | dd | 2.3862 (dd), 2.6 (dd), 4.292 (dd) | 1 × 10−12 | Malic acid |
| 13 | 3.2155 | s | 3.2155 (s), 3.517 (m), 4.107 (m), 3.2713 (dd), 3.415 (m), 3.474 (m), 3.565 (dd) | 1 × 10−13 | Choline |
| 14 | 3.2713 | dd | 3.72 (m), 3.889 (m), 4.026 (dd), 4.646 (d), 5.275 (d) | 1 × 10−13 | Glucose |
| 15 | 3.6165 | s | 3.617 (s) | 1 × 10−14 | Propanedinitrile |
| 16 | 3.632 | s | 3.632 (s) | 1 × 10−14 | Oxalacetic acid |
| 17 | 4.102 | m | 3.824 (s), 3.961 (t), 4.102 (m), 4.55 (d) | 1 × 10−13 | 6-phosphogluconic acid |
| 18 | 6.531 | s | 6.531 (s) | 1 × 10−14 | Fumarate |
| 19 | 8.481 | s | 8.481 (s) | 1 × 10−14 | Formic acid |
| 20 | 9.142 | d | 9.1418 (s), 8.848 (d), 7.595 (td) | 1 × 10−11 | Pyrimidine |
| 21 | 5.8222 | d | 7.88 (s), 5.8222 (d), 4.378 (dd), 4.255 (q), 3.872 (m), 3.791 (m) | 1 × 10−11 | Xanthosine |
| 22 | 7.5948 | d | 8.2657 (m), 7.595 (d), 6.933 (d), 3.902 (d) | 1 × 10−11 | 4-Aminohippuric acid |
| 23 | 7.4317 | m | 7.389 (d), 7.4137 (m) | 1 × 10−11 | Cinnamic acid |
| 24 | 8.0389 | d | 8.039 (d), 8.089 (d), 8.118 (d) | 1 × 10−11 | Adenine |
| Control | CUS | FMC40 + CUS | FMC80 + CUS | |
|---|---|---|---|---|
| Body weight | ||||
| Initial BW (g) | 381.33 ± 8.43 | 382.43 ± 12.71 | 377.71 ± 9.36 | 382.83 ± 18.10 |
| Final BW (g) | 478.22 ± 23.66 | 416.32 ± 22.97 **** | 425.13 ± 6.58 | 460.87 ± 10.74 ## |
| Percentage change in BW | 25.42 ± 5.83 | 10.62 ± 3.06 ** | 14.25 ± 5.37 | 19.54 ± 4.07 |
| Testicular weight | ||||
| Absolute weight (g) | 1.887 ± 0.10 | 1.693 ± 0.14 *** | 1.841 ± 0.09 # | 1.881 ± 0.15 ### |
| Relative weight (g/100 g) | 0.407 ± 0.01 | 0.397 ± 0.00 | 0.434 ± 0.01 ### | 0.433 ± 0.02 ### |
| Sperm quality | ||||
| Sperm count (×106) | 28.790 ± 1.18 | 24.229 ± 4.80 | 40.700 ± 4.19 ### | 38.012 ± 5.88 # |
| Sperm viability (%) | 95.667 ± 3.69 | 93.333 ± 1.04 | 94.167 ± 1.61 | 91.000 ± 4.77 |
| Serum hormones | ||||
| Cortisol level (ng/mL) | 1086.670 ± 248.46 | 1170.000 ± 52.92 | 993.330 ± 151.44 | 1436.670 ± 49.33 |
| Testosterone level (ng/mL) | 1.930 ± 0.04 | 0.679 ± 0.12 **** | 1.078 ± 0.18 ## | 1.163 ± 0.01 ### |
| Morphometrics | ||||
| Tubular diameters (μm) | 295.949 ± 53.23 | 266.346 ± 37.77 * | 302.169 ± 50.85 ## | 300.336 ± 54.70 # |
| Epithelial heights (μm) | 66.410 ± 11.75 | 57.406 ± 11.61 *** | 71.308 ± 15.01 #### | 70.395 ± 17.29 #### |
| Testicular MDA level (ng/mg.protein) | 0.663 ± 0.17 | 1.047 ± 0.14 * | 0.511 ± 0.11 ## | 0.987 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamollerd, T.; Uopasai, S.; Sawatpanich, T.; Uabundit, N.; Arun, S.; Tangsrisakda, N.; Taoto, C.; Chaimontri, C.; Lapyuneyong, N.; Thukhammee, W.; et al. Protective Effects of Momordica charantia Fruit Extract on Male Sexual Dysfunction and Testicular Damage in Rats Induced by Chronic Unpredictable Stressors. Life 2025, 15, 1559. https://doi.org/10.3390/life15101559
Kamollerd T, Uopasai S, Sawatpanich T, Uabundit N, Arun S, Tangsrisakda N, Taoto C, Chaimontri C, Lapyuneyong N, Thukhammee W, et al. Protective Effects of Momordica charantia Fruit Extract on Male Sexual Dysfunction and Testicular Damage in Rats Induced by Chronic Unpredictable Stressors. Life. 2025; 15(10):1559. https://doi.org/10.3390/life15101559
Chicago/Turabian StyleKamollerd, Therachon, Suwit Uopasai, Tarinee Sawatpanich, Nongnut Uabundit, Supatcharee Arun, Nareelak Tangsrisakda, Chayakorn Taoto, Chadaporn Chaimontri, Natthapol Lapyuneyong, Wipawee Thukhammee, and et al. 2025. "Protective Effects of Momordica charantia Fruit Extract on Male Sexual Dysfunction and Testicular Damage in Rats Induced by Chronic Unpredictable Stressors" Life 15, no. 10: 1559. https://doi.org/10.3390/life15101559
APA StyleKamollerd, T., Uopasai, S., Sawatpanich, T., Uabundit, N., Arun, S., Tangsrisakda, N., Taoto, C., Chaimontri, C., Lapyuneyong, N., Thukhammee, W., Innoi, S., & Iamsaard, S. (2025). Protective Effects of Momordica charantia Fruit Extract on Male Sexual Dysfunction and Testicular Damage in Rats Induced by Chronic Unpredictable Stressors. Life, 15(10), 1559. https://doi.org/10.3390/life15101559






