Effect of Cervical Manual Therapy on Sleep Quality: A Scoping Review of Randomized Controlled Trials
Abstract
1. Background
2. Methods
2.1. Research Questions of Scoping Review
- Main question
- -
- What is the current status of RCTs utilizing CMT that include sleep quality-related outcome measures?
- Secondary questions
- -
- What CMT techniques have demonstrated improvements in sleep quality?
- -
- What are the characteristics of patient populations that showed or did not show improvement in sleep quality?
- -
- In what aspects does sleep quality improve (e.g., sleep latency, duration, or depth)?
- -
- Based on the above analyses, what directions should future research take to investigate the mechanisms underlying the effects of CMT on sleep quality?
2.2. Literature Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Data Analysis
3. Results
3.1. Characteristics of RCTs Meeting the Inclusion Criteria
3.2. Interventions and Outcome Measurements for Sleep Quality in RCTs
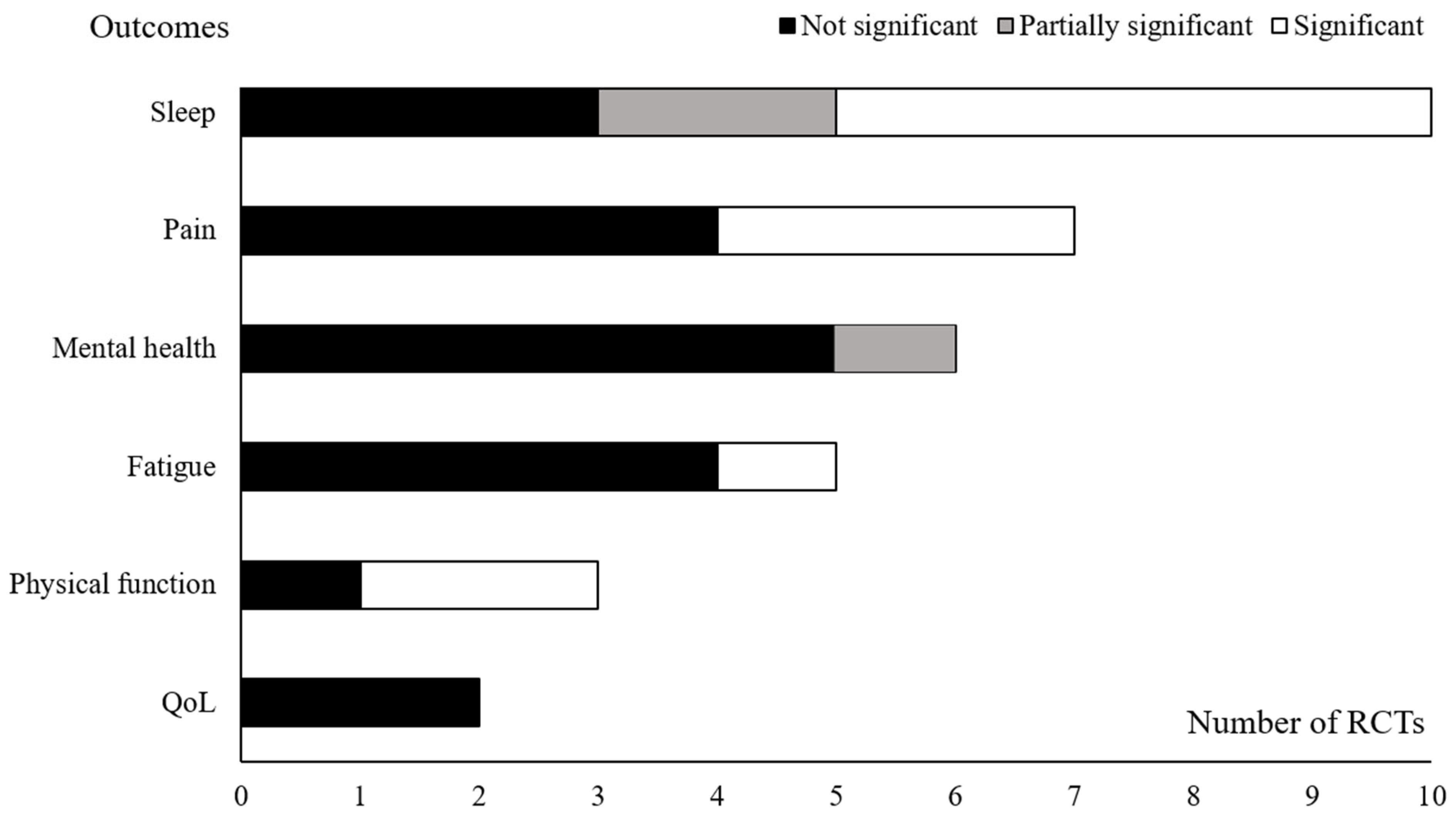
3.3. Effects on Sleep Quality in RCTs
3.4. Subscale Analysis
4. Discussion
4.1. Summary of Evidence
4.1.1. Overall Efficacy of CMT on Sleep Quality
4.1.2. Efficacy of CMT Across Conditions and Techniques
4.1.3. Effect on Sleep Depth, Efficiency, and Duration
4.2. Modulating 5-HTergic Activity
4.2.1. Potential Linkage Between 5-HT, Sleep and CMT
4.2.2. Pathologic Conditions of 5-HT Modulation and CMT
4.3. Regulation of the HPA Axis
4.3.1. Efficacy of CMT on HPA Axis and Sleep Architecture
4.3.2. Dysregulation of HPA Axis in FM
4.4. Limitation and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ANS | Autonomic nervous system |
| CMT | Cervical manual therapy |
| FM | Fibromyalgia |
| HPA | Hypothalamic–pituitary–adrenal axis |
| ME/CFS | Myalgic encephalomyelitis/chronic fatigue syndrome |
| MET | Muscle energy technique |
| MFT | Myofascial technique |
| RCTs | Randomized controlled trials |
| QoL | Quality of life |
| SCI | Spinal cord injury |
| STT | Soft tissue technique |
| 5-HT | 5-hydroxytryptamine |
References
- Krueger, J.M.; Frank, M.G.; Wisor, J.P.; Roy, S. Sleep function: Toward elucidating an enigma. Sleep Med. Rev. 2016, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, M.; Wang, X.; Jiang, H.; Huang, J.; Liang, S. Association of sleep duration and risk of mental disorder: A systematic review and meta-analysis. Sleep Breath. 2024, 28, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hoshide, S.; Kario, K. Sleep duration as a risk factor for cardiovascular disease-a review of the recent literature. Curr. Cardiol. Rev. 2010, 6, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Prevention CfDCa. Behavioral Risk Factor Surveillance System Survey Data; Services DoHaH, Ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/brfss/annual_data/annual_2022.html (accessed on 1 April 2024).
- Huyett, P.; Bhattacharyya, N. Incremental health care utilization and expenditures for sleep disorders in the United States. J. Clin. Sleep Med. 2021, 17, 1981–1986. [Google Scholar] [CrossRef]
- Stuck, B.A.; Maurer, J.T.; Schlarb, A.A.; Schredl, M.; Weeß, H.-G. Practice of Sleep Medicine: Sleep Disorders in Children and Adults; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Duo, L.; Yu, X.; Hu, R.; Duan, X.; Zhou, J.; Wang, K. Sleep disorders in chronic pain and its neurochemical mechanisms: A narrative review. Front. Psychiatry 2023, 14, 1157790. [Google Scholar] [CrossRef]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, insomnia and mental health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef]
- Espie, C.A. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu. Rev. Psychol. 2002, 53, 215–243. [Google Scholar] [CrossRef]
- McCrae, C.S.; Lichstein, K.L. Secondary insomnia: A heuristic model and behavioral approaches to assessment, treatment, and prevention. Appl. Prev. Psychol. 2001, 10, 107–123. [Google Scholar]
- Zee, P.C.; Bertisch, S.M.; Morin, C.M.; Pelayo, R.; Watson, N.F.; Winkelman, J.W.; Krystal, A.D. Long-term use of insomnia medications: An appraisal of the current clinical and scientific evidence. J. Clin. Med. 2023, 12, 1629. [Google Scholar] [CrossRef]
- Natelson, B.H. Myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia: Definitions, similarities, and differences. Clin. Ther. 2019, 41, 612–618. [Google Scholar] [CrossRef]
- Williams, N.H.; Hendry, M.; Lewis, R.; Russell, I.; Westmoreland, A.; Wilkinson, C. Psychological response in spinal manipulation (PRISM): A systematic review of psychological outcomes in randomised controlled trials. Complement. Ther. Med. 2007, 15, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Colombi, A.; Testa, M. The effects induced by spinal manipulative therapy on the immune and endocrine systems. Medicina 2019, 55, 448. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Wang, Z.; Zhou, H.; Zhang, L.; Wang, Y.; Li, M.; Zhou, Y. Effect of tuina on sleep quality, psychological state and neurotransmitter level in patients with insomnia: A systematic review and meta-analysis. Front. Neurol. 2024, 15, 1273194. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.-w.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The serotonergic raphe promote sleep in zebrafish and mice. Neuron 2019, 103, 686–701.e8. [Google Scholar] [CrossRef]
- Meyer, A.-L.; Amorim, M.-A.; Schubert, M.; Schweinhardt, P.; Leboeuf-Yde, C. Unravelling functional neurology: Does spinal manipulation have an effect on the brain?—A systematic literature review. Chiropr. Man. Ther. 2019, 27, 60. [Google Scholar] [CrossRef]
- Kingston, J.; Raggio, C.; Spencer, K.; Stalaker, K.; Tuchin, P.J. A review of the literature on chiropractic and insomnia. J. Chiropr. Med. 2010, 9, 121–126. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Glover, J. Educational Council on Osteopathic Principles. Glossary of Osteopathic Terminology; American Association of Colleges of Osteopathic Medicine: Chicago, IL, USA, 2006. [Google Scholar]
- Paolucci, T.; Ferrillo, M.; Pezzi, L.; Agostini, F.; Di Matteo, A.; Prosperi, P.; Mangone, M.; Bernetti, A.; Spacone, A.; de Sire, A. Efficacy of orofacial myofunctional therapy combined with myofascial release in patients with mild obstructive sleep apnoea: A randomized controlled trial. J. Oral Rehabil. 2023, 50, 555–565. [Google Scholar] [CrossRef]
- Kadıoğlu, M.B.; Sezer, M.; Elbasan, B. Effects of Manual Therapy and Home Exercise Treatment on Pain, Stress, Sleep, and Life Quality in Patients with Bruxism: A Randomized Clinical Trial. Medicina 2024, 60, 2007. [Google Scholar] [CrossRef] [PubMed]
- Örenler, S.D.; Tuncer, A.; Najafov, E. A comparison of manual therapy and splint therapy in patients diagnosed with myofascial temporomandibular dysfunction with sleep bruxism. Türk Fiz. Ve Rehabil. Derg. 2022, 33, 89–97. [Google Scholar] [CrossRef]
- Cholewicki, J.; Popovich, J.M., Jr.; Reeves, N.P.; DeStefano, L.A.; Rowan, J.J.; Francisco, T.J.; Prokop, L.L.; Zatkin, M.A.; Lee, A.S.; Sikorskii, A. The effects of osteopathic manipulative treatment on pain and disability in patients with chronic neck pain: A single--blinded randomized controlled trial. PM&R 2022, 14, 1417–1429. [Google Scholar]
- Hadamus, A.; Wojda, A.; Białoszewski, D. Can the sleep quality of patients with chronic neck pain be improved by muscle energy techniques combined with Swedish massage? Complement. Ther. Clin. Pract. 2021, 44, 101421. [Google Scholar] [CrossRef]
- Ughreja, R.A.; Venkatesan, P.; Gopalakrishna, D.B.; Singh, Y.P.; Lakshmi, R.V. Effectiveness of craniosacral therapy, Bowen therapy, static touch and standard exercise program on sleep quality in fibromyalgia syndrome: A randomized controlled trial. J. Integr. Med. 2024, 22, 473–483. [Google Scholar] [CrossRef]
- Nadal-Nicolás, Y.; Rubio-Arias, J.Á.; Martínez-Olcina, M.; Reche-García, C.; Hernández-García, M.; Martínez-Rodríguez, A. Effects of manual therapy on fatigue, pain, and psychological aspects in women with fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 4611. [Google Scholar] [CrossRef] [PubMed]
- Castro Sánchez, A.M.; García López, H.; Fernández Sánchez, M.; Pérez Mármol, J.M.; Aguilar-Ferrándiz, M.E.; Luque Suárez, A.; Matarán Peñarrocha, G.A. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil. Rehabil. 2019, 41, 2235–2246. [Google Scholar] [CrossRef]
- Moustafa, I.M.; Diab, A.A. The addition of upper cervical manipulative therapy in the treatment of patients with fibromyalgia: A randomized controlled trial. Rheumatol. Int. 2015, 35, 1163–1174. [Google Scholar] [CrossRef]
- Nerbass, F.B.; Feltrim, M.I.Z.; de Souza, S.A.; Ykeda, D.S.; Lorenzi-Filho, G. Effects of massage therapy on sleep quality after coronary artery bypass graft surgery. Clinics 2010, 65, 1105–1110. [Google Scholar] [CrossRef]
- Jamison, J.R. Insomnia: Does chiropractic help? J. Manip. Physiol. Ther. 2005, 28, 179–186. [Google Scholar] [CrossRef]
- Kim, D.; Baek, G.G.; Shin, B.-C. An umbrella review of systematic reviews for Chuna (or Tuina) manual therapy on musculoskeletal disorders. Perspect. Integr. Med. 2023, 2, 142–154. [Google Scholar] [CrossRef]
- Voogt, L.; de Vries, J.; Meeus, M.; Struyf, F.; Meuffels, D.; Nijs, J. Analgesic effects of manual therapy in patients with musculoskeletal pain: A systematic review. Man. Ther. 2015, 20, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kamath, J.; Prpich, G.; Jillani, S. Sleep disturbances in patients with medical conditions. Psychiatr. Clin. 2015, 38, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Vgontzas, A.N.; Kritikou, I.; Chrousos, G. HPA axis and sleep. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Bomholt, S.F.; Harbuz, M.S.; Blackburn-Munro, G.; Blackburn-Munro, R.E. Involvement and role of the hypothalamo-pituitary-adrenal (HPA) stress axis in animal models of chronic pain and inflammation. Stress 2004, 7, 1–14. [Google Scholar] [CrossRef]
- Chemelo, V.d.S.; Né, Y.G.d.S.; Frazão, D.R.; Souza-Rodrigues, R.D.d.; Fagundes, N.C.F.; Magno, M.B.; Silva, C.M.T.d.; Maia, L.C.; Lima, R.R. Is there association between stress and bruxism? A systematic review and meta-analysis. Front. Neurol. 2020, 11, 590779. [Google Scholar] [CrossRef]
- Mukherjee, S.; Saxena, R.; Palmer, L.J. The genetics of obstructive sleep apnoea. Respirology 2018, 23, 18–27. [Google Scholar] [CrossRef]
- Tegegne, S.S.; Alemnew, E.F. Postoperative poor sleep quality and its associated factors among adult patients: A multicenter cross-sectional study. Ann. Med. Surg. 2022, 74, 103273. [Google Scholar] [CrossRef] [PubMed]
- Nim, C.; Aspinall, S.L.; Cook, C.E.; Corrêa, L.A.; Donaldson, M.; Downie, A.S.; Harsted, S.; Hansen, S.; Jenkins, H.J.; McNaughton, D. The effectiveness of spinal manipulative therapy in treating spinal pain does not depend on the application procedures: A systematic review and network meta-analysis. J. Orthop. Sports Phys. Ther. 2025, 55, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Nim, C.G.; Downie, A.; O’Neill, S.; Kawchuk, G.N.; Perle, S.M.; Leboeuf-Yde, C. The importance of selecting the correct site to apply spinal manipulation when treating spinal pain: Myth or reality? A systematic review. Sci. Rep. 2021, 11, 23415. [Google Scholar] [CrossRef]
- Barry, R.L.; Smith, S.A.; Dula, A.N.; Gore, J.C. Resting state functional connectivity in the human spinal cord. eLife 2014, 3, e02812. [Google Scholar] [CrossRef]
- Park, I.; Díaz, J.; Matsumoto, S.; Iwayama, K.; Nabekura, Y.; Ogata, H.; Kayaba, M.; Aoyagi, A.; Yajima, K.; Satoh, M. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci. Rep. 2021, 11, 4410. [Google Scholar] [CrossRef]
- Chen, T.-L.; Chang, S.-C.; Hsieh, H.-F.; Huang, C.-Y.; Chuang, J.-H.; Wang, H.-H. Effects of mindfulness-based stress reduction on sleep quality and mental health for insomnia patients: A meta-analysis. J. Psychosom. Res. 2020, 135, 110144. [Google Scholar] [CrossRef]
- Van Maanen, A.; Meijer, A.M.; van der Heijden, K.B.; Oort, F.J. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 29, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.; Daville, R.; Jonathan, L.; Raverot, V.; Di Maria, J.; Bossard, I.; Bensmail, D.; Quera-Salva, M.; Leotard, A. Melatonin secretion and sleep disorders in patients with spinal cord injuries. Spinal Cord 2024, 62, 143–148. [Google Scholar] [CrossRef]
- Scheer, F.; Zeitzer, J.; Ayas, N.; Brown, R.; Czeisler, C.; Shea, S. Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord 2006, 44, 78–81. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Kim, D.-Y.; Lee, J.-S.; Hwang, S.-J.; Kim, G.-H.; Hyun, S.-H.; Son, C.-G. Korean red ginseng ameliorates fatigue via modulation of 5-HT and corticosterone in a sleep-deprived mouse model. Nutrients 2021, 13, 3121. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, F.; Wilson, C.; Maidment, N.; Poland, R.; Engel, J., Jr. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience 2003, 121, 523–530. [Google Scholar] [CrossRef]
- Witkowska, A.; Jaromirska, J.; Gabryelska, A.; Sochal, M. Obstructive Sleep Apnea and Serotoninergic Signalling Pathway: Pathomechanism and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 9427. [Google Scholar] [CrossRef]
- Garrett, A.R.; Hawley, J.S. SSRI-associated bruxism: A systematic review of published case reports. Neurol. Clin. Pract. 2018, 8, 135–141. [Google Scholar] [CrossRef]
- Choy, E.H. The role of sleep in pain and fibromyalgia. Nat. Rev. Rheumatol. 2015, 11, 513–520. [Google Scholar] [CrossRef]
- Shan, Z.Y.; Barnden, L.R.; Kwiatek, R.A.; Bhuta, S.; Hermens, D.F.; Lagopoulos, J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. J. Transl. Med. 2020, 18, 335. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Rodríguez, A.; Reyes-Long, S.; Roldan-Valadez, E.; González-Torres, M.; Bonilla-Jaime, H.; Bandala, C.; Avila-Luna, A.; Bueno-Nava, A.; Cabrera-Ruiz, E.; Sanchez-Aparicio, P. Association of the Serotonin and Kynurenine Pathways as Possible Therapeutic Targets to Modulate Pain in Patients with Fibromyalgia. Pharmaceuticals 2024, 17, 1205. [Google Scholar] [CrossRef]
- Kashi, A.A.; Davis, R.W.; Phair, R.D. The IDO metabolic trap hypothesis for the etiology of ME/CFS. Diagnostics 2019, 9, 82. [Google Scholar] [CrossRef]
- Rowe, P.C.; Marden, C.L.; Heinlein, S.; Edwards, C.C. Improvement of severe myalgic encephalomyelitis/chronic fatigue syndrome symptoms following surgical treatment of cervical spinal stenosis. J. Transl. Med. 2018, 16, 21. [Google Scholar] [CrossRef]
- Bush, B.; Hudson, T. The role of cortisol in sleep. Nat. Med. J. 2010, 2, 2006–2010. [Google Scholar]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 2020, 41, bnaa002. [Google Scholar] [CrossRef] [PubMed]
- Kovanur Sampath, K.; Treffel, L.; Thomson, O.P.; Rodi, J.D.; Fleischmann, M.; Tumilty, S. Changes in biochemical markers following a spinal manipulation–a systematic review update. J. Man. Manip. Ther. 2024, 32, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Follenius, M.; Brandenberger, G.; Bandesapt, J.; Libert, J.; Ehrhart, J. Nocturnal cortisol release in relation to sleep structure. Sleep 1992, 15, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Leproult, R.; Plat, L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000, 284, 861–868. [Google Scholar] [CrossRef]
- Born, J.; Muth, S.; Fehm, H. The significance of sleep onset and slow wave sleep for nocturnal release of growth hormone (GH) and cortisol. Psychoneuroendocrinology 1988, 13, 233–243. [Google Scholar] [CrossRef]
- Wei, F.; Song, J.; Zhang, C.; Lin, J.; Xue, R.; Shan, L.-D.; Gong, S.; Zhang, G.-X.; Qin, Z.-H.; Xu, G.-Y. Chronic stress impairs the aquaporin-4-mediated glymphatic transport through glucocorticoid signaling. Psychopharmacology 2019, 236, 1367–1384. [Google Scholar] [CrossRef]
- Jin, H.; Yoon, J.-H.; Hong, S.P.; Hwang, Y.S.; Yang, M.J.; Choi, J.; Kang, H.J.; Baek, S.E.; Jin, C.; Jung, J. Increased CSF drainage by non-invasive manipulation of cervical lymphatics. Nature 2025, 643, 755–767. [Google Scholar] [CrossRef]
- Crofford, L.J.; Demitrack, M.A. Evidence that abnormalities of central neurohormonal systems are key to understanding fibromyalgia and chronic fatigue syndrome. Rheum. Dis. Clin. 1996, 22, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, A.E.; Bergeman, C.; Clark, L.A.; Wirth, M.M. Aging and the HPA axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev. 2016, 68, 928–945. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Lee, J.-S.; Park, S.-Y.; Kim, S.-J.; Son, C.-G. Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 7. [Google Scholar] [CrossRef]
- Lautenschläger, J. Present state of medication therapy in fibromyalgia syndrome. Scand. J. Rheumatol. 2000, 29, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]

| Items | Count |
|---|---|
| N. of RCT | 10 |
| N. of participants (female) | 552 (383) |
| Mean N. of participant (±SD) | 55.2 ± 30.1 |
| Mean age (±SD) a | 49.6 ± 12.0 |
| Condition of participants (N. of RCT, %) | 10 (100.0) |
| Fibromyalgia | 4 (40.0) |
| Chronic neck pain | 2 (20.0) |
| Sleep bruxism | 2 (20.0) |
| Obstructive sleep apnea | 1 (10.0) |
| Post-cardiac surgery | 1 (10.0) |
| Type of intervention (N. of RCTs, %) b | 10 (100.0) |
| Soft tissue technique (STT) | 7 (70.0) |
| Joint manipulation (JM) | 6 (60.0) |
| Myofascial technique (MFT) | 6 (60.0) |
| Muscle energy technique (MET) | 2 (20.0) |
| Mean treatment time per session (minutes ± SD) | 51.9 ± 41.8 |
| Mean N. of treatment time (±SD) | 11.6 ± 5.1 |
| Mean treatment period (weeks ± SD) | 5.6 ± 3.9 |
| Mean N. of measurements per RCT (±SD) | 6.2 ± 2.4 |
| Measurement for sleep quality (N. of RCTs, %) b | |
| Pittsburgh Sleep Quality Index (PSQI) | 9 (90.0) |
| Epworth Sleepiness Scale (ESS) | 2 (20.0) |
| Patient Reported Outcomes Measurement Information System (PROMIS-sleep disturbance subscale) | 1 (10.0) |
| Polysomnography (PSG) | 1 (10.0) |
| Disorder (Author, Year) | N. of Participants (Female, Mean Age) | Intervention (Control, Tx. Period) | Type of Tx. a | Finding (Statistical Significance) | |
|---|---|---|---|---|---|
| Subject Sleep Quality | Others | ||||
| Primary Sleep Disorder | |||||
| Obstructive sleep apnea (Paolucci, 2023) [21] | 52 (30, 61.0) | MFT d (myofunctional therapy, 4 weeks) | MFT | PSQI (total score): p < 0.01 ESS (total score): NS | snoring, SpO2: p < 0.05 AHI c, N. of apneas, ODI, heart rate: NS |
| Sleep bruxism (Kadıoğlu, 2024) [22] | 30 (21, 18–25 b) | manual therapy (exercise, 8 weeks) | JM, MFT, STT | PSQI (total score) c: p < 0.01 | pain c, stress c, QoL c, bruxism symptom, trigger point: NS |
| Sleep bruxism (Örenler, 2022) [23] | 29 (29, 28.4) | manual therapy (occlusal splint, 8 weeks) | JM, MFT | PSQI (total score): p < 0.05 | TMJ ROM, satisfaction: p < 0.05 pain, physical function: p < 0.01 |
| Secondary Sleep Disorder | |||||
| Chronic neck pain (Cholewicki, 2022) [24] | 87 (66, 42.1) | osteopathic manual therapy (wait-list, 4–6 weeks) | JM, MET, MFT, STT | PROMIS (sleep disturbance): p < 0.01 | pain c: p < 0.01 physical function c, fatigue, depression: p < 0.05 activity, work, social role, anxiety: NS |
| Chronic neck pain (Hadamus, 2021) [25] | 40 (32, 63.5) | MET d (Swedish massage, 2 weeks) | MET | PSQI (total score) c: p < 0.01 | - |
| Fibromyalgia (Ughreja, 2024) [26] | 66 (58, 37.3) | craniosacral therapy (sham Tx., 12 weeks) | JM, MFT, STT | PSQI (total score) c: p < 0.05 | PPT, FM symptom, physical function, fatigue, kinesiophobia, emotional distress: NS |
| Fibromyalgia (Nadal-Nicolás, 2020) [27] | 24 (24, 53.0) | massage (placebo, 4 weeks) | STT | PSQI (total score) c: NS | pain c: p < 0.05 fatigue c, mood c: NS |
| Fibromyalgia (Castro Sánchez, 2019) [28] | 64 (58, 47.1) | MFT (dry needling, 4 weeks) | MFT, STT | PSQI (total score): NS | trigger pont c, QoL, FM symptom, pain, anxiety, depression, fatigue: NS |
| Fibromyalgia (Moustafa, 2015) [29] | 120 (52, 52.5) | manual therapy d (rehabilitative program 12 weeks) | JM | PSQI (total score): NS | spinal posture: p < 0.01 FM symptom c, pain, PPT, anxiety, depression: NS |
| Post-cardiac surgery (Nerbass, 2010) [30] | 40 (13, 61.9) | massage (no Tx., 3 days) | JM, STT | Subscales of PSQI, ESS sleep effectiveness c: p < 0.05 total sleep time c, sleep disorder c: NS | fatigue, pain: NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Go, D.-H.; Kim, H.-J.; Lee, N.-W.; Lee, Y.J.; Lee, S.-H.; Ha, I.-H. Effect of Cervical Manual Therapy on Sleep Quality: A Scoping Review of Randomized Controlled Trials. Life 2025, 15, 1557. https://doi.org/10.3390/life15101557
Kim D-Y, Go D-H, Kim H-J, Lee N-W, Lee YJ, Lee S-H, Ha I-H. Effect of Cervical Manual Therapy on Sleep Quality: A Scoping Review of Randomized Controlled Trials. Life. 2025; 15(10):1557. https://doi.org/10.3390/life15101557
Chicago/Turabian StyleKim, Do-Young, Dong-Hyun Go, Hak-Jae Kim, Nam-Woo Lee, Yoon Jae Lee, Sook-Hyun Lee, and In-Hyuk Ha. 2025. "Effect of Cervical Manual Therapy on Sleep Quality: A Scoping Review of Randomized Controlled Trials" Life 15, no. 10: 1557. https://doi.org/10.3390/life15101557
APA StyleKim, D.-Y., Go, D.-H., Kim, H.-J., Lee, N.-W., Lee, Y. J., Lee, S.-H., & Ha, I.-H. (2025). Effect of Cervical Manual Therapy on Sleep Quality: A Scoping Review of Randomized Controlled Trials. Life, 15(10), 1557. https://doi.org/10.3390/life15101557






