Ischemia with No Obstructive Coronary Artery Disease (INOCA): A Review
Abstract
1. Introduction
1.1. Pathophysiology of INOCA
1.2. Diagnostic Approach to INOCA
2. Material and Methods
2.1. Conventional Non-Surgical Treatment of INOCA
2.1.1. Pharmacologic Therapy
2.1.2. Lifestyle Modifications
2.2. Nonpharmacological Treatment Options for INOCA
2.3. Transmyocardial Revascularization (TMR)
2.4. Sympathectomy
2.4.1. Percutaneous Sympathetic Nerve Ablation
2.4.2. Surgical Sympathectomy
2.5. Coronary Sinus Reducer
2.5.1. Autologous Stem Cell Therapy
2.5.2. Selective Surgical Cardiac Vein Retroperfusion
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar]
- Hansen, B.; Holtzman, J.N.; Juszczynski, C.; Khan, N.; Kaur, G.; Varma, B.; Gulati, M. Ischemia with No Obstructive Arteries (INOCA): A Review of the Prevalence, Diagnosis and Management. Curr. Probl. Cardiol. 2023, 48, 101420. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Shaw, R.E.; Merz, C.N.B.; Brindis, R.G.; Klein, L.W.; Nallamothu, B.; Douglas, P.S.; Krone, R.J.; McKay, C.R.; Block, P.C.; et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008, 117, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Fu, B.; Wei, X.; Lin, Y.; Chen, J.; Yu, D. Pathophysiologic Basis and Diagnostic Approaches for Ischemia With Non-obstructive Coronary Arteries: A Literature Review. Front. Cardiovasc. Med. 2022, 9, 731059. [Google Scholar] [CrossRef]
- Benenati, S.; Campo, G.; Seitun, S.; Caglioni, S.; Leone, A.M.; Porto, I. Ischemia with non-obstructive coronary artery (INOCA): Non-invasive versus invasive techniques for diagnosis and the role of #FullPhysiology. Eur. J. Intern. Med. 2024, 127, 15–24. [Google Scholar]
- Montone, R.A.; Caffè, A.; Yasumura, K.; Kini, A. Routine diagnosis of ANOCA/INOCA: Pros and cons. EuroIntervention 2025, 21, e293–e295. [Google Scholar] [CrossRef]
- Sadaba, J.R.; Nair, U.R. Selective arterialization of the coronary venous system. Ann. Thorac. Surg. 2004, 78, 1458–1460. [Google Scholar] [CrossRef]
- Briones, E.; Lacalle, J.R.; Marin-Leon, I.; Rueda, J.R. Transmyocardial laser revascularization versus medical therapy for refractory angina. Cochrane Database Syst Rev 2015, 2015, CD003712. [Google Scholar] [CrossRef]
- Verheye, S.; Jolicœur, E.M.; Behan, M.W.; Pettersson, T.; Sainsbury, P.; Hill, J.; Vrolix, M.; Agostoni, P.; Engstrom, T.; Labinaz, M.; et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N. Engl. J. Med. 2015, 372, 519–527. [Google Scholar] [CrossRef]
- Corban, M.T.; Toya, T.; Albers, D.; Sebaali, F.; Lewis, B.R.; Bois, J.; Gulati, R.; Prasad, A.; Best, P.J.; Bell, M.R.; et al. IMPROvE-CED Trial: Intracoronary Autologous CD34+ Cell Therapy for Treatment of Coronary Endothelial Dysfunction in Patients With Angina and Nonobstructive Coronary Arteries. Circ. Res. 2022, 130, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Keramida, G.; Baksi, A.J.; de Silva, R. Implantation of the coronary sinus reducer for refractory angina due to coronary microvascular dysfunction in the context of apical hypertrophic cardiomyopathy-a case report. Eur. Heart J.-Case Rep. 2022, 6, ytac440. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Merz, C.N.B.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients With Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, e010802. [Google Scholar] [CrossRef] [PubMed]
- Tryon, D.; Corban, M.T.; Alkhouli, M.; Prasad, A.; Raphael, C.E.; Rihal, C.S.; Reeder, G.S.; Lewis, B.; Albers, D.; Gulati, R.; et al. Coronary Sinus Reducer Improves Angina, Quality of Life, and Coronary Flow Reserve in Microvascular Dysfunction. JACC Cardiovasc. Interv. 2024, 17, 2893–2904. [Google Scholar] [CrossRef]
- Foley, M.J.; Rajkumar, C.A.; Ahmed-Jushuf, F.; Simader, F.A.; Chotai, S.; Pathimagaraj, R.H.; Mohsin, M.; Salih, A.; Wang, D.; Dixit, P.; et al. Coronary sinus reducer for the treatment of refractory angina (ORBITA-COSMIC): A randomised, placebo-controlled trial. Lancet 2024, 403, 1543–1553. [Google Scholar] [CrossRef]
- Verheye, S.; Agostoni, P.; Giannini, F.; Hill, J.M.; Jensen, C.; Lindsay, S.; Stella, P.R.; Redwood, S.; Banai, S.; Konigstein, M. Coronary sinus narrowing for the treatment of refractory angina: A multicentre prospective open-label clinical study (the REDUCER-I study). EuroIntervention 2021, 17, 561–568. [Google Scholar] [CrossRef]
- Allen, K.B.; Kelly, J.; Borkon, A.M.; Stuart, R.S.; Daon, E.; Pak, A.F.; Zorn, G.L.; Haines, M. Transmyocardial laser revascularization: From randomized trials to clinical practice. A review of techniques, evidence-based outcomes, and future directions. Anesthesiol. Clin. 2008, 26, 501–519. [Google Scholar] [CrossRef]
- Horvath, K.A. Transmyocardial laser revascularization. J. Card. Surg. 2008, 23, 266–276. [Google Scholar] [CrossRef]
- Allen, K.B.; Dowling, R.D.; DelRossi, A.J.; Realyvasques, F.; Lefrak, E.A.; Pfeffer, T.A.; Fudge, T.L.; Mostovych, M.; Schuch, D.; Szentpetery, S.; et al. Transmyocardial laser revascularization combined with coronary artery bypass grafting: A multicenter, blinded, prospective, randomized, controlled trial. J. Thorac. Cardiovasc. Surg. 2000, 119, 540–549. [Google Scholar] [CrossRef]
- Coote, J.H.; Chauhan, R.A. The sympathetic innervation of the heart: Important new insights. Auton. Neurosci. 2016, 199, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hadaya, J.; Ardell, J.L. Autonomic Modulation for Cardiovascular Disease. Front. Physiol. 2020, 11, 617459. [Google Scholar] [CrossRef] [PubMed]
- Sungur, M.A.; Zeren, G.; Yılmaz, M.F.; Avcı, İ.İ.; Can, F.; Çetin, T.; Sungur, A.; Tezen, O.; Yücel, E.; Karagöz, A.; et al. Endoscopic Thoracic Sympathectomy in the Treatment of Vasospastic Angina Resistant to Medical Therapy. Anatol. J. Cardiol. 2024, 28, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.C.; Navaratnarajah, M.; Taggart, D.P. Does surgical sympathectomy improve clinical outcomes in patients with refractory angina pectoris? Interact. Cardiovasc. Thorac. Surg. 2016, 22, 488–492. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. ESC Scientific Document Group. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Harig, F.; Schmidt, J.; Hoyer, E.; Eckl, S.; Adamek, E.; Ertel, D.; Nooh, E.; Amann, K.; Weyand, M.; Ensminger, S.M. Long-term evaluation of a selective retrograde coronary venous perfusion model in pigs (Sus scrofa domestica). Comp. Med. 2011, 61, 150–157. [Google Scholar]
- Harig, F.; Hoyer, E.; Labahn, D.; Schmidt, J.; Weyand, M.; Ensminger, S.M. Refinement of pig retroperfusion technique: Global retroperfusion with ligation of the azygos connection preserves hemodynamic function in an acute infarction model in pigs (Sus scrofa domestica). Comp. Med. 2010, 60, 38–44. [Google Scholar]
- Munz, M.; Amorim, M.J.; Faria, M.; Vicente, C.; Pinto, A.; Monteiro, J.; Leite-Moreira, A.F.; Águas, A.P. Cardiac venous arterialization in acute myocardial infarction: How great is the benefit? Interact. Cardiovasc. Thorac. Surg. 2013, 16, 307–313. [Google Scholar] [CrossRef]
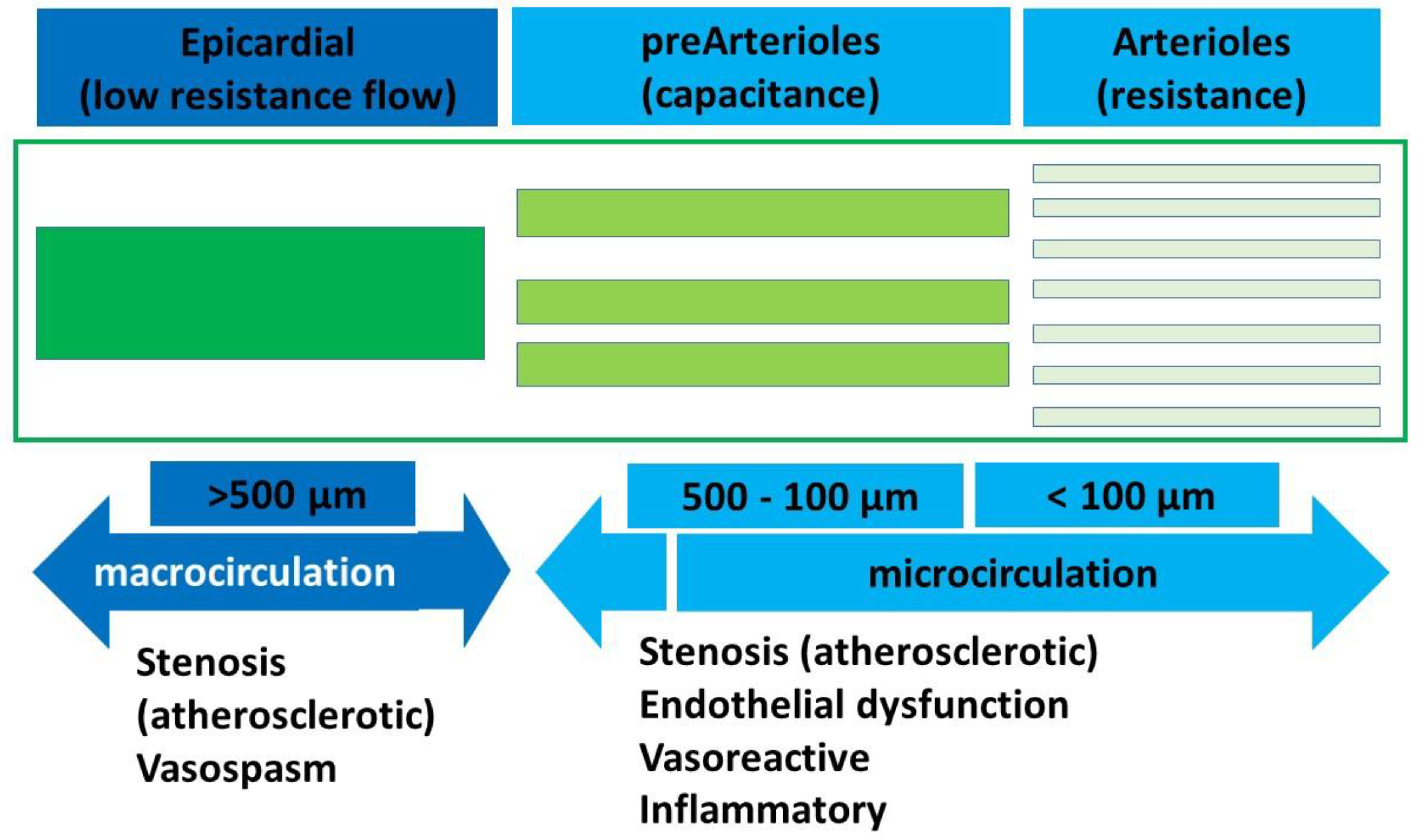
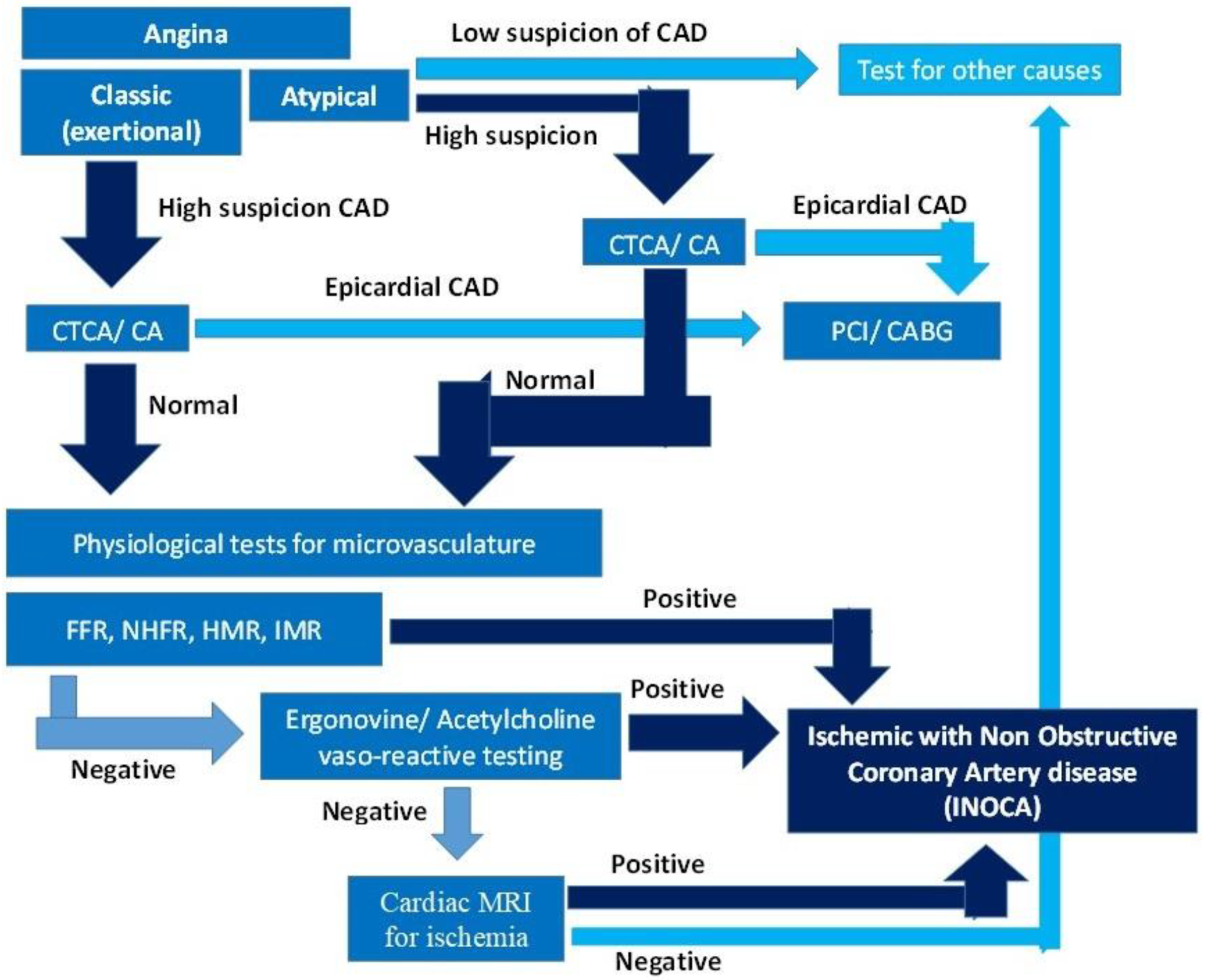

| Macrovasculature (Epicardial CAD) | Microvasculature (INOCA) | DUAL Micro- and Macrovasculature | |||
|---|---|---|---|---|---|
| Pathophysiology | Investigations | Pathophysiology | Investigations | Pathophysiology | Investigations |
| Stenotic | CTCA/CA shows discreet stenosis/diffuse disease FFR positive | Structural/functional -Cardiovascular disease -Endothelial dysfunction -Ventricular hypertrophy -Cardiomyopathies | Invasive physiologic assessment FFR > 0.80 or NHPR > 0.89 CFR < 2.0–2.5 IMR > 25 U or HMR >2.5 mm Hg/cm/s | Atherosclerotic disease Vasospastic disease | Positive CA/CTCA with positive microvascular physiological assessment |
| Ectatic | CTCA/CA | Microvascular vasospastic | Ischemia on vasoreactive testing No epicardial artery constriction Chest pain Ischemic ECG changes (ST-segment depression or elevation >0.1 mV) in at least two contiguous leads | ||
| Myocardial bridging/anomalous course | CTCA shows external compression CMR/stress echo shows Ischemic positive | Diffuse mixed/isolated microvascular -Cardiovascular disease -Atherosclerosis | FFR < 0.80 or NHPR < 0.89 with gradual step-up on pull-back Intravascular imaging may show diffuse disease | ||
| Epicardial vasospastic | Vasoreactive test positive | ||||
| Imaging Tests | Physiological Tests | ||
|---|---|---|---|
| Coronary Angiography (CA) | Gold standard for epicardial disease resolution—0.1–0.2 mm cannot image microvasculature | Fractional Flow Reserve (FFR) | Invasive. Measures pressure drops across the circulation. Typically values less than 0.80 are considered significant (indexed FFR < 0.89). |
| CT Coronary Angiogram (CTCA) | Less invasive than CA—lower resolution of 0.3–0.4 mm | Coronary Flow Reserve (CFR) | Microvascular dysfunction, key marker of INOCA. <2.0 is impaired reserve. Correlates with clinical outcomes. |
| Cardiac Magnetic Resonance Imaging (cMRI) | Myocardial perfusion and ischemic areas Late Gadolinium Enhancement (LGE) identify myocardial injury or fibrosis | Non-Hyperemic Pressure Ratio (NHPR) | Indicative of coronary microvascular resistance. NHPR < 0.89 with gradual step-up on pull-back. |
| Positron Emission Tomography (PET) | Myocardial perfusion, coronary blood flow, and metabolic activity highly sensitive in detecting subclinical myocardial ischemia | Hyperemic Microvascular Resistance (HMR) | Indicative of microvascular dysfunction HMR > 2.5 mm Hg/cm/s is high. |
| Intravascular Ultrasound (IVUS) | Cross-sectional plaque burden and signs of microvascular disease in surrounding tissue. | Index of Microcirculatory Resistance (IMR) | IMR > 25 U is high. |
| Optical Coherence Tomography (OCT) | Endothelial and microvascular abnormalities with infra-red light | Stress Testing— Exercise Treadmill Testing and Dobutamine Stress Testing (Stress Echocardiography) | Assesses silent myocardial ischemia. |
| Nuclear Stress Tests (SPECT/PET) | Show regional myocardial perfusion defects. |
| Anti-Anginal Agents | Lifestyle Modification | |
|---|---|---|
| Nitrates | Improve coronary arteries dilatation and myocardial perfusion. Relieve symptoms of chest pain or discomfort due to reduced coronary blood flow Do not treat underlying microvascular dysfunction in INOCA. | Exercise and Physical Activity |
| Beta-Blockers | Reduce heart rate, myocardial contractility and myocardial oxygen demand. Improve endothelial function by reducing sympathetic stimulation. Decreasing oxidative stress in the coronary microcirculation. | |
| Calcium Channel Blockers | Reduce vascular resistance by coronary dilatation. Reduce myocardial oxygen demand by lowering heart rate and contractility. Effective in treating both microvascular dysfunction and vasospasm. | |
| Ranolazine | Reduces intracellular calcium overload, improves myocardial relaxation and perfusion. Active in refractory angina and non-responders to other anti-anginals. Improves microvascular dysfunction without affecting heart rate or blood pressure. | |
| Nicorandil | Dual properties of nitrates at low doses (epicardial vessels) and ATP-sensitive K+ channel opener at high doses (decreases microvascular resistance). Effective even where nitrates are not effective. | |
| Antiplatelet therapy Aspirin | reduces inflammation and risk of thrombotic events stabilizes non-obstructive atherosclerotic plaques by platelet inhibition. | Weight Management and Healthy Diet |
| Clopidogrel (P2Y12 inhibitors) | reduce increased thrombotic risk imrove thrombotic microvascular injury. | |
| Statins | lower cholesterol levels by HMGCoA reductase inhibition. improve endothelial function and reduce inflammation. reduce the overall risk of cardiovascular events. | Stress Management and Mental Health |
| ACE Inhibitors and ARBs | lower blood pressure, reduce strain by inhibiting renin-angiotensin-aldosterone system. promote vasodilation and improve endothelial function. | Smoking Cessation and Alcohol Moderation |
| If current inhibitors | New class of drugs; blocks If pacemaker current (funny current) in the sinus node (SA) reduces heart rate without affecting cardiac contractility by blocking the HCN channels and delaying the repolarization. Mainly indicated for BB/CCB intolerance as acts through SA node | |
| Antiinflammatory drugs and steroids | Reduce inflammation and tissue odema and improve microcirculation in inflammatory cardiomyopathies |
| Study Name Author Year (PMID) [Ref] | Design | Population | No. Patients | Intervention | Primary Outcomes | Results |
|---|---|---|---|---|---|---|
| Sadaba et al. 2004 (15464519) [9] | Case report | Refractory angina | 1 | CABG | Angina | Complete relief of angina. |
| Briones et al. 2015 (25721946) [10] | Meta-analysis (seven studies) | Refractory angina—not for PCI/CABG | 1137 | TMR vs. OMT | Mortality angina score | The 30-day mortality was at 6.8% following TMLR compared with 0.8% OMT, and subjective improvements in angina score were compounded by bias. |
| CORSIRA Verheye et al. 2015 (25651246) [11] | Randomized control trial | Refractory angina | 104 52 CSR 52 Sham | CSR vs. Sham | +2 CCS angina class improvement | Primary endpoint was achieved in 35% (18/52) of the reducer-treated patients versus 15% (8/52) of the sham (p = 0.02). At least one CCS angina class improvement in 71% vs. 42% in the sham (p = 0.003).QoL improved significantly in the reducer group compared to the sham (17.6 vs. 7.6 points, p = 0.03). |
| IMPROvE-CED Corban et al. 2022 (34923853) [12] | Clinical trial | INOCA | 20 | Intracoronary (LAD) autologous CD34+ cell therapy | Safety, angina score, sublingual GTN usage, and exercise tolerance | The infusion was safe and resulted in statistically significant decrease in Canadian Cardiovascular Society angina class (p = 0.00018) and sublingual GTN use (p = 0.00047), but no significant improvement in exercise tolerance compared with historical control patients, following a one-off infusion via the LAD |
| Cheng et al. 2022 (36415685) [13] | Case report | INOCA | 1 | CSR | Symptom relief, MPR, and ischemia burden at six months | A marked reduction in ischemia burden, improved global MPR, symptoms, and quality of life |
| NCT03508609 Henry et al. 2022 (35067072) [14] | Clinical trial | INOCA | 20 | Intracoronary (LAD) autologous CD34+ cell therapy | Efficacy: coronary flow reserve, angina score, and exercise tolerance | Improved coronary flow reserve (CRF) at six months (p < 0.005); decreased angina (p < 0004); improved angina class (p < 0.001); and improved quality of life score p < 0.03). No serious adverse events noted. Comparisons made with patient scores at baseline (pre-intervention). |
| G200153 Tyron et al. 2024 (39520443) [15] | Clinical trial | INOCA | 30 | CSR | Coronary flow reserve (CFR); coronary blood flow (CBF) | Increased CFR from 2.1 to 2.7 (p = 0.0011); increased CBF from 11% to 11.5% (p = 0.042); improved CCS angina class from 4 to 2 (p < 0.0001); and improved quality of life across all questionnaire domains (p < 0.0006). |
| ORBITA-COSMIC Foley et al. 2024 (38604209) [16] | Randomized control trial | Refractory angina | 51 25—CSR 26—placebo | CSR vs. placebo | Angina improvement at 6 m (ORBITA app) | Myocardial blood flow (MBF) in ischaemic segments did not improve with CSR compared with placebo (difference 0·06 mL/min per g [95% CI −0.09 to 0.20]; Pr(Benefit) = 78.8%). Daily angina episodes reduced with CSR compared with placebo (OR 1.40 [95% CI 1.08 to 1.83]; Pr(Benefit) = 99.4%). |
| REDUCER-I Verheye et al. 2024 (39520437) [17] | Real world multicenter registry | Refractory angina | 400 | CSR | Improved CCS score at six months and improved Seattle Angina Questionnaire score at six months | 69.8% patients improved by >1 CCS class at six months and interim three year results showed sustained improvements (p < 0.0001). |
| Study Name, Author Year (PMID) [Ref] | Evidence Level | Population | Strengths | Limitations | Pathophysiological Groups Likely to Benefit |
|---|---|---|---|---|---|
| Sadaba et al. 2004 (15464519) [9] | Case report | Refractory angina | - | Limited evidence Pathophysiology is unclear Subjective endpoint | Unclear |
| Briones et al. 2015 (25721946) [10] | Meta-analysis (seven studies) | Refractory angina—not for PCI/CABG | Large evidence base Meta-analysis of seven studies Control arm | Mainly observational studies Cohorts not INOCA Pathophysiology unclear No physiological evaluation for pathophysiology No post intervention physiological tests Subjective endpoints Placebo effects | Most with refractory angina with multiple pathophysiologies |
| CORSIRA Verheye et al. 2015 (25651246) [11] | Randomized control trial | Refractory angina | Control group Randomized groups First RCT for CSR | Small numbers Not INOCA Pathophysiology unclear Not generalizable No physiological measures Subjective endpoints | Diffuse coronary disease Ungraftable vessels Microvascular disease Failed OMT |
| IMPROvE-CED Corban et al. 2022 (34923853) [12] | Clinical trial | INOCA | Clinical trail setting Well-defined INOCA group Clear methodology and endpoints | Limited numbers Very controlled group Confounders with other treatments No imaging Subjective endpoints | Diffuse coronary disease Ungraftable vessels Microvascular disease Failed OMT |
| Cheng et al. 2022 (36415685) [13] | Case report | INOCA | - | Limited report Subjective assessment Not generalizable | - |
| NCT03508609 Henry et al. 2022 (35067072) [14] | Clinical trial | INOCA | Clinical trail setting Well-defined INOCA group Clear methodology and endpoints CRF measured | Limited numbers Very controlled group Confounders with other treatments Subjective endpoints Results not generalizable | Microvascular disease Failed OMT |
| G200153 Tyron et al. 2024 (39520443) [15] | Clinical trial | INOCA | Clinical trail setting Well-defined group Clear methodology and endpoints CFR, CBR measured | Limited numbers Very controlled group Confounders with other treatments No imaging Subjective endpoints Results not generalizable | Microvascular disease Failed OMT |
| ORBITA-COSMIC Foley et al. 2024 (38604209) [16] | Randomized control trial | Refractory angina | Clinical trail setting Well-defined group Clear methodology and endpoints MBF measurements | Limited numbers Very controlled group Confounders with other treatments No imaging No physiological assessment Subjective endpoints Results may be generalizable | Microvascular disease Failed OMT |
| REDUCER-I Verheye et al. 2024 (39520437) [17] | Real world multicenter registry | Refractory angina | Real world setting Large number Long term follow-up | Limited numbers Ill defined group Confounders with other treatments No imaging No physiological assessment Subjective endpoints Results may be generalizable | Microvascular disease Failed OMT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, L.; Masters, M.; Shafiq, U.; Jujjavarapu, K.R.; Luthra, S. Ischemia with No Obstructive Coronary Artery Disease (INOCA): A Review. Life 2025, 15, 1554. https://doi.org/10.3390/life15101554
Viola L, Masters M, Shafiq U, Jujjavarapu KR, Luthra S. Ischemia with No Obstructive Coronary Artery Disease (INOCA): A Review. Life. 2025; 15(10):1554. https://doi.org/10.3390/life15101554
Chicago/Turabian StyleViola, Laura, Megan Masters, Umar Shafiq, Krishnam Raju Jujjavarapu, and Suvitesh Luthra. 2025. "Ischemia with No Obstructive Coronary Artery Disease (INOCA): A Review" Life 15, no. 10: 1554. https://doi.org/10.3390/life15101554
APA StyleViola, L., Masters, M., Shafiq, U., Jujjavarapu, K. R., & Luthra, S. (2025). Ischemia with No Obstructive Coronary Artery Disease (INOCA): A Review. Life, 15(10), 1554. https://doi.org/10.3390/life15101554







