Integrative Phenotyping of Knee Osteoarthritis: Linking WOMAC Cut-Offs, Kellgren–Lawrence Grades, and Cluster Analysis for Personalized Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design: Participants
2.2. Clinical Outcome Measures
2.3. Metabolic Assessments
2.4. Radiographic Assessments
2.5. Statistical Analysis
3. Results
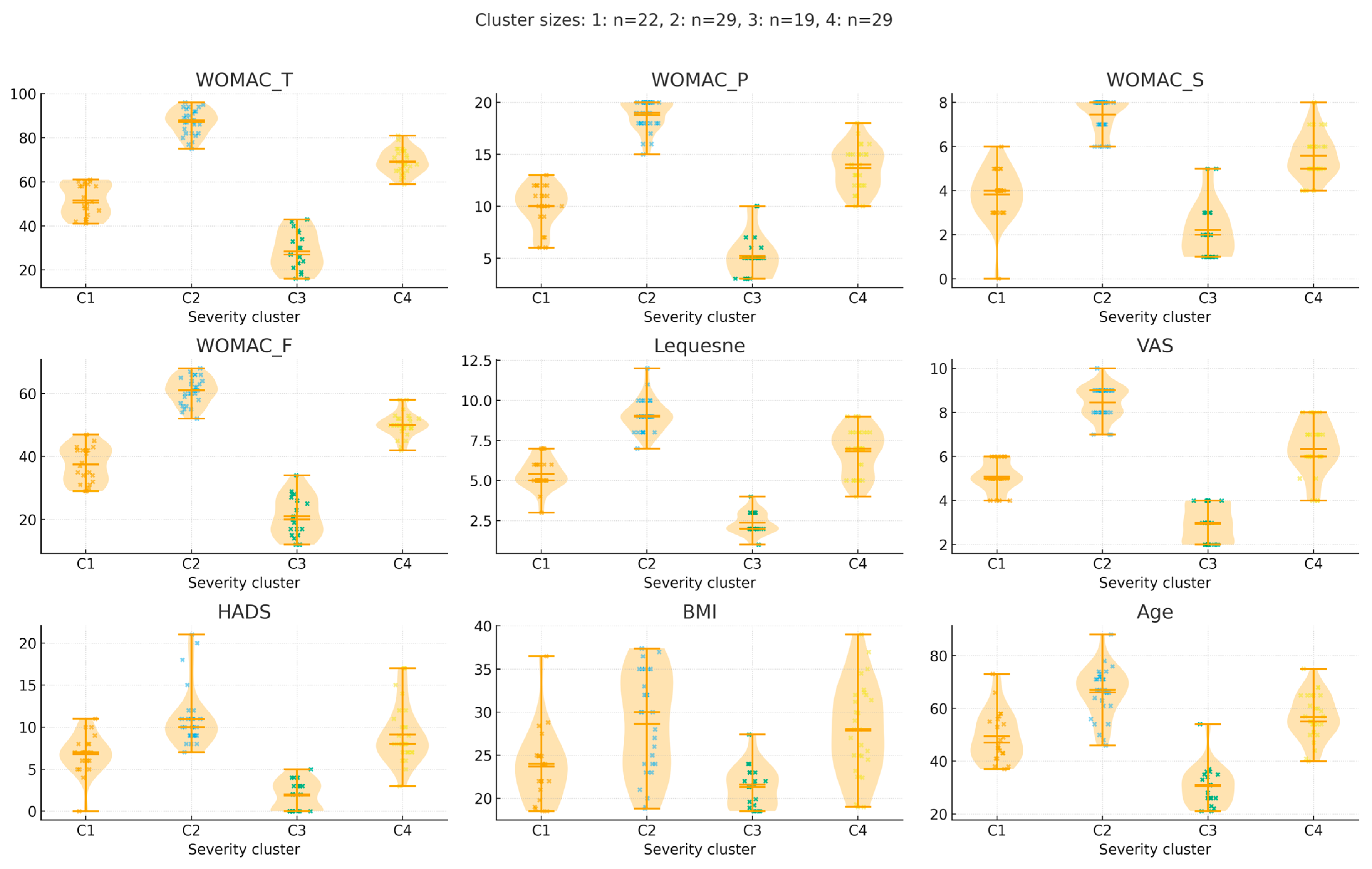
Descriptive Analysis
- WOMAC Total Score: Mild OA patients had significantly lower scores than all other groups (p < 0.001), confirming increasing severity.
- WOMAC Pain Score: Mild OA cluster differed significantly from Moderate, Moderate-Severe, and Severe OA (p < 0.001).
- WOMAC Functional and Stiffness Scores: Stepwise increases were observed across groups, all of which were statistically significant (p < 0.001).
- Joint Space Narrowing and Osteophyte Scores: Progressively higher in more severe clusters (p < 0.001).
- BMI: Clusters 3 and 4 had significantly higher BMI than Clusters 1 and 2 (p < 0.001), consistent with a metabolic phenotype in more severe OA.
4. Discussion
4.1. Clinical Interpretation of WOMAC Cut-Offs and KL Grades
4.2. Psychological and Metabolic Influences on OA Severity
4.3. Inflammation, Depression, and OA Phenotype Overlap
4.4. Personalized Management Strategies for Identified Clusters
4.4.1. Non-Pharmacological Interventions
4.4.2. Pharmacological Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Alshami, A.M. Knee osteoarthritis related pain: A narrative review of diagnosis and treatment. Int. J. Health Sci. 2014, 8, 85–104. [Google Scholar] [CrossRef]
- Nelson, A.E. How feasible is the stratification of osteoarthritis phenotypes by means of artificial intelligence? Expert Rev. Precis. Med. Drug Dev. 2020, 6, 83–85. [Google Scholar] [CrossRef]
- Bedson, J.; Croft, P.R. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet. Disord. 2008, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Son, K.M.; Hong, J.I.; Kim, D.-H.; Jang, D.-G.; Crema, M.D.; Kim, H.A. Absence of pain in subjects with advanced radiographic knee osteoarthritis. BMC Musculoskelet. Disord. 2020, 21, 640. [Google Scholar] [CrossRef] [PubMed]
- Vargas e Silva, N.C.O.; dos Anjos, R.L.; Santana, M.M.C.; Battistella, L.R.; Alfieri, F.M. Discordance between radiographic findings, pain, and superficial temperature in knee osteoarthritis. Rheumatology 2020, 58, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Eble, S.; Moschetti, W.E.; Schilling, P.L. The Discordance Between Pain and Imaging in Knee Osteoarthritis. J. Am. Acad. Orthop. Surg. 2025, 33, e786–e794. [Google Scholar] [CrossRef]
- Sonobe, T.; Otani, K.; Sekiguchi, M.; Otoshi, K.; Nikaido, T.; Konno, S.; Matsumoto, Y. Influence of Knee Osteoarthritis Severity, Knee Pain, and Depression on Physical Function: A Cross-Sectional Study. Clin. Interv. Aging 2024, 19, 1653–1662. [Google Scholar] [CrossRef]
- Sougu, C.; Diallo, M.; Laurent, Y.; Ayouba Tinni, I.; Kaboré, F.; Zabsonré Tiendrebeogo, W.J.S.; Dakouré, P.W.H.; Ouedraogo, D.D. Clinical phenotypes and associated factors in knee osteoarthritis in an African black population. Osteoarth. Cartil. 2025, 7, 100570. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Steultjens, M. Classification of patients with knee osteoarthritis in clinical phenotypes: Data from the osteoarthritis initiative. PLoS ONE 2018, 13, e0191045. [Google Scholar] [CrossRef]
- Segarra-Queralt, M.; Galofré, M.; Tio, L.; Monfort, J.; Monllau, J.C.; Piella, G.; Noailly, J. Characterization of clinical data for patient stratification in moderate osteoarthritis with support vector machines, regulatory network models, and verification against osteoarthritis Initiative data. Sci. Rep. 2024, 14, 11797. [Google Scholar] [CrossRef]
- Shtroblia, V.; Petakh, P.; Kamyshna, I.; Halabitska, I.; Kamyshnyi, O. Recent advances in the management of knee osteoarthritis: A narrative review. Front. Med. 2025, 12, 1523027. [Google Scholar] [CrossRef] [PubMed]
- Knoop, J.; van der Leeden, M.; Thorstensson, C.A.; Roorda, L.D.; Lems, W.F.; Knol, D.L.; Steultjens, M.P.M.; Dekker, J. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: Data from the osteoarthritis initiative. Arthritis Care Res. 2011, 63, 1535–1542. [Google Scholar] [CrossRef]
- Roemer, F.W.; Jarraya, M.; Collins, J.E.; Kwoh, C.K.; Hayashi, D.; Hunter, D.J.; Guermazi, A. Structural phenotypes of knee osteoarthritis: Potential clinical and research relevance. Skelet. Radiol. 2023, 52, 2021–2030. [Google Scholar] [CrossRef]
- Innmann, M.M.; Lunz, A.; Fröhlich, L.; Bruckner, T.; Merle, C.; Reiner, T.; Schiltenwolf, M. What Is the Correlation between Clinical and Radiographic Findings in Patients with Advanced Osteoarthritis of the Knee? J. Clin. Med. 2023, 12, 5420. [Google Scholar] [CrossRef]
- Szebenyi, B.; Hollander, A.P.; Dieppe, P.; Quilty, B.; Duddy, J.; Clarke, S.; Kirwan, J.R. Associations between pain, function, and radiographic features in osteoarthritis of the knee. Arthritis Rheum. 2006, 54, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Novita, M.; Fatimah, N. Correlation of Radiological Severity of Genu Osteoarthritis (Kellgren and Lawrence) with Functional Disabilities (Womac Score). Sriwijaya J. Med. 2020, 3. [Google Scholar] [CrossRef]
- Longo, U.G.; Papalia, R.; Campi, S.; De Salvatore, S.; Piergentili, I.; Bandini, B.; Lalli, A.; Denaro, V. Evaluating the Minimum Clinically Important Difference and Patient Acceptable Symptom State for the Womac Osteoarthritis Index after Unicompartmental Knee Arthroplasty. J. Clin. Med. 2023, 12, 7618. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Engen, C.N.; Øiestad, B.E.; Engebretsen, L.; Risberg, M.A. Defining the presence of radiographic knee osteoarthritis: A comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3532–3539. [Google Scholar] [CrossRef]
- Felson, D.T.; Niu, J.; Guermazi, A.; Sack, B.; Aliabadi, P. Defining radiographic incidence and progression of knee osteoarthritis: Suggested modifications of the Kellgren and Lawrence scale. Ann. Rheum. Dis. 2011, 70, 1884–1886. [Google Scholar] [CrossRef]
- Neogi, T.; Felson, D.; Niu, J.; Nevitt, M.; Lewis, C.E.; Aliabadi, P.; Sack, B.; Torner, J.; Bradley, L.; Zhang, Y. Association between radiographic features of knee osteoarthritis and pain: Results from two cohort studies. BMJ 2009, 339, b2844. [Google Scholar] [CrossRef]
- Elhamid, Y.A.A.; Elazkalany, G.S.; Niazy, M.H.; Afifi, A.Y. Anxiety and depression in primary knee osteoarthritis patients: Are they related to clinical features and radiographic changes? Rheumatology 2024, 62, 421–429. [Google Scholar] [CrossRef] [PubMed]
- White, D.K.; Master, H. Patient-Reported Measures of Physical Function in Knee Osteoarthritis. Rheum. Dis. Clin. N. Am. 2016, 42, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Lequesne, M.G.; Mery, C.; Samson, M.; Gerard, P. Indexes of severity for osteoarthritis of the hip and knee. Validation—Value in comparison with other assessment tests. Scand. J. Rheumatol. Suppl. 1987, 65, 85–89. [Google Scholar] [CrossRef]
- da Costa, B.; Saadat, P.; Basciani, R.; Agarwal, A.; Johnston, B.; Jüni, P. Visual Analogue Scale has higher assay sensitivity than WOMAC pain in detecting between-group differences in treatment effects: A meta-epidemiological study. Osteoarthr. Cartil. 2021, 29, 304–312. [Google Scholar]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Sakellariou, G.; Conaghan, P.G.; Zhang, W.; Bijlsma, J.W.J.; Boyesen, P.; D’AGostino, M.A.; Doherty, M.; Fodor, D.; Kloppenburg, M.; Miese, F.; et al. EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann. Rheum. Dis. 2017, 76, 1484–1494. [Google Scholar] [CrossRef]
- Fox, M.G.; Chang, E.Y.; Amini, B.; Bernard, S.A.; Gorbachova, T.; Ha, A.S.; Iyer, R.S.; Lee, K.S.; Metter, D.F.; Mooar, P.A.; et al. ACR Appropriateness Criteria® Chronic Knee Pain. J. Am. Coll. Radiol. 2018, 15, S302–S312. [Google Scholar] [CrossRef]
- Steyerberg, E.W. Chapter on internal validation, bootstrap methods. In Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Garcia-Vidal, C.; Teijón-Lumbreras, C.; Aiello, T.F.; Chumbita, M.; Menendez, R.; Mateu-Subirà, A.; Peyrony, O.; Monzó, P.; Lopera, C.; Gallardo-Pizarro, A.; et al. K-Means Clustering Identifies Diverse Clinical Phenotypes in COVID-19 Patients: Implications for Mortality Risks and Remdesivir Impact. Infect. Dis. Ther. 2024, 13, 715–726. [Google Scholar] [CrossRef]
- Jiang, Y.; Dang, Y.; Wu, Q.; Yuan, B.; Gao, L.; You, C. Using a k-means clustering to identify novel phenotypes of acute ischemic stroke and development of its Clinlabomics models. Front. Neurol. 2024, 27, 1366307. [Google Scholar] [CrossRef]
- Chan, A.K.; Wozny, T.A.; Bisson, E.F.; Pennicooke, B.H.; Bydon, M.; Glassman, S.D.; Foley, K.T.; Shaffrey, C.I.; Potts, E.A.; Shaffrey, M.E.; et al. Classifying Patients Operated for Spondylolisthesis: A K-Means Clustering Analysis of Clinical Presentation Phenotypes. Neurosurgery 2021, 89, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khanna, V. Correlation between clinical and radiological grading of osteoarthritis. Sci. J. Med. Sci. 2014, 3, 320–324. [Google Scholar] [CrossRef]
- Pereira, D.; Severo, M.; Barros, H.; Branco, J.; Santos, R.A.; Ramos, E. The effect of depressive symptoms on the association between radiographic osteoarthritis and knee pain: A cross-sectional study. BMC Musculoskelet. Disord. 2013, 14, 214. [Google Scholar] [CrossRef]
- Creamer, P.; Lethbridge-Cejku, M.; Hochberg, M.C. Factors associated with functional impairment in symptomatic knee os-teoarthritis. Rheumatology 2000, 39, 490–496. [Google Scholar] [CrossRef]
- Kumar, D.; Wyatt, C.; Lee, S.; Nardo, L.; Link, T.; Majumdar, S.; Souza, R. Association of cartilage defects, and other MRI findings with pain and function in individuals with mild–moderate radiographic hip osteoarthritis and controls. Osteoarthr. Cartil. 2013, 21, 1685–1692. [Google Scholar] [CrossRef]
- Kim, K.W.; Han, J.W.; Cho, H.J.; Chang, C.B.; Park, J.H.; Lee, J.J.; Lee, S.B.; Seong, S.C.; Kim, T.K. Association between comorbid depression and osteoarthritis symptom severity in patients with knee osteoarthritis. J. Bone Jt. Surg. 2011, 93, 556–563. [Google Scholar] [CrossRef]
- Solomon, L.; Warwick, D.; Nayagam, S. Apley’s System of Orthopaedics and Fractures, 9th ed.; Hodder Arnold: London, UK, 2010. [Google Scholar] [CrossRef]
- Konstantinidis, G.A.; Aletras, V.H.; Kanakari, K.-A.; Natsis, K.; Bellamy, N.; Niakas, D. Comparative validation of the WOMAC osteoarthritis and Lequesne algofunctional indices in Greek patients with hip or knee osteoarthritis. Qual. Life Res. 2014, 23, 539–548. [Google Scholar] [CrossRef]
- El Monaem, S.M.A.; Hashaad, N.I.; Ibrahim, N.H. Correlations between ultrasonographic findings, clinical scores, and depression in patients with knee osteoarthritis. Eur. J. Rheumatol. 2017, 4, 205–209. [Google Scholar] [CrossRef]
- Shumnalieva, R.; Kotov, G.; Monov, S. Obesity-Related Knee Osteoarthritis—Current Concepts. Life 2023, 13, 1650. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.; Au, M.; Ni, J.; Wen, C. Association between hypertension and osteoarthritis: A systematic review and meta-analysis of observational studies. J. Orthop. Transl. 2021, 32, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Bierma-Zeinstra, S.; Dell’Accio, F.; Kraus, V.B.; Nakata, K.; Sekiya, I.; Arden, N.K.; Lohmander, L.S. Toward classification criteria for early osteoarthritis of the knee. Semin. Arthritis Rheum. 2018, 47, 457–463. [Google Scholar] [CrossRef]
- Mahmoudian, A.; Lohmander, L.S.; Jafari, H.; Luyten, F.P. Towards classification criteria for early-stage knee osteoarthritis: A population-based study to enrich for progressors. Semin. Arthritis Rheum. 2021, 51, 285–291. [Google Scholar] [CrossRef]
- Migliore, A.; Scirè, C.A.; Carmona, L.; Herrero-Beaumont, G.; Bizzi, E.; Branco, J.; Carrara, G.; Chevalier, X.; Collaku, L.; Aslanidis, S.; et al. The challenge of the definition of early symptomatic knee osteoarthritis: A proposal of criteria and red flags from an international initiative promoted by the Italian Society for Rheumatology. Rheumatol Int. 2017, 37, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2022, 35, 128–134. [Google Scholar] [CrossRef]
- Tarasovs, M.; Skuja, S.; Svirskis, S.; Sokolovska, L.; Vikmanis, A.; Lejnieks, A.; Shoenfeld, Y.; Groma, V. Interconnected Pathways: Exploring Inflammation, Pain, and Cognitive Decline in Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 11918. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Z.Y.; Tao, X.X.; Mei, Y.L.; Zhou, D.Q.; Cheng, K.; Gao, S.L.; Shi, H.Y.; Song, C.; Zhang, X.M. Research Progress on the Pathogenesis of Knee Osteoarthritis. Orthop. Surg. 2023, 15, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, P.; Zhou, X.; Wang, J.; Wang, R. A recommended exercise program appropriate for patients with knee osteoarthritis: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 934511. [Google Scholar] [CrossRef]
- Bell, E.; Wallis, J.; Goff, A.; Crossley, K.; O’HAlloran, P.; Barton, C. Does land-based exercise-therapy improve physical activity in people with knee osteoarthritis? A systematic review with meta-analyses. Osteoarthr. Cartil. 2022, 30, 1420–1433. [Google Scholar] [CrossRef]
- Duca, L.; Roman, N.; Teodorescu, A.; Ifteni, P. Association between Inflammation and Thrombotic Pathway Link with Pathogenesis of Depression and Anxiety in SLE Patients. Biomolecules 2023, 13, 567. [Google Scholar] [CrossRef]
- Holden, M.; Metcalf, B.; Lawford, B.; Hinman, R.; Boyd, M.; Button, K.; Collins, N.; Cottrell, E.; Henrotin, Y.; Larsen, J.; et al. Recommendations for the delivery of therapeutic exercise for people with knee and/or hip osteoarthritis. An international consensus study from the OARSI Rehabilitation Discussion Group. Osteoarthr. Cartil. 2022, 31, 386–396. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Messier, S.P.; Resnik, A.E.; Beavers, D.P.; Mihalko, S.L.; Miller, G.D.; Nicklas, B.J.; Devita, P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Intentional Weight Loss in Overweight and Obese Patients With Knee Osteoarthritis: Is More Better? Arthritis Care Res. 2018, 70, 1569–1575. [Google Scholar] [CrossRef]
- Lim, Y.Z.; Wong, J.; Hussain, S.M.; Estee, M.M.; Zolio, L.; Page, M.J.; Harrison, C.L.; Wluka, A.E.; Wang, Y.; Cicuttini, F.M. Recommendations for weight management in osteoarthritis: A systematic review of clinical practice guidelines. Osteoarthr. Cartil. Open 2022, 4, 100298. [Google Scholar] [CrossRef]
- Uritani, D.; Koda, H.; Sugita, S. Effects of self-management education programmes on self-efficacy for osteoarthritis of the knee: A systematic review of randomised controlled trials. BMC Musculoskelet. Disord. 2021, 22, 515. [Google Scholar] [CrossRef] [PubMed]
- Holm, I.; Pripp, A.H.; Risberg, M.A. The Active with OsteoArthritis (AktivA) Physiotherapy Implementation Model: A Patient Education, Supervised Exercise and Self-Management Program for Patients with Mild to Moderate Osteoarthritis of the Knee or Hip Joint. A National Register Study with a Two-Year Follow-Up. J. Clin. Med. 2020, 9, 3112. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Szwedowski, D.; Dallo, I.; Nowaczyk, J. Overview of First-Line and Second-Line Pharmacotherapies for Osteoarthritis with Special Focus on Intra-Articular Treatment. Int. J. Mol. Sci. 2022, 23, 1566. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, T.J.; Robinson, R.L.; Viktrup, L.; Cappelleri, J.C.; Bushmakin, A.G.; Tive, L.; Berry, M.; Walker, C.; Jackson, J. Opioids for Osteoarthritis: Cross-Sectional Survey of Patient Perspectives and Satisfaction. J. Clin. Med. 2023, 12, 2733. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, G.; Zhou, G.; Hong, K.; Yang, W.; Liu, J.; Zeng, L. Efficacy and safety of curcumin therapy for knee osteoarthritis: A Bayesian network meta-analysis. J. Ethnopharmacol. 2023, 321, 117493. [Google Scholar] [CrossRef] [PubMed]
- Glinkowski, W.M.; Tomaszewski, W. Intra-Articular Hyaluronic Acid for Knee Osteoarthritis: A Systematic Umbrella Review. J. Clin. Med. 2025, 14, 1272. [Google Scholar] [CrossRef]
- Pojala, C.V.; Toma, S.; Costache, C.; Peter, T.; Pojala, C.E.; Roman, N.A.; Dima, L. The Potential of Intra-Articular Therapies in Managing Knee Osteoarthritis: A Systematic Review. Clin. Pract. 2024, 14, 1970–1996. [Google Scholar] [CrossRef] [PubMed]
- Mende, E.; Love, R.J.; Young, J.-L. A Comprehensive Summary of the Meta-Analyses and Systematic Reviews on Platelet-Rich Plasma Therapies for Knee Osteoarthritis. Mil. Med. 2024, 189, e2347–e2356. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, K.; Ma, X.; Wang, H.; Ma, C.; Zhang, Y.; Liu, R. Clinical phenotypes of comorbidities in end-stage knee osteoarthritis: A cluster analysis. BMC Musculoskelet. Disord. 2024, 25, 299. [Google Scholar] [CrossRef] [PubMed]
- Isop, L.M.; Neculau, A.E.; Necula, R.D.; Kakucs, C.; Moga, M.A.; Dima, L. Metformin: The Winding Path from Understanding Its Molecular Mechanisms to Proving Therapeutic Benefits in Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 1714. [Google Scholar] [CrossRef]

| Characteristics | Mean ± SD/n (%) |
|---|---|
| Age (years) | 52.84 ± 15.22 |
| BMI (kg/m2) | 25.91 ± 5.61 |
| Lequesne Index | 6.30 ± 2.58 |
| WOMAC Pain | 12.74 ± 5.22 |
| WOMAC Stiffness | 5.09 ± 2.18 |
| WOMAC Function | 44.85 ± 15.18 |
| WOMAC Total | 62.75 ± 22.10 |
| VAS Pain (0–10) | 6.03 ± 2.14 |
| HADS (total score) | 7.74 ± 4.34 |
| KL | 2.35 ± 0.87 |
| Joint space narrowing, grade (0–3) | 1.61 ± 1 |
| Joint space narrowing present, n (%) | 84 (84.8%) |
| Osteophytes, grade (0–3) | 1.67 ± 0.7 |
| Osteophytes present, n (%) | 94 (94.9%) |
| Hypertension (yes) | 40 (40.4%) |
| Diabetes (yes) | 20 (20.2%) |
| Category | WOMAC Score Interval | KL Grade Association | Specific Cut-Off (Youden J) | AUC (95% CI) | Clinical Interpretation |
|---|---|---|---|---|---|
| No symptoms | ≤24 | KL ≥ 1 vs. KL = 0 | 24 | 0.976 (0.938–1.000) | Minimal or no symptoms, no radiographic changes |
| Mild | 25–41 | KL ≥ 2 vs. KL ≤ 1 | 41 | 1.000 (1.000–1.000) | Mild symptoms, early structural joint changes |
| Moderate | 42–69 | KL ≥ 3 vs. KL ≤ 2 | 69 | 0.943 (0.892–0.980) | Clear pain and stiffness, moderate functional limitations |
| Severe | 70–86 | KL = 4 vs. KL ≤ 3 | 87 | 0.944 (0.832–1.000) | Severe pain and significant mobility loss |
| Extreme | ≥87 | KL 4 | 87 | 0.944 (0.832–1.000) | End-stage OA, high likelihood of disability. |
| Variable | 1 Mild OA (n = 22) | 2 Moderate OA (n = 29) | 3 Moderate-Severe OA (n = 19) | 4 Severe OA (n = 29) |
|---|---|---|---|---|
| KL Grade | 0–2 | 2–3 | 3 | 3–4 |
| WOMAC Total | 29.15 ± 9.18 | 56.81 ± 8.73 | 71.83 ± 7.22 | 88.64 ± 4.55 |
| WOMAC Pain | 5.45 ± 2.35 | 10.74 ± 2.08 | 14.74 ± 2.07 | 19.20 ± 1.00 |
| WOMAC Stiffness | 2.25 ± 1.25 | 4.29 ± 1.24 | 5.91 ± 1.04 | 7.60 ± 0.71 |
| WOMAC Functional | 21.45 ± 6.59 | 41.58 ± 6.85 | 51.13 ± 5.22 | 61.84 ± 3.79 |
| Joint Space Narrowing | 0.25 ± 0.44 | 1.23 ± 0.50 | 2.48 ± 0.51 | 2.40 ± 0.50 |
| Osteophytes | 0.85 ± 0.59 | 1.32 ± 0.48 | 2.22 ± 0.42 | 2.24 ± 0.44 |
| Lequesne Index | 2.40 ± 0.68 | 5.48 ± 0.85 | 7.65 ± 0.98 | 9.20 ± 0.96 |
| BMI | 21.24 ± 2.41 | 23.95 ± 3.75 | 30.38 ± 5.30 | 27.97 ± 5.82 |
| Age (Years) | 31.30 ± 8.30 | 50.58 ± 8.74 | 59.91 ± 8.58 | 66.36 ± 9.83 |
| Pain (VAS) | 3.00 ± 0.86 | 5.16 ± 0.73 | 7.00 ± 0.74 | 8.64 ± 0.57 |
| HADS | 1.75 ± 1.77 | 6.77 ± 1.63 | 10.26 ± 2.96 | 11.40 ± 3.46 |
| Hypertension % | 0.00% | 19.35% | 69.57% | 72.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pojala, C.-V.; Moga, M.A.; Pojala, C.-E.; Roman, N.A.; Necula, R.D.; Toma, S.I.; Manea, R.M.; Dima, L. Integrative Phenotyping of Knee Osteoarthritis: Linking WOMAC Cut-Offs, Kellgren–Lawrence Grades, and Cluster Analysis for Personalized Care. Life 2025, 15, 1542. https://doi.org/10.3390/life15101542
Pojala C-V, Moga MA, Pojala C-E, Roman NA, Necula RD, Toma SI, Manea RM, Dima L. Integrative Phenotyping of Knee Osteoarthritis: Linking WOMAC Cut-Offs, Kellgren–Lawrence Grades, and Cluster Analysis for Personalized Care. Life. 2025; 15(10):1542. https://doi.org/10.3390/life15101542
Chicago/Turabian StylePojala, Ciprian-Vasile, Marius Alexandru Moga, Cristiana-Elena Pojala, Nadinne Alexandra Roman, Radu Dan Necula, Sebastian Ionut Toma, Rosana Mihaela Manea, and Lorena Dima. 2025. "Integrative Phenotyping of Knee Osteoarthritis: Linking WOMAC Cut-Offs, Kellgren–Lawrence Grades, and Cluster Analysis for Personalized Care" Life 15, no. 10: 1542. https://doi.org/10.3390/life15101542
APA StylePojala, C.-V., Moga, M. A., Pojala, C.-E., Roman, N. A., Necula, R. D., Toma, S. I., Manea, R. M., & Dima, L. (2025). Integrative Phenotyping of Knee Osteoarthritis: Linking WOMAC Cut-Offs, Kellgren–Lawrence Grades, and Cluster Analysis for Personalized Care. Life, 15(10), 1542. https://doi.org/10.3390/life15101542






