Increased Pre-Transplant Carotid Intima-Media Thickness Is Associated with Early Post-Transplant Atrial Fibrillation, Stroke, and Reduced Survival After Heart Transplantation
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Follow-Up
2.3. Post-Transplant Medication
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Initial Post-Transplant Medications
3.3. Post-Transplant Primary Outcome
3.4. Post-Transplant Secondary Outcomes
3.5. Sensitivity Analysis
4. Discussion
4.1. Carotid Intima-Media Thickness and Cardiovascular Risk
4.2. Mortality and Causes of Death After Heart Transplantation
4.3. Cardiovascular Events After Heart Transplantation
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, M.; Schibilsky, D.; Zeh, W.; Berchtold-Herz, M.; Beyersdorf, F.; Siepe, M. Does the heart transplant have a future? Eur. J. Cardiothorac. Surg. 2019, 55 (Suppl. 1), i38–i48. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.A. Taking heart-cardiac transplantation past, present, and future. N. Engl. J. Med. 2006, 355, 231–235. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Stehlik, J.; Kobashigawa, J.; Hunt, S.A.; Reichenspurner, H.; Kirklin, J.K. Honoring 50 Years of Clinical Heart Transplantation in Circulation: In-Depth State-of-the-Art Review. Circulation 2018, 137, 71–87. [Google Scholar] [CrossRef]
- Zhu, Y.; Lingala, B.; Baiocchi, M.; Toro Arana, V.; Williams, K.M.; Shudo, Y.; Oyer, P.E.; Woo, Y.J. The Stanford experience of heart transplantation over five decades. Eur. Heart J. 2021, 42, 4934–4943. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Bourge, R.C.; McGiffin, D.C.; Hill, J.A.; Rodeheffer, R.J.; Jaski, B.E.; Hauptman, P.J.; Weston, M.; White-Williams, C. Evolving trends in risk profiles and causes of death after heart transplantation: A ten-year multi-institutional study. J. Thorac. Cardiovasc. Surg. 2003, 125, 881–890. [Google Scholar] [CrossRef]
- Ortega-Legaspi, J.M.; Bravo, P.E. Diagnosis and management of cardiac allograft vasculopathy. Heart 2021, 108, 586–592. [Google Scholar] [CrossRef]
- Valantine, H. Cardiac allograft vasculopathy after heart transplantation: Risk factors and management. J. Heart Lung Transplant. 2004, 23 (Suppl. 5), S187–S193. [Google Scholar] [CrossRef]
- Ahmari, S.A.; Bunch, T.J.; Chandra, A.; Chandra, V.; Ujino, K.; Daly, R.C.; Kushwaha, S.S.; Edwards, B.S.; Maalouf, Y.F.; Seward, J.B.; et al. Prevalence, pathophysiology, and clinical significance of post-heart transplant atrial fibrillation and atrial flutter. J. Heart Lung Transplant. 2006, 25, 53–60. [Google Scholar] [CrossRef]
- Joglar, J.A.; Wan, E.Y.; Chung, M.K.; Gutierrez, A.; Slaughter, M.S.; Bateson, B.P.; Loguidice, M.; Drazner, M.; Kistler, P.M.; Saour, B.; et al. Management of Arrhythmias After Heart Transplant: Current State and Considerations for Future Research. Circ. Arrhythm. Electrophysiol. 2021, 14, e007954. [Google Scholar] [CrossRef]
- Thajudeen, A.; Stecker, E.C.; Shehata, M.; Patel, J.; Wang, X.; McAnulty, J.H., Jr.; Kobashigawa, J.; Chugh, S.S. Arrhythmias after heart transplantation: Mechanisms and management. J. Am. Heart Assoc. 2012, 1, e001461. [Google Scholar]
- Choi, H.I.; Kim, Y.T.; Kang, J.G.; Kim, Y.; Lee, J.Y.; Sung, K.C. Segment-Specific Analysis of Carotid Intima-Media Thickness and Its Association with Cardiovascular Risk Factors in a Large Healthy Cohort. J. Clin. Med. 2025, 14, 1918. [Google Scholar]
- Den Ruijter, H.M.; Peters, S.A.; Anderson, T.J.; Britton, A.R.; Dekker, J.M.; Eijkemans, M.J.; Engström, G.; Evans, G.W.; de Graaf, J.; Grobbee, D.E.; et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. JAMA 2012, 308, 796–803. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Van den Oord, S.C.H.; Sijbrands, E.J.G.; ten Kate, G.L.; van Klaveren, D.; van Domburg, R.T.; van der Steen, A.F.W.; Schinkel, A.F.L. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis 2013, 228, 1–11. [Google Scholar] [CrossRef]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: Meta-Analysis of 119 Clinical Trials Involving 100,667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar]
- Bots, M.L.; Hoes, A.W.; Koudstaal, P.J.; Hofman, A.; Grobbee, D.E. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997, 96, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.L.; Salles, G.C.; Leite, N.C.; Salles, G.F. Prognostic impact of carotid intima-media thickness and carotid plaques on the development of micro- and macrovascular complications in individuals with type 2 diabetes: The Rio de Janeiro type 2 diabetes cohort study. Cardiovasc. Diabetol. 2019, 18, 2. [Google Scholar] [CrossRef]
- Ham, S.Y.; Song, J.W.; Shim, J.K.; Soh, S.; Kim, H.J.; Kwak, Y.L. Prognostic role of carotid intima-media thickness in off-pump coronary artery bypass surgery. Sci. Rep. 2018, 8, 11385. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pozza, R.; Greil, S.; Januszewska, K.; Netz, H.; Kozlik-Feldmann, R. Carotid intima media thickness and cardiac allograft vasculopathy after heart transplantation in childhood. Transplantation 2011, 91, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Ventura, H.; Smart, F.; Hewitt, R.; Stapleton, D.; Akers, D.; Augustine, S.; Lumpkin, D. Peripheral vascular disease in cardiac transplantation: Abnormal carotid wall thickness. Transplantation 1999, 67, S106. [Google Scholar] [CrossRef]
- Heil, K.M.; Helmschrott, M.; Darche, F.F.; Bruckner, T.; Ehlermann, P.; Kreusser, M.M.; Doesch, A.O.; Sommer, W.; Warnecke, G.; Frey, N.; et al. Risk Factors, Treatment and Prognosis of Patients with Lung Cancer after Heart Transplantation. Life 2021, 11, 1344. [Google Scholar] [CrossRef]
- Darche, F.F.; Helmschrott, M.; Rahm, A.K.; Thomas, D.; Schweizer, P.A.; Bruckner, T.; Ehlermann, P.; Kreusser, M.M.; Warnecke, G.; Frey, N.; et al. Atrial fibrillation before heart transplantation is a risk factor for post-transplant atrial fibrillation and mortality. ESC Heart Fail. 2021, 8, 4265–4277. [Google Scholar] [CrossRef]
- Darche, F.F.; Fabricius, L.C.; Helmschrott, M.; Rahm, A.K.; Ehlermann, P.; Bruckner, T.; Sommer, W.; Warnecke, G.; Frey, N.; Rivinius, R. Oral Anticoagulants after Heart Transplantation-Comparison between Vitamin K Antagonists and Direct Oral Anticoagulants. J. Clin. Med. 2023, 12, 4334. [Google Scholar] [CrossRef]
- Darche, F.F.; Heil, K.M.; Rivinius, R.; Helmschrott, M.; Ehlermann, P.; Frey, N.; Rahm, A.K. Early Pacemaker Dependency After Heart Transplantation Is Associated with Permanent Pacemaker Implantation, Graft Failure and Mortality. J. Cardiovasc. Dev. Dis. 2024, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Rivinius, R.; Helmschrott, M.; Ruhparwar, A.; Schmack, B.; Darche, F.F.; Thomas, D.; Bruckner, T.; Katus, H.A.; Ehlermann, P.; Doesch, A.O. COPD in patients after heart transplantation is associated with a prolonged hospital stay, early posttransplant atrial fibrillation, and impaired posttransplant survival. Clin. Epidemiol. 2018, 10, 1359–1369. [Google Scholar] [CrossRef]
- Rivinius, R.; Gralla, C.; Helmschrott, M.; Darche, F.F.; Ehlermann, P.; Bruckner, T.; Sommer, W.; Warnecke, G.; Kopf, S.; Szendroedi, J.; et al. Pre-transplant Type 2 Diabetes Mellitus Is Associated with Higher Graft Failure and Increased 5-Year Mortality After Heart Transplantation. Front. Cardiovasc. Med. 2022, 9, 890359. [Google Scholar] [CrossRef]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar]
- Chen, L.Y.; Leening, M.J.; Norby, F.L.; Roetker, N.S.; Hofman, A.; Franco, O.H.; Pan, W.; Polak, J.F.; Witteman, J.C.; Kronmal, R.A.; et al. Carotid Intima-Media Thickness and Arterial Stiffness and the Risk of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study, Multi-Ethnic Study of Atherosclerosis (MESA), and the Rotterdam Study. J. Am. Heart Assoc. 2016, 5, e002907. [Google Scholar] [CrossRef]
- Mitra, S.; Biswas, R.K.; Hooijenga, P.; Cassidy, S.; Nova, A.; De Ciutiis, I.; Wang, T.; Kroeger, C.M.; Stamatakis, E.; Masedunskas, A.; et al. Carotid intima-media thickness, cardiovascular disease, and risk factors in 29,000 UK Biobank adults. Am. J. Prev. Cardiol. 2025, 22, 101011. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, J.; van der Kuip, D.A.; Hofman, A.; Kors, J.A.; van Rooij, F.J.; Lip, G.Y.; Witteman, J.C. Subclinical atherosclerosis and risk of atrial fibrillation: The rotterdam study. Arch. Intern. Med. 2007, 167, 382–387. [Google Scholar] [CrossRef]
- Wilhelm, M.J. Long-term outcome following heart transplantation: Current perspective. J. Thorac. Dis. 2015, 7, 549–551. [Google Scholar] [PubMed]
- Huang, X.; Yuzefpolskaya, M.; Colombo, P.C.; Choe, J.; Shertel, T.; Jennings, D.L. The Impact of Statin Intensity on the Early Progression of Cardiac Allograft Vasculopathy. Clin. Transplant. 2024, 38, e70030. [Google Scholar] [CrossRef]
- Alnsasra, H.; Asleh, R.; Kumar, N.; Lopez, C.; Toya, T.; Kremers, W.K.; Edwards, B.; Daly, R.C.; Kushwaha, S.S. Incidence, Risk Factors, and Outcomes of Stroke Following Cardiac Transplantation. Stroke 2021, 52, e720–e724. [Google Scholar] [CrossRef]
- Dasari, T.W.; Pavlovic-Surjancev, B.; Patel, N.; Williams, A.A.; Ezidinma, P.; Rupani, A.; Sinacore, J.L.; Heroux, A.L. Incidence, risk factors, and clinical outcomes of atrial fibrillation and atrial flutter after heart transplantation. Am. J. Cardiol. 2010, 106, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Pavri, B.B.; O’Nunain, S.S.; Newell, J.B.; Ruskin, J.N.; William, G. Prevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantation. J. Am. Coll. Cardiol. 1995, 25, 1673–1680. [Google Scholar]
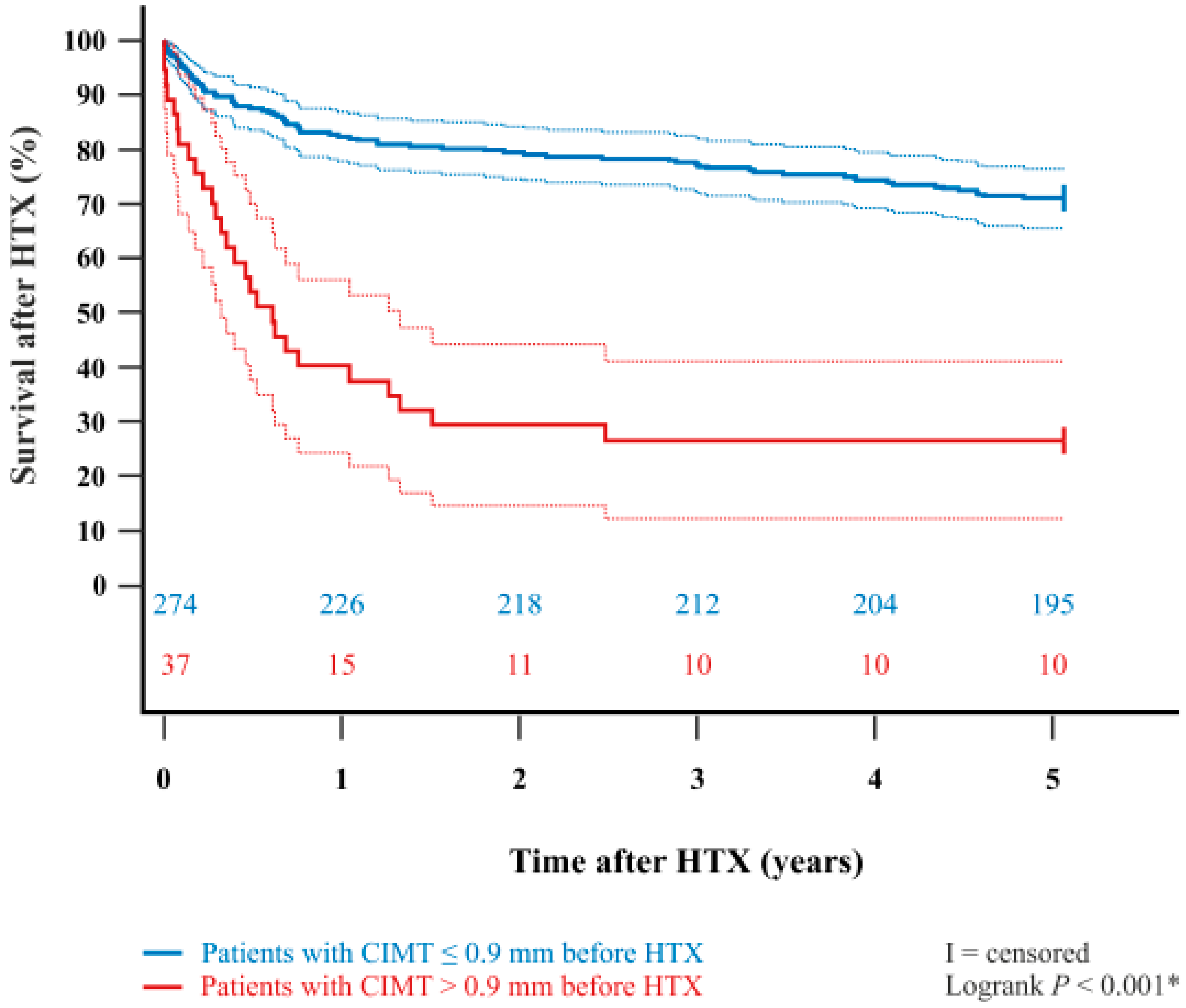
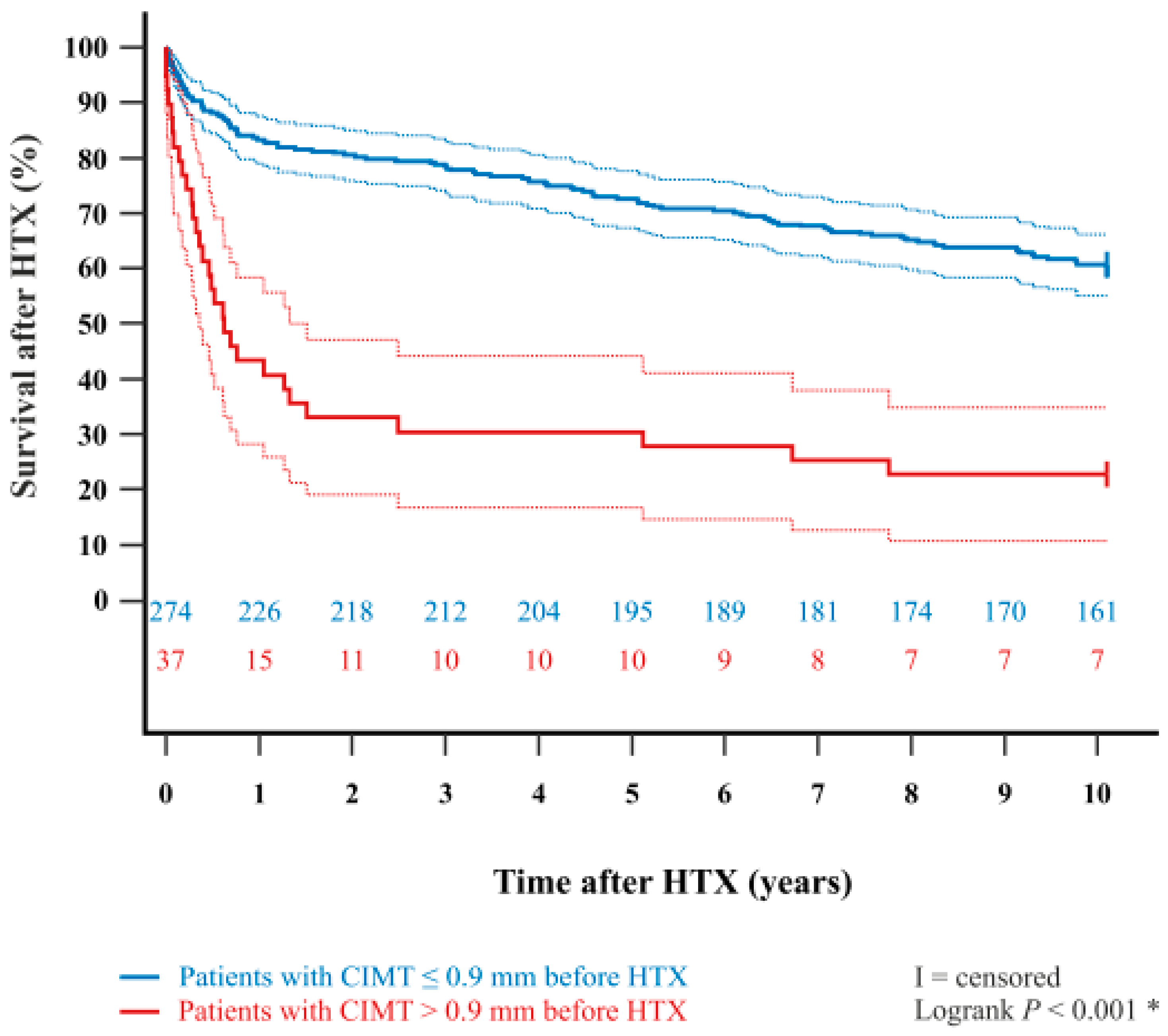
| Parameter | All Patients (n = 311) | CIMT ≤ 0.9 mm (n = 274) | CIMT > 0.9 mm (n = 37) | Difference | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Recipient data | |||||||
| Age (years), mean ± SD | 51.9 ± 10.6 | 51.3 ± 10.9 | 56.5 ± 6.2 | 5.2 | 2.8–7.6 | <0.001 | * |
| Male sex, n (%) | 239 (76.8%) | 208 (75.9%) | 31 (83.8%) | 7.9% | −5.0–20.8% | 0.287 | |
| BMI (kg/m2), mean ± SD | 25.1 ± 4.4 | 25.0 ± 4.4 | 25.4 ± 3.9 | 0.4 | −1.0–1.8 | 0.558 | |
| Arterial hypertension, n (%) | 174 (55.9%) | 146 (53.3%) | 28 (75.7%) | 22.4% | 7.4–37.4% | 0.010 | * |
| Dyslipidemia, n (%) | 198 (63.7%) | 169 (61.7%) | 29 (78.4%) | 16.7% | 2.2–31.2% | 0.047 | * |
| Diabetes mellitus, n (%) | 103 (33.1%) | 84 (30.7%) | 19 (51.4%) | 20.7% | 3.7–37.7% | 0.012 | * |
| Peripheral artery disease, n (%) | 28 (9.0%) | 22 (8.0%) | 6 (16.2%) | 8.2% | −4.1–20.5% | 0.102 | |
| COPD, n (%) | 82 (26.4%) | 62 (22.6%) | 20 (54.1%) | 31.5% | 14.7–48.3% | <0.001 | * |
| History of smoking, n (%) | 178 (57.2%) | 150 (54.7%) | 28 (75.7%) | 21.0% | 6.0–36.0% | 0.016 | * |
| Chronic kidney disease ^, n (%) | 173 (55.6%) | 149 (54.4%) | 24 (64.9%) | 10.5% | −6.0–27.0% | 0.228 | |
| eGFR (ml/min/1.73 m2), mean ± SD | 58.3 ± 23.6 | 59.0 ± 24.3 | 53.3 ± 16.7 | 5.7 | −0.4–11.8 | 0.073 | |
| Previous open-heart surgery | |||||||
| Overall open-heart surgery, n (%) | 87 (28.0%) | 69 (25.2%) | 18 (48.6%) | 23.4% | 6.5–40.3% | 0.003 | * |
| CABG surgery, n (%) | 41 (13.2%) | 29 (10.6%) | 12 (32.4%) | 21.8% | 6.3–37.3% | <0.001 | * |
| Other surgery °, n (%) | 34 (10.9%) | 30 (10.9%) | 4 (10.8%) | 0.1% | −10.6–10.8% | 0.980 | |
| VAD surgery, n (%) | 18 (5.8%) | 16 (5.8%) | 2 (5.4%) | 0.4% | −7.4–8.2% | 0.915 | |
| Principal diagnosis for HTX | |||||||
| Ischemic CMP, n (%) | 108 (34.7%) | 84 (30.6%) | 24 (64.9%) | 34.3% | 18.0–50.6% | <0.001 | * |
| Non-ischemic CMP, n (%) | 147 (47.3%) | 138 (50.4%) | 9 (24.3%) | 26.1% | 11.1–41.1% | 0.003 | * |
| Valvular heart disease, n (%) | 14 (4.5%) | 12 (4.4%) | 2 (5.4%) | 1.0% | −6.7–8.7% | 0.778 | |
| Cardiac amyloidosis, n (%) | 42 (13.5%) | 40 (14.6%) | 2 (5.4%) | 9.2% | −0.8–19.2% | 0.125 | |
| Donor data | |||||||
| Age (years), mean ± SD | 45.0 ± 12.5 | 44.9 ± 12.6 | 46.2 ± 12.0 | 1.3 | −2.8–5.4 | 0.528 | |
| Male sex, n (%) | 95 (30.5%) | 82 (29.9%) | 13 (35.1%) | 5.2% | −11.1–21.5% | 0.519 | |
| BMI (kg/m2), mean ± SD | 25.0 ± 4.7 | 25.1 ± 4.8 | 24.2 ± 3.4 | 0.9 | −0.4–2.2 | 0.193 | |
| Transplant sex mismatch | |||||||
| Mismatch, n (%) | 163 (52.4%) | 143 (52.2%) | 20 (54.1%) | 1.9% | −15.2–19.0% | 0.831 | |
| Donor (m) to recipient (f), n (%) | 9 (2.9%) | 8 (2.9%) | 1 (2.7%) | 0.2% | −5.4–5.8% | 0.941 | |
| Donor (f) to recipient (m), n (%) | 154 (49.5%) | 135 (49.3%) | 19 (51.4%) | 2.1% | −15.1–19.3% | 0.812 | |
| Perioperative data | |||||||
| High-urgent listing status, n (%) | 247 (79.4%) | 218 (79.6%) | 29 (78.4%) | 1.2% | −12.9–15.3% | 0.867 | |
| Ischemic time (min), mean ± SD | 253.0 ± 58.4 | 253.6 ± 58.4 | 248.0 ± 58.9 | 5.6 | −14.6–25.8 | 0.586 | |
| Biatrial anastomosis, n (%) | 4 (1.3%) | 4 (1.5%) | 0 (0.0%) | 1.5% | −0.1–3.1% | 0.459 | |
| Bicaval anastomosis, n (%) | 116 (37.3%) | 101 (36.8%) | 15 (40.5%) | 3.7% | −13.1–20.5% | 0.664 | |
| Total orthotopic anastomosis, n (%) | 191 (61.4%) | 169 (61.7%) | 22 (59.5%) | 2.2% | −14.6–19.0% | 0.795 |
| Parameter | All Patients (n = 311) | CIMT ≤ 0.9 mm (n = 274) | CIMT > 0.9 mm (n = 37) | Difference | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Immunosuppressive drug therapy | ||||||
| Cyclosporine A, n (%) | 86 (27.7%) | 72 (26.3%) | 14 (37.8%) | 11.5% | −5.0–28.0% | 0.140 |
| Tacrolimus, n (%) | 225 (72.3%) | 202 (73.7%) | 23 (62.2%) | 11.5% | −5.0–28.0% | 0.140 |
| Azathioprine, n (%) | 10 (3.2%) | 9 (3.3%) | 1 (2.7%) | 0.6% | −5.0–6.2% | 0.851 |
| Mycophenolic acid, n (%) | 301 (96.8%) | 265 (96.7%) | 36 (97.3%) | 0.6% | −5.0–6.2% | 0.851 |
| Steroids, n (%) | 311 (100.0%) | 274 (100.0%) | 37 (100.0%) | 0.0% | n.a. | n.a. |
| Concomitant medications | ||||||
| ASA, n (%) | 42 (13.5%) | 39 (14.2%) | 3 (8.1%) | 6.1% | −3.6–15.8% | 0.306 |
| Beta blocker, n (%) | 71 (22.8%) | 66 (24.1%) | 5 (13.5%) | 10.6% | −1.5–22.7% | 0.150 |
| Ivabradine, n (%) | 39 (12.5%) | 36 (13.1%) | 3 (8.1%) | 5.0% | −4.7–14.7% | 0.386 |
| Calcium channel blocker, n (%) | 85 (27.3%) | 71 (25.9%) | 14 (37.8%) | 11.9% | −4.6–28.4% | 0.127 |
| ACE inhibitor/ARB, n (%) | 132 (42.4%) | 114 (41.6%) | 18 (48.6%) | 7.0% | −10.1–24.1% | 0.416 |
| Diuretic, n (%) | 311 (100.0%) | 274 (100.0%) | 37 (100.0%) | 0.0% | n.a. | n.a. |
| Statin, n (%) | 194 (62.4%) | 172 (62.8%) | 22 (59.5%) | 3.3% | −13.5–20.1% | 0.696 |
| Gastric protection drug †, n (%) | 311 (100.0%) | 274 (100.0%) | 37 (100.0%) | 0.0% | n.a. | n.a. |
| Parameter | All Patients (n = 311) | CIMT ≤ 0.9 mm (n = 274) | CIMT > 0.9 mm (n = 37) | Difference | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| 30-day mortality after HTX, n (%) | 17 (5.5%) | 10 (3.6%) | 7 (18.9%) | 15.3% | 2.5–28.1% | <0.001 | * |
| 1-year mortality after HTX, n (%) | 70 (22.5%) | 48 (17.5%) | 22 (59.5%) | 42.0% | 25.6–58.4% | <0.001 | * |
| 2-year mortality after HTX, n (%) | 82 (26.4%) | 56 (20.4%) | 26 (70.3%) | 49.9% | 34.4–65.4% | <0.001 | * |
| 5-year mortality after HTX, n (%) | 106 (34.1%) | 79 (28.8%) | 27 (72.9%) | 44.1% | 28.8–59.4% | <0.001 | * |
| 10-year mortality after HTX, n (%) | 143 (46.0%) | 113 (41.2%) | 30 (81.1%) | 39.9% | 26.0–53.8% | <0.001 | * |
| (a) Within 5 years after HTX. | |||||||
| Parameter | All Patients (n = 311) | CIMT ≤ 0.9 mm (n = 274) | CIMT > 0.9 mm (n = 37) | Difference | 95% CI | p-Value | |
| Graft failure, n (%) | 30 (9.6%) | 22 (8.0%) | 8 (21.6%) | 13.6% | 0.4–26.8% | 0.009 | * |
| Acute rejection, n (%) | 2 (0.6%) | 2 (0.7%) | 0 (0.0%) | 0.7% | −0.3–1.7% | 0.602 | |
| Infection/Sepsis, n (%) | 59 (19.0%) | 45 (16.4%) | 14 (37.8%) | 21.4% | 5.2–37.6% | 0.002 | * |
| Malignancy, n (%) | 7 (2.3%) | 6 (2.2%) | 1 (2.7%) | 0.5% | −5.0–6.0% | 0.843 | |
| Thromboembolic event/bleeding, n (%) | 8 (2.6%) | 4 (1.5%) | 4 (10.8%) | 9.3% | 0.8–17.8% | 0.001 | * |
| All causes, n (%) | 106 (34.1%) | 79 (28.8%) | 27 (72.9%) | 44.1% | 28.8–59.4% | <0.001 | * |
| (b) Within 10 years after HTX. | |||||||
| Parameter | All Patients (n = 311) | CIMT ≤ 0.9 mm (n = 274) | CIMT > 0.9 mm (n = 37) | Difference | 95% CI | p-Value | |
| Graft failure, n (%) | 38 (12.2%) | 29 (10.6%) | 9 (24.3%) | 13.7% | 0.6–26.8% | 0.017 | * |
| Acute rejection, n (%) | 3 (1.0%) | 2 (0.7%) | 1 (2.7%) | 2.0% | −3.3–7.3% | 0.249 | |
| Infection/Sepsis, n (%) | 70 (22.5%) | 56 (20.4%) | 14 (37.8%) | 17.4% | 1.1–33.7% | 0.017 | * |
| Malignancy, n (%) | 20 (6.4%) | 18 (6.6%) | 2 (5.4%) | 1.2% | −6.7–9.1% | 0.786 | |
| Thromboembolic event/bleeding, n (%) | 12 (3.9%) | 8 (2.9%) | 4 (10.8%) | 7.9% | 0.2–15.6% | 0.019 | * |
| All causes, n (%) | 143 (46.0%) | 113 (41.2%) | 30 (81.0%) | 39.8% | 25.9–53.7% | <0.001 | * |
| (a) Within 5 years after HTX. | ||||
| Parameter | Hazard Ratio | 95% CI | p-Value | |
| Recipient age (years) | 1.022 | 0.998–1.046 | 0.068 | |
| Arterial hypertension (in total) | 1.235 | 0.660–2.310 | 0.509 | |
| Dyslipidemia (in total) | 0.632 | 0.338–1.179 | 0.149 | |
| Diabetes mellitus (in total) | 1.435 | 0.949–2.171 | 0.087 | |
| COPD (in total) | 4.748 | 2.850–7.910 | <0.001 | * |
| History of smoking (in total) | 0.588 | 0.336–1.028 | 0.062 | |
| CABG surgery ^ (in total) | 0.993 | 0.532–1.852 | 0.981 | |
| Ischemic CMP ° (in total) | 0.970 | 0.535–1.760 | 0.921 | |
| CIMT > 0.9 mm (in total) | 2.899 | 1.802–4.664 | <0.001 | * |
| (b) Within 10 years after HTX. | ||||
| Parameter | Hazard Ratio | 95% CI | p-Value | |
| Recipient age (years) | 1.013 | 0.994–1.033 | 0.192 | |
| Arterial hypertension (in total) | 1.163 | 0.674–2.008 | 0.588 | |
| Dyslipidemia (in total) | 0.667 | 0.389–1.143 | 0.141 | |
| Diabetes mellitus (in total) | 1.223 | 0.852–1.756 | 0.275 | |
| COPD (in total) | 4.695 | 3.098–7.115 | <0.001 | * |
| History of smoking (in total) | 0.843 | 0.530–1.341 | 0.471 | |
| CABG surgery ^ (in total) | 1.212 | 0.713–2.059 | 0.478 | |
| Ischemic CMP ° (in total) | 0.943 | 0.561–1.584 | 0.823 | |
| CIMT > 0.9 mm (in total) | 2.599 | 1.683–4.014 | <0.001 | * |
| Parameter | All Patients (n = 311) | CIMT ≤ 0.9 mm (n = 274) | CIMT > 0.9 mm (n = 37) | Difference | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| 30-day atrial fibrillation after HTX, n (%) | 40 (12.9%) | 30 (10.9%) | 10 (27.0%) | 16.1% | 1.3–30.9% | 0.006 | * |
| 30-day rejection episode after HTX, n (%) | 40 (12.9%) | 36 (13.1%) | 4 (10.8%) | 2.3% | −8.5–13.1% | 0.691 | |
| 30-day TIA after HTX, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% | n.a. | n.a. | |
| 30-day stroke after HTX, n (%) | 7 (2.3%) | 3 (1.1%) | 4 (10.8%) | 9.7% | 0.4–19.0% | <0.001 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heil, K.M.; Rivinius, R.; Helmschrott, M.; Rahm, A.-K.; Ehlermann, P.; Frey, N.; Darche, F.F. Increased Pre-Transplant Carotid Intima-Media Thickness Is Associated with Early Post-Transplant Atrial Fibrillation, Stroke, and Reduced Survival After Heart Transplantation. Life 2025, 15, 1539. https://doi.org/10.3390/life15101539
Heil KM, Rivinius R, Helmschrott M, Rahm A-K, Ehlermann P, Frey N, Darche FF. Increased Pre-Transplant Carotid Intima-Media Thickness Is Associated with Early Post-Transplant Atrial Fibrillation, Stroke, and Reduced Survival After Heart Transplantation. Life. 2025; 15(10):1539. https://doi.org/10.3390/life15101539
Chicago/Turabian StyleHeil, Karsten M., Rasmus Rivinius, Matthias Helmschrott, Ann-Kathrin Rahm, Philipp Ehlermann, Norbert Frey, and Fabrice F. Darche. 2025. "Increased Pre-Transplant Carotid Intima-Media Thickness Is Associated with Early Post-Transplant Atrial Fibrillation, Stroke, and Reduced Survival After Heart Transplantation" Life 15, no. 10: 1539. https://doi.org/10.3390/life15101539
APA StyleHeil, K. M., Rivinius, R., Helmschrott, M., Rahm, A.-K., Ehlermann, P., Frey, N., & Darche, F. F. (2025). Increased Pre-Transplant Carotid Intima-Media Thickness Is Associated with Early Post-Transplant Atrial Fibrillation, Stroke, and Reduced Survival After Heart Transplantation. Life, 15(10), 1539. https://doi.org/10.3390/life15101539








