Abstract
Fetal alcohol spectrum disorder (FASD/FAS) is a chronic inflammatory process of the fetal brain induced by alcohol and mediated by pro-inflammatory (PILM) and pro-resolving (PRLM) lipid mediators of inflammation. DHA (docosahexaenoic acid) is an essential precursor of PRLM. A study examining the response of lipid mediators of inflammation to alcohol insult and DHA supplementation can provide vital information on the pathogenesis of FASD/FAS and the potential ameliorative role of DHA. Four groups of timed pregnant rats were studied: control, low-dose (1.6 g/kg/day) and high-dose (2.4 g/kg/day) alcohol, and high-dose alcohol (2.4 g/kg/day) + DHA (1250 mg/kg/day). The pups were delivered on day 20, and their whole brain was examined for lipid mediators by liquid chromatography mass spectroscopy. The following biomarkers of brain lipid mediators were studied, namely, PILM (LTB4, PGE2, PGF2α, TXB2) and PRLM (LXA5, 4-HDoHE, 17-HDoHE, and MaR1n-3, DPA). The brain PILM and PRLM concentrations decreased significantly (p < 0.001) with high-dose alcohol. However, high-dose alcohol + DHA resulted in a significant (p < 0.001) increase in PRLM levels, viz., LXA5, MaR1n-3 DPA, 17-HDoHE, and a threefold increase in 4-HDoHE. We conclude that DHA supplementation in alcohol-exposed pregnant rats significantly increased levels of brain pro-resolving lipid mediators in the offspring, suggesting a potential role in modulating the inflammatory response.
1. Introduction
The body responds to noxious insults through inflammation, initially through the release of pro-inflammatory lipid mediators, such as prostaglandins and leukotrienes, followed by the release of pro-resolving mediators, e.g., resolvins, protectins, epoxins, etc. [1]. The precursor of the pro-inflammatory lipid mediators is Ω-6 arachidonic acid, whereas the Ω-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are the principal precursors of the pro-resolving lipid mediators [1].
Alcohol abuse among pregnant women is a serious health problem. In the United States, 1 in 7 pregnant women reported drinking alcohol within the past 30 days, and 1 in 20 engaged in binge drinking [2]. One of the serious complications of maternal alcoholism in the offspring is Fetal Alcohol Spectrum Disorder (FASD) and the Fetal Alcohol Syndrome (FAS) [3,4,5]. The pooled prevalence of FAS and FASD in the United States is approximately 2 per 1000 and 15 per 1000, respectively, in the general population [6,7].
The mechanism underlying FASD is still poorly understood, although unresolved inflammation, the generation of reactive oxygen species, and the lipid mediators of inflammation are important contributing factors [8,9,10,11]. The aim of our study was to assess, in a rat model, the pro-inflammatory and pro-resolving brain lipid mediators in response to chronic alcohol exposure at low (1.6 g/kg/day) and high (2.4 g/kg/day) alcohol doses and the effect of DHA supplementation in mitigating the inflammation process of alcohol at the high alcohol dose.
2. Materials and Methods
This study consisted of 4 groups of timed-pregnant Sprague-Dawley rats (dams)- See Figure 1.
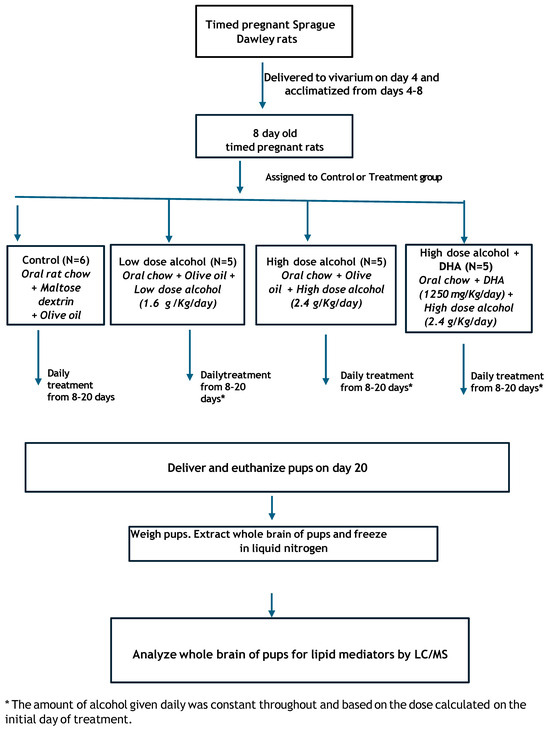
Figure 1.
Schematic diagram of this study.
Group 1. Control (C).
Group 2. Low-dose alcohol (LD) at 1.6 g alcohol/kg/day.
Group 3. High-dose alcohol (HD) at 2.4 g alcohol/kg/day.
Group 4. High-dose alcohol + DHA (HD/DHA) at 2.4 g alcohol/kg/day + DHA (1250 mg/kg/day).
The dams were delivered from the Charles River Laboratories to the vivarium on gestational day (GD) 4. Each dam was randomly assigned (Research Randomizer, www.randomizer.org) to either the control or treatment group. The animals were placed in individual cages and provided with standard rat chow and water ad libitum. The room was at a constant (22 °C) temperature on a 16 h dark/8 h light cycle (lights off from 5:30 p.m. to 9:30 a.m. the following day).
The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University, Detroit, Michigan. (Protocol # IACUC-19-08-1222, Wayne State University).
2.1. Control Group (N = 6)
The control group was given daily gavage feedings in 2 divided doses of maltose/dextrin solution and olive oil starting from GD 8 to day 20. An equivolume of olive oil was given to the dams in groups 1–3 as a lipid control for the docosahexaenoic acid (DHA) given to group 4. Olive oil had a caloric load of 120 calories per 15 mL, and DHA had a caloric load of 123 calories per 15 mL. A maltose/dextrin solution served as the control for the caloric load of 2.4 g/kg/d of alcohol. Alcohol had a caloric load of 7 calories per gram, and maltose/dextrin had a caloric load of 95 calories per 25 g.
The total volume of maltose/dextrin/olive oil was approximately equal to the total volume of alcohol+olive oil given to the alcohol group and given by gavage in 2 divided doses, not to exceed the maximum safety limit of 10 mL/kg per gavage. Based on the initial weight of the dams, the volume of olive oil administered by gavage was 0.8 mL per day, and maltose dextrin was 1.8 mL per day.
Nine dams were initially assigned to the control group to anticipate the possible replacement of rats in the treatment groups that resisted initial gavage feedings. Three dams were subsequently reassigned to the treatment group, reducing the number of dams in the control group to 6. There was no pair-fed control group nor a group given alcohol alone, since our previous study showed that prenatal alcohol alone caused adverse effects of low birth and brain weights in the fetus, and low placental weight [12].
2.2. Alcohol Groups
A constant daily dose of alcohol was given to the treatment group based on the initial weight of the dam at the start of treatment (day 8). The study design was similar to our previous study [12] due to our concern that varying daily alcohol doses in the treatment group based on the changing weight of the dams would introduce an important confounder to the alcohol effect.
2.2.1. Low-Dose (LD) Alcohol at 1.6 g Alcohol/kg/day (N = 5)
The stock solution of 29.85% (volume/volume) was prepared by adding 70 mL of distilled water to 30 mL of 99.5% ethanol solution (Spectrum Chemical MFG Corporation, New Brunswick, NJ, USA). At a density of alcohol of 0.7892 g/mL, the stock solution contained 0.7892 g/mL of alcohol. The low alcohol dose was 1.6 g/kg/d plus olive oil (volume for volume with DHA) given daily by gavage in 2 divided doses from gestational day 8 to day 20. Based on the different initial weights of the dams, the gavage volume of 29.85% alcohol that the dams received ranged between 1.6 and 1.9 mL per day and the volume of olive oil given was 0.8 mL per day.
2.2.2. High-Dose (HD) Alcohol at 2.4 g Alcohol/kg/day (N = 5)
As with the low-dose alcohol group, a 29.85% stock solution of alcohol was used for the high alcohol dose of 2.4 g/kg/day, administered by gavage with olive oil (volume-to-volume with DHA) in two divided doses from gestational day 8 to day 20. Based on the initial weight of the dams, the gavage volume of 29.85% alcohol given to the dams in the alcohol + DHA group ranged from 2.2 to 2.5 mL per day. The volume of olive oil administered was 0.8 mL per day. We did not measure the initial blood alcohol level in the dams and estimated the alcohol level based on a similar study by Bielawski [13]. At an alcohol dose of 3 g/kg, administered in two divided doses to Sprague-Dawley rats, Bielawski et al. found the peak blood alcohol level in the rat at 0.5 h to be 53 mg/dL. In our study, the high-dose alcohol group was 2.4 g/day, which was below the 3 g/kg dose in the Bielawski paper, and we assumed that the BAC would be slightly below 53 mg/dL.
2.2.3. High Dose Alcohol + DHA (HD/DHA) at 2.4 g Alcohol/kg/day + DHA at 1250 mg/kg/day (N = 5)
The DHA dose of 1250–2500 mg/kg was found to be safe and did not cause implantation losses, resorptions, abnormal sex ratios in live births, or fetal malformations [14]. DHA was obtained from DHASCO oil (DSM Nutritional Products, Columbia, MD, USA), which contains approximately 35% DHA (350 mg/mL DHA), 15% omega-6 docosapentaenoic acid (DPAn-6, 22:5n-6), and saturated and monounsaturated fatty acids. The full composition of the DHASCO oil is provided in Supplementary Materials S1. DHA was administered at a daily dose of 1250 mg/kg of body weight, along with 2.4 g/kg/day of alcohol, in two divided doses from gestational day 8 to day 20. Based on the initial weight of the dams, the gavage volume of 29.7% alcohol that the dams received in the alcohol + DHA group ranged from 2.2 to 2.5 mL per day. DHA was given at 0.8 mL per day.
2.2.4. Gavage Feeding
Intubation of the dams for gavage feeding was initiated on gestational day 8 and continued until day 20. The feedings were given in two separate sessions, two hours apart, starting at 10 am. The gavage feedings were delivered through a curved, stainless steel, blunt needle (Perfectum #7916 CVD). No anesthesia or sedation of the dams was required, since the research members were well-trained in gavage insertion and feeding and the dams were gently and manually restrained with a dry towel.
The dams tolerated well the volume of ethanol plus DHA or olive oil that was administered at the high dose of 2.4 g alcohol/kg/day. Some dams were slightly intoxicated approximately 30 min after the alcohol feeding but regained motor ability, including normal feeding and drinking, within 4 h. They were monitored for 15 min after alcohol feeding to look for signs of labored breathing or distress, per the protocol of the Institutional Animal Care and Use Committee (IACUC) of Wayne State University.
2.2.5. Delivery of the Dams and Pups
The usual gestational period for the dams is about 21 days, but we delivered the fetuses on GD 20 to prevent the spontaneously delivered pups on GD 21 from breastfeeding. On GD 20, the dams were quickly anesthetized with CO2 gas, and death was induced by puncturing the chest wall to cause a pneumothorax, opening the chest cavity, and cutting the heart. A laparotomy was immediately performed and the uterine horns exteriorized and opened to deliver the fetuses and the placenta. A total of 8 pups, closest to the uterus, were delivered per dam, four pups from each uterine horn. The pups were immediately weighed, decapitated, and their heads frozen in liquid nitrogen and stored at −80 °C for future analysis of brain lipid mediators of inflammation.
We could not determine the sex of the pups based on the standard measurement of anogenital distance, since the pups were very small and the key structures were difficult to identify. Thus, the offspring were not sex-stratified.
Since the brains of the pups were very small and soft, it was not possible obtain individual parts of the brain for separate analysis of lipid mediators. Instead, for each pup, we took the whole brain and analyzed it as one sample. However, we did not pool the brain of all the pups from each litter into one sample. Rather, we analyzed the whole brain of each pup separately.
2.2.6. Brain Lipid Analysis
Thawed brain tissue from rat pups was used to prepare samples for liquid chromatography–mass spectrometry (LCMS) analysis. The pup’s head was retrieved from the deep freeze. The skull was opened to obtain the entire brain content, and the brain was weighed and thawed on ice. The thawed brain tissue was suspended in ice-cold phosphate-buffered saline (50 mM phosphate, 0.9% NaCl, pH 7.4) containing 10 mM butylated hydroxytoluene at a ratio of 1:9 (w/v, tissue to buffer). Zirconium beads were added to the sample and homogenized by high-frequency oscillation (PreCellys, Bertin Instruments, Rockville, MD, USA). The homogenate was centrifuged at 1000× g for 10 min and the supernatant was collected for lipid mediator extraction and analysis as previously reported [15,16]. Briefly, the homogenate was supplemented with 5 ng each of stable isotope-labeled internal standards (Prostaglandin E1-d4, Resolvin D2-d5, Leukotriene B4-d4, 15-hydroxyeicosatetraenoic acid-d18 (HETE), and 14R,15S-epoxyeicosatrienoic acid (14,15-EpEtrE-d11)—Cayman Chemicals, Ann Arbor, MI, USA) and applied to StrataX Solid Phase Extraction columns (Phenomenex, Torrance, CA, USA). The eluates were analyzed by LCMS on a QTRAP5500 mass spectrometer following high-performance liquid chromatography (HPLC) separation on a Luna C18(2) (2 × 150 mm, 3µ) column as previously described [15,16]. Lipid mediator concentrations were calculated against the internal standards and normalized to the protein concentration in the homogenate (ng/mg protein).
2.2.7. Statistical Analysis
We enrolled eight pups from the litter of each dam consisting of four pups from each uterine horn. The sample size provided us with 90–95% power to detect a medium effect size among groups and at p < 0.05 for statistical significance. Each lipid metabolite was an independent measure and was compared to the type of treatment for groupwise changes, since the aim of this study was to compare specific lipid metabolite concentrations based on treatment groups and not between metabolites. Homogeneity of variance was assessed using Levene’s test; if variances were equal, the means were compared using one-way analysis of variance (ANOVA). If variances were unequal, the non-parametric Kruskal–Wallis (H test) was used for pairwise comparisons. p < 0.05 in either the ANOVA or Kruskal–Wallis tests was considered as the level of statistical significance.
An unadjusted Dunn’s test was used to compare the dependent variable across each pairing of the independent variable to examine possible significant differences. To reduce the risk of Type I errors (or false positives), a Bonferroni correction was applied to adjust the p values.
Levene’s test was used to assess the equality of variances across different groups. The test evaluates whether the variances in different samples are significantly different from each other. This is particularly important because many statistical tests assume that the variances of the populations from which the samples are drawn are equal. Given the number of groups examined in the model, we chose Levene’s test as an appropriate selection. Although the Welch’s ANOVA is generally more powerful than the Kruskal–Wallis test under specific conditions related to data characteristics, the Welch’s ANOVA is more powerful than the Kruskal–Wallis test when the data is normally distributed, variances are unequal, sample sizes are large, and effect sizes are significant. The graphs shown in Figure 2 and Figure 3 display positively skewed distributions, and since the sample sizes used in our study within each group were small, we interpreted these as sufficient evidence to perform the non-parametric Kruskal–Wallis test.
Statistical analysis was performed using SPSS version 29.
3. Results
3.1. Birth Weight
The mean birth weight of the pups when delivered on day 20 of gestation is shown in Table 1 [17].

Table 1.
The mean birth weight (±SD) of rat pups at the delivery date of day 20 in the control, low-dose alcohol (LD), high-dose alcohol (HD), and high-dose alcohol + DHA (HD/DHA) treatment groups. Comparison was carried out by one-way ANOVA with Bonferroni correction. All dams were also given olive oil except the HD/DHA group.
The number of fetuses reported is the number of fetuses obtained per litter and not the litter size. The pups in the high-dose alcohol group had a significantly higher mean birth weight compared to the control group (4.10 g vs. 3.06 g, p <0.05). Similarly, the pups in the high-dose alcohol/DHA group had a significantly higher birth weight compared to the control group (3.54 g vs. 3.06 g, p = 0.05). The pups in the high-dose alcohol group had a significantly higher birth weight than the pups in the high-dose alcohol/DHA group (4.10 g vs. 3.54 g, p = 0.02). There was a significant difference in the birth weight between the pups in the LD alcohol vs. HD alcohol group (3.08 g vs. 4.10 g, p = 0.05) and the LD vs. HD alcohol/DHA group (3.08 g vs. 3.54 g, p = 0.05). There was no significant difference in the mean birth weight of pups in the LD alcohol vs. control group (3.08 vs. 3.06, p > 0.05).
3.2. Dam Weights
The mean weight of the dams on gestation days 8 and 20 are shown in Table 2 [17]. There was no significant difference in the dam weight in the different treatment groups on GDs 8 and 20. The alcohol dose was calculated from the initial weight of the dams.

Table 2.
Mean body weight (gms) ± SD of the dams on GDs 8 and 20 in the control, low-dose alcohol (LD), high-dose alcohol (HD), and high-dose alcohol + DHA (HD/DHA) treatment groups. Comparison was carried out by one-way ANOVA with Bonferroni correction. All dams were given olive oil except the HD/DHA group.
3.3. Placental Weights
The mean placental weights from each treatment group are shown in Table 3 and Table 4 [17]. The mean placental weight in the control group was significantly smaller in the control group compared to the treatment groups (ANOVA, p < 0.001).

Table 3.
Mean placental weight (±SD) in the control, low-dose alcohol (LD), high-dose alcohol (HD), and high-dose alcohol + DHA (HD/DHA) groups. Comparison of means was carried out by one-way ANOVA with post hoc Duncan’s test.

Table 4.
Duncan’s post hoc statistical test.
3.4. Brain Lipid Mediators of Inflammation
Many cyclooxygenase- and lipoxygenase-derived pro-inflammatory and pro-resolving lipid mediators were analyzed in the pups’ brains in response to alcohol dose and DHA supplementation of the dams. The different brain lipid mediators were compared based on their treatment group, and only those mediators with a significant difference (p < 0.05) when comparing group treatments are presented in this report. These brain lipid mediators included 1) the pro-inflammatory lipid mediators (PILMs), e.g., leukotriene B4 (LTB4), prostaglandin E2 (PGE2), prostaglandin F2 (PGF2), and thromboxane B2 (TXB2); 2) the pro-resolving lipid mediators (PRLMs), e.g., lipoxin A5 (LXA5), 4-Hydroxydocosahexaenoic acid (4-HDoHE), 17-Hydroxydocosahexaenoic acid (17-HDoHE), and 7R,14S-dihydroxy-docosapentaenoic acid (MaR1n-3 DPA).
The pro-inflammatory lipid mediators are derived from the cyclooxygenase pathway (PGE2, PGF2alpha, and TxB2) and the lipoxygenase pathway (LTB4). The pro-resolving mediators (LXA4, 4 and 17 HDoHE, and MaR1) originate from the lipoxygenase pathway.
The mean concentrations of the brain lipid mediators were significantly skewed, and their variances were non-homogeneous. Therefore, the Kruskal–Wallis H test was used to compare the groups based on treatment.
The results of the Kruskal–Wallis H test comparing median brain lipid mediator concentrations across treatment groups are shown in Figure 2 and Figure 3. There were three degrees of freedom.
(Note: DHASCO, which was the primary source of DHA in this study, contains other polyunsaturated fatty acids, as shown in Supplementary Materials S1. Therefore, the possible contribution of these other fatty acids cannot be excluded from the beneficial effects attributed to DHA alone. Although the DHA dose given to the dams was based on its actual content in DHASCO, the term “DHA” in the study refers to the combined effect of DHA and other polyunsaturated fatty acids present in DHASCO.)
3.4.1. Pro-Inflammatory Brain Lipid Mediators
The effects of alcohol with or without DHA on pro-inflammatory lipid mediators, such as LTB4 (Leukotriene B4), PGE2 (Prostaglandin E2), PGF2a (Prostaglandin F2), and TXB2 (Thromboxane B2), in the fetal rat brain are shown in Figure 2. All data are expressed in ng/mg protein of pup brain homogenates and are presented as the median with interquartile range.
Paired comparisons and p-values were analyzed using the Kruskal–Wallis test, with the red line in the figure indicating no significant relationship at p > 0.05 and the blue line indicating a significant relationship at p < 0.05.
- Legend: Control = olive oil + maltose/dextrin;
- Low-dose alcohol = 1.6 g/kg/day alcohol + olive oil;
- High-dose alcohol = 2.4 g/kg/day alcohol + olive oil;
- Alcohol + DHA = alcohol (2.4 g/kg/day) + DHA (1250 mg/kg).
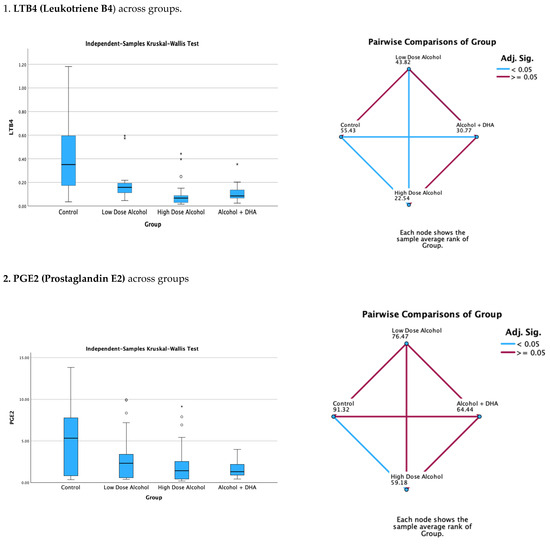
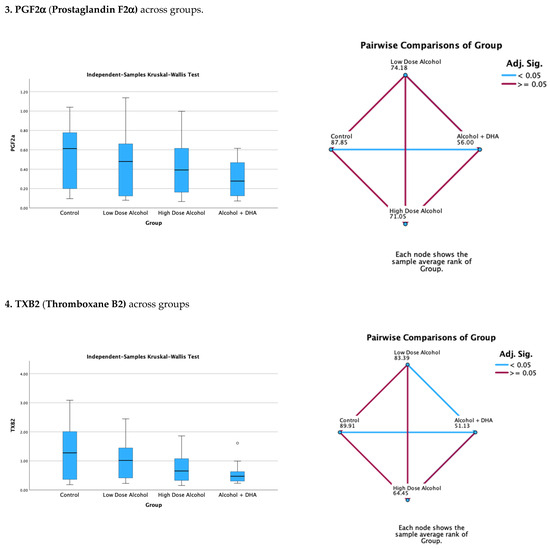
Figure 2.
The effect of alcohol with or without DHA on the pro-inflammatory lipid mediators of inflammation, viz., LTB4 (Leukotriene B4), PGE2 (Prostaglandin E2), PGF2a (Prostaglandin F2α), and TXB2 (Thromboxane B2), in the fetal rat brain. All data are in ng/mg protein of pup brain homogenates and presented as median and interquartile range. Asterisks in bar graphs stand for extreme values.
Summary of paired comparisons of pro-inflammatory brain lipid mediators based on treatment:
- The low-dose alcohol group was not significantly lower than the control in the four pro-inflammatory lipid mediators.
- The high-dose alcohol group was significantly lower than the control in LTB4 and PGE2 (Figure 2).
- The high-dose alcohol + DHA group was significantly lower than the control in LTB4, PGF2α, and TXB2.
- High-dose alcohol was significantly lower than low-dose alcohol in LTB4.
- High-dose alcohol + DHA was not significantly different compared to high-dose alcohol in any of the pro-inflammatory lipid mediators.
3.4.2. Pro-Resolving Brain Lipid Mediators
The effect of alcohol, with or without DHA, on the pro-resolving lipid mediators, e.g., LXA5 (Lipoxin A5), 17-HDoHE (17-Hydroxydocosahexaenoic acid), 4-HDoHE (4-Hydroxydocosahexaenoic acid), and MaR1n-3 DPA (7R,14S-dihydroxy-docosapentaenoic acid), in the fetal rat brain are shown in Figure 3. All data are expressed in ng/mg protein of pup brain homogenates and are presented as medians with interquartile range.
Paired comparisons and “p” values were analyzed using the Kruskal–Wallis test, with the red line in the figure indicating no significant relationship at p > 0.05 and the blue line indicating a significant relationship at p < 0.05.
- Legend: control = olive oil + maltose/dextrin;
- Low-dose alcohol = 1.6 g/kg/day alcohol + olive oil;
- High-dose alcohol = 2.4 g/kg/day alcohol + olive oil;
- Alcohol + DHA = alcohol (2.4 g/kg/day) + DHA (1250 mg/kg).
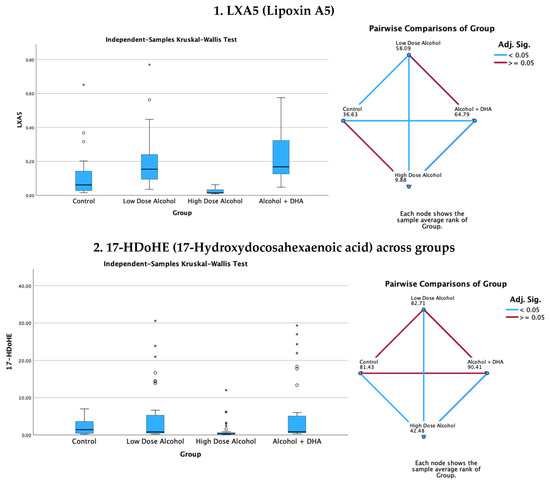
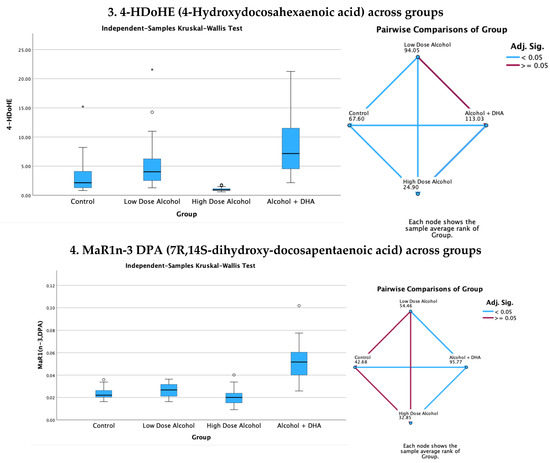
Figure 3.
Effect of alcohol with or without DHA on the pro-resolving lipid mediators, e.g., LXA5 (Lipoxin A5), 17-HDoHE (17-Hydroxydocosahexaenoic acid), 4-HDoHE (4-Hydroxydocosahexaenoic acid), and MaR1n-3 DPA (7R,14S-dihydroxy-docosapentaenoic acid), in the fetal rat brain. All data are in ng/mg protein of pup brain homogenates and presented as the median and interquartile range. Paired comparisons and “p” values were analyzed by the Kruskal–Wallis test with the red line in the figure indicating no significant relationship at p > 0.05 and the blue line indicating a significant relationship at p < 0.05.
Summary of paired comparisons of pro-resolving brain lipid mediators based on treatment:
- The low-dose alcohol group was significantly higher than the control in LAX5 and 4-HDoHE.
- The high-dose alcohol group was significantly lower than the control in 4-HDoHE and 17-HDoHE. Alcohol + DHA was significantly higher than the control in LXA5, MaR1n-3 DPA, and 4-HDoHE.
- High-dose alcohol was significantly lower than low-dose alcohol in LXA5, 4-HDoHE, and 17-HDoHE and vs. the control in 4-HDoHE and 17-HDoHE.
- High-dose alcohol combined with DHA, compared to high-dose alcohol alone, showed a significant increase in LXA5 and MaR1 (n-3 DPA), and a two- to three-fold rise in 4-HDoHE (7.163 vs. 2.155 ng/mg, p < 0.001) and 17-HDoHE levels (0.850 vs. 0.317 ng/mg, p < 0.001), despite the high alcohol dose.
4. Discussion
Inflammation is a major protective response of the body to noxious insults [1]. The initial inflammatory response involves specific cellular infiltrates, such as neutrophils, and the release of pro-inflammatory lipid mediators, including prostaglandins, cytokines, and leukotrienes [1]. This is soon followed by the cessation of PMN influx, the clearance of debris by macrophages, and the production of pro-resolving lipid mediators known as specialized pro-resolving mediators (SPMs), such as lipoxins, resolvins, protectins, and maresins, which evoke potent anti-inflammatory and pro-resolving responses as well as enhance microbial clearance [1]. SPMs include ω-6 arachidonic acid-derived lipoxins, ω-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)-derived resolvins, protectins and maresins, cysteinyl-SPMs, and n-3 docosapentaenoic acid (DPA)-derived SPMs [1,18].
In a rat model, our study found many of the cyclooxygenase and lipoxygenase-derived pro-inflammatory and pro-resolving lipid mediators in the brain of rat pups chronically exposed to alcohol and DHA during gestation. These included pro-inflammatory lipid mediators such as LTB4, PGE2, PGF2α, and TXB2; pro-resolving mediators such as lipoxin LXA5 and MaR1n-3DPA; and resolvin precursors such as 4-HDoHE and 17-HDoHE (Figure 2 and Figure 3).
The study showed no significant increase in pro-inflammatory lipid mediators in the low-dose alcohol group (1.6 g/kg/day) compared to the control, likely due to the low alcohol dose and the potential beneficial effect of olive oil. Conversely, high-dose alcohol (2.4 g/kg/day) without DHA supplementation resulted in a significant (p < 0.005) reduction in pro-inflammatory lipid mediators, such as LTB4, PGE2, and TXB2, as well as a significant decrease in pro-resolving lipid mediators such as LXB4, 4-HDoHE, and 17-HDoHE.
Although the expectation was that both pro-inflammatory and pro-resolving brain lipid mediators would increase in response to alcohol exposure, this was not observed, likely due to significant neuronal degeneration and death caused by prolonged alcohol toxicity. Alcohol has been shown to induce apoptotic neuronal degeneration and death through the activation of caspase 3 and 9 [19,20], DNA fragmentation, nuclear disruption [21], oxidative stress that coincides with the induction of pro-inflammatory cytokines TNF-α, IL-1β and TGF-β [22], enhanced acetylcholinesterase activity, and increased oxidative-nitrosative stress [22,23].
Although a high dose of alcohol alone caused a significant decrease in both pro-inflammatory and pro-resolving lipid mediators in the pups’ brains, the opposite was observed with alcohol + DHA supplementation. Despite the high alcohol dose, many pro-resolving brain lipid mediator levels were significantly increased with high alcohol combined with DHA. These included (1) a significant rise in LXA5 and MaR1n-3DPA, (2) a threefold increase in 4-HDoHE, and (3) an increase in 7-HDoHE concentration (Figure 3). Therefore, DHA’s ability to increase pro-resolving brain lipid mediators provides strong evidence of its protective role in counteracting the harmful effects of high alcohol intake on the fetal brain.
Resolvin precursors were enhanced by DHA supplementation in the alcohol-exposed fetal pups. Resolvin D-series are produced from the oxidation of DHA by 15-lipooxygenase to 17-hydroperoxydocosahexaenoic acid and metabolized further into resolvin Ds. The resolvin E series are produced from EPA by acetylated cyclooxegenase-2 or cytochrome P450 [24]. Resolvin was discovered by Serhan and his team in their search for potential endogenous bioactive compounds derived from omega-3 fatty acids [25]. The pro-resolving and anti-inflammatory effects of resolvins are predominately achieved through specific G-protein-coupled receptors [26]. Resolvins can inhibit microglia activation and reduce the pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1β, iNOS, and nitric oxide, through the MAPK, NF-κB, PI3K/Akt, and caspase-3 signaling pathways [25]. The many actions of resolvin in resolving inflammation-mediated diseases are extensively discussed in several review articles [23,24,25,26,27].
In a previously published report [17], we measured cytokine levels in the dams, which showed higher pro-inflammatory cytokines (principally interleukin-1β, interleukin-12p70, and TNF-α) in the alcohol-exposed pregnant rats compared to the control. However, the difference was not statistically significant, due to the large variance in the mean concentrations and the small sample size. Nonetheless, the literature shows an increase in brain cytokines from alcohol insult secondary to microglia activation [28] and an increase in the brain cytokines, TNF-α, IL-1β, and TGF-β in alcohol-exposed rat pups [21].
DHA is one of the important long-chain polyunsaturated fatty acids (LCPUFAs) in the body. It is a major component of the pro-resolving lipid mediators of inflammation [18] and an essential element in the growth and development of the brain and retinal development in infants and normal brain function in adults [28,29]. DHA deficiencies are associated with Fetal Alcohol Syndrome, attention deficit hyperactivity disorder, cystic fibrosis, phenylketonuria, unipolar depression, aggressive hostility, and adrenoleukodystrophy [30].
Deficiency in DHA can occur in the fetus secondary to several conditions. Prematurity is a significant factor since the premature infant cannot sufficiently synthesize DHA, and a maternal source is necessary [31]. LCPUFA is increasingly transferred from the mother to fetus late in pregnancy, reaching peak accretion rates between 42 and 75 mg/d at 35–40 weeks of gestational age [32,33]. Thus, premature delivery of the infant results in lower levels of DHA and other vital PUFAs due to the insufficient transfer of these fatty acids from the mother. In maternal alcohol disorder, the deficiency of DHA in the fetus is further aggravated since alcohol depletes DHA by the esterification of DHA into fatty acid ethyl esters (FAEEs), which is excreted in the urine [34]. DHA deficiency has been observed in the plasma levels of women with high alcohol intake during pregnancy and, consequently, a low placental transport of DHA to the fetus [35].
Prenatal alcohol exposure in the developing rat brain appears to decrease PUFA concentrations of membrane phospholipids [36,37], and the deficiency of DHA in the fetus has been shown to result in poor cognitive and behavioral performance [33,38,39,40,41]. Supplementation of pregnant women in their diet with n-3 PUFA has appeared to have increased DHA levels, reduced placental oxidative stress, and enhanced placental and fetal growth [42,43]. The postnatal omega-3 supplementation of pups exposed to prenatal alcohol also partially reduces oxidative stress and reverses long-term deficits in hippocampal synaptic plasticity [44,45].
Although our primary aim in this study was to determine the salutary role of DHA on brain lipid mediators of inflammation in rat pups prenatally exposed to alcohol with olive oil as the lipid control for DHA, there was an unexpected secondary finding of a significant increase in birth weight with olive oil, even in the alcohol-exposed pups [17]. The rise in birth weight could have resulted from the calories provided by alcohol and olive oil, since olive oil has 8.5 calories per gram and alcohol has 7 calories per gram. Likewise, the positive effect of olive oil on birth weight was more noticeable in the high-dose compared to low-dose alcohol groups, probably due to the higher caloric intake in the high-dose alcohol group. The pups’ birth weight was also higher in the high-dose alcohol/olive oil group compared to the high-dose alcohol/DHA group, possibly because of DHA loss from esterification with alcohol [34]. It is also possible that increased visceral fat biosynthesis and accumulation, due to the interaction of alcohol and the high saturated fat content in olive oil, contributed to these results [46].
Olive oil contains hydroxytyrosol (HT), a key polyphenol with anti-inflammatory and neuroprotective effects. Hydroxytyrosol is quickly absorbed and demonstrates cytoprotective properties by scavenging free radicals and reducing inflammation [47,48]. In animal studies, maternal HT supplementation has also been associated with improved neurogenesis and cognitive function [49,50], as well as increasing average birth weight [49]. Thus, it is probably worthwhile to explore the potential of olive oil supplementation in improving the birth weight of infants born to women who consume alcohol during pregnancy. However, unlike DHA, olive oil, when combined with alcohol in our study, did not increase the levels of pro-resolving lipid mediators in the brains of the alcohol-exposed pups (Figure 3). Existing evidence from the literature does not support any benefits of olive oil in counteracting alcohol’s inflammatory effects.
5. Limitations
There are some limitations in this study.
- (1).
- This paper is essentially an exploratory study that demonstrates the response of pro-inflammatory and pro-resolving brain lipid mediators to alcohol, and the protective role of DHA. However, as an exploratory study, it is not comprehensive and many biomarkers of alcohol-induced degeneration—such as caspase 3 and 9 activation, DNA fragmentation, nuclear disruption, and pro-inflammatory cytokines like TNF-α, IL-1β, and TGF-β—are not included. Similarly, exploring the relationship with FASD phenotypes and brain pathology was beyond the initial aims of this study. Nonetheless, the research, especially its findings on the beneficial effects of DHA on pro-resolving brain lipid mediators, provides a foundation for future studies. These future investigations could offer valuable insight into the mechanisms of alcohol toxicity and potential treatment strategies in relation to prevention of the serious fetal and infant sequelae associated with maternal alcoholism. The Special Issue on “The Biological Impacts of Fetal Alcohol Exposure” aims to “bring together cutting-edge research from a range of disciplines to deepen our understanding of how prenatal alcohol exposure affects fetal development and long-term health outcomes”. Furthermore, despite decades of research, many questions remain about how alcohol disrupts critical biological processes during gestation, and how these disruptions result in the clinical features seen in Fetal Alcohol Spectrum Disorders (FASDs). We hope our study, though exploratory, aligns well with these goals.
- (2).
- Adverse sex-specific responses to prenatal alcohol are known, such as in cognitive control [51], performance in differential tasks [52], and neuroimmune function [53]. Unfortunately, we were unable to determine the sex of the pups in this study, due to the difficulty we encountered in accurately measuring the anogenital distance in the extremely small pups. Molecular sex determination using Spry gene PCR determination on tissues has been suggested, and we can explore this option on frozen brain tissues of the pups, once funding becomes available.
- (3).
- The present study of brain lipid mediators in the pups is only the first step in assessing the beneficial effects of DHA in mitigating the adverse impacts of alcohol on the fetal brain. Demonstrating an improvement in pups’ behavior is also necessary. We have an ongoing follow-up study using the same protocol which examines the anxiety behavior in rat pups at 6 weeks of postnatal age. Preliminary observations indicate that female rats perform better than male pups in the anxiety tests, which highlights the importance of sex.
- (4).
- We could not determine sex and measure brain alcohol concentrations in the rat pups, nor blood alcohol concentrations in the dams, which are important factors that can directly reflect fetal brain exposure and response to alcohol. These factors will be determined in future studies.
6. Conclusions
We conclude that a high dose of 2.4 g/kg/day of alcohol significantly reduced the levels of pro-inflammatory and pro-resolving brain lipid mediators in rat pups prenatally exposed to alcohol. However, even at a high alcohol dose, the substantial decrease in pro-resolving brain lipid mediators was significantly reversed by DHA supplementation of the dams.
This study has potential implications for translational research in humans by using DHA to treat pregnant women who consume alcohol, aiming to prevent serious sequalae of alcohol injury such as FASD/FAS and intellectual disability in their offspring. To date, aside from abstaining from alcohol during pregnancy, there has been no effective intervention in humans to prevent these severe consequences of maternal alcoholism. DHA supplementation could represent a promising alternative to address this problem.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life15101530/s1, Supplementary Materials S1. DHASCO oil composition.
Author Contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript. E.M.O.J. was responsible for the concept, protocol design, and draft of the manuscript. D.Y. contributed significantly to protocol design, the animal experiment, data collection, data analysis, and interpretation of the article. C.T.C. and E.D.K. substantially participated in the animal experiment. K.R.M. was responsible for the lipid analysis in his laboratory and the analysis of the lipid data. R.L.T. contributed significantly to statistical design and data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by a fellow grant from the Sarnaik Endowment Research Foundation (Reference # 2018-01) at the Children’s Research Center of the Children’s Hospital of Michigan. Detroit, Michigan. We thank the support of the Nutritional Product Division of DSM Corporation for providing the DHASCO oil for the study.
Institutional Review Board Statement
The were no human subjects in the study. The animal study protocol was approved by the Wayne State University Institutional Animal Care and Use Committee, Detroit, Michigan (Protocol # IACUC-17-05-271, 13 October 2017) and the study was conducted in accordance with the local legislation and institutional requirements.
Informed Consent Statement
Not applicable. There were no human subjects in the study to require informed consent.
Data Availability Statement
The datasets presented in this article are not readily available because the results of the paper are not published elsewhere and are not linked to archived database which can be shared.
Acknowledgments
The authors are indebted to the valuable suggestions and advice of Robert Sokol of the Department of Obstetrics and Gynecology, Wayne State University School of Medicine, Detroit, Michigan, and Kenneth V. Honn of the Bioactive Lipids Research Program, Wayne State University, Detroit, Michigan, for the study design.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Abbreviations
The following abbreviations were used in this manuscript:
| FASD | Fetal alcohol spectrum disorder |
| FAS | Fetal alcohol syndrome |
| PILMs | Pro-inflammatory lipid mediators |
| LTB4 | Leukotriene B4 |
| PGE2 | ProstaglandinE2 |
| PGF2 | Prostaglandin F2α |
| TXB2 | Thromboxane B2 |
| PRLMs | Pro-resolving lipid mediators |
| LXA5 | Lipoxin A5 |
| 4-HDoHE | 4-Hydroxydocosahexaenoic acid |
| 17-HDoHE | 17-Hydroxydocosahexaenoic acid |
| MaR1n-3 DPA | 7R,14S-dihydroxy-docosapentaenoic acid |
| PUFA | Polyunsaturated fatty acid |
References
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Gosdin, L.K.; Deputy, N.P.; Kim, S.Y.; Dang, E.P.; Denny, C.H. Alcohol consumption and binge drinking during pregnancy among adults aged 18–49 Years—United States. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 10–13. [Google Scholar] [CrossRef]
- Mead, E.A.; Sarkar, D.K. Fetal alcohol spectrum disorders and their transmission through genetic and epigenetic mechanisms. Front. Genet. 2014, 5, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Dörrie, N.; Föcker, M.; Freunscht, I.; Hebebrand, J. Fetal alcohol spectrum disorders. Eur. Child. Adol Psych. 2014, 23, 863–875. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Jones, K.L. A review of the physical features of the fetal alcohol spectrum disorders. Eur. J. Med. Genet. 2017, 60, 55–64. [Google Scholar]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Parunashvili, N.; Rehm, J. Prevalence of alcohol consumption during pregnancy and fetal alcohol spectrum disorders among the general and aboriginal populations in Canada and the United States. Eur. J. Med. Genet. 2017, 60, 32–48. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health J. 2003, 27, 277–284. [Google Scholar]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Valles, S.L.; Blanco, A.M.; Pascual, M.; Guerri, C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004, 14, 365–371. [Google Scholar] [CrossRef]
- Dejong, K.; Olyaei, A.; Lo, J.O. Alcohol Use in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 142–155. [Google Scholar] [CrossRef]
- Cheng, C.T.; Ostrea, E.M.; Alviedo, J.N.; Banadera, F.P.; Thomas, R.L. Fatty acid ethyl esters in meconium: A biomarker of fetal alcohol exposure and effect. Exp. Biol. Med. 2021, 246, 380–386. [Google Scholar]
- Bielawski, D.M.; Abel, E.L. The effect of administering ethanol as single vs. divided doses on blood alcohol levels in the rat. Neurotoxicol. Teratol. 2002, 24, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Boswell, K.D.; Henwood, S.M.; Kyle, D.J. A developmental safety study in rats using DHA-and ARA-rich single-cell oils. Food Chem. Toxicol. 2000, 38, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Maddipati, K.R.; Romero, R.; Chaiworapongsa, T.; Chaemsaithong, P.; Zhou, S.L.; Xu, Z.; Tarca, A.L.; Kusanovic, J.P.; Gomez, R.; Chaiyasit, N.; et al. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4. Faseb J. 2016, 30, 3296–3307. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Romero, R.; Chaiworapongsa, T.; Zhou, S.L.; Xu, Z.; Tarca, A.L.; Kusanovic, J.P.; Munoz, H.; Honn, K.V. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. Faseb J. 2014, 28, 4835–4846. [Google Scholar]
- Yadav, D.; Ostrea, E.M.; Cheng, C.T.; Kisseih, E.; Maddipati, K.R.; Thomas, R.L. Effect of docosahexaenoic acid and olive oil supplementation on pup weight in alcohol-exposed pregnant rats. Front. Pediatr. 2024, 12, 1334285. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. Faseb J. 2017, 31, 1273–1888. [Google Scholar] [PubMed]
- Balaszczuk, V.; Bender, C.; Pereno, G.L.; Beltramino, C.A. Alcohol-induced neuronal death in central extended amygdala and pyriform cortex during the postnatal period of the rat. Int. J. Dev. Neurosci. 2011, 29, 733–742. [Google Scholar] [PubMed]
- Olney, J.W.; Wozniak, D.F.; Jevtovic-Todorovic, V.; Farber, N.B.; Bittigau, P.; Ikonomidou, C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002, 12, 488–498. [Google Scholar]
- Moon, Y.; Kwon, Y.; Yu, S. How does ethanol induce apoptotic cell death of SK-N-SH neuroblastoma cells. Neural Regen. Res. 2013, 8, 1853–1862. [Google Scholar] [PubMed]
- Tiwari, V.; Chopra, K. Resveratrol prevents alcohol-induced cognitive deficits and brain damage by blocking inflammatory signaling and cell death cascade in neonatal rat brain. J. Neurochem. 2011, 117, 678–690. [Google Scholar] [CrossRef]
- Crews, F.T.; Nixon, K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol. Alcohol. 2009, 44, 115–127. [Google Scholar] [CrossRef]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.H.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of Resolvins in the inflammatory resolution of neurological giseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Terasaki, L.S.; Schwarz, J.M. Effects of moderate prenatal alcohol exposure during early gestation in rats on inflammation across the maternal-fetal-immune interface and later-life immune function in the offspring. J. Neuroimmune Pharmacol. 2016, 11, 680–692. [Google Scholar] [CrossRef]
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Baack, M.L.; Puumala, S.E.; Messier, S.E.; Pritchett, D.K.; Harris, W.S. Daily enteral DHA supplementation alleviates deficiency in premature infants. Lipids 2016, 51, 423–433. [Google Scholar] [CrossRef]
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.A.; Muskiet, F.A. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 3–20. [Google Scholar] [CrossRef]
- Innis, S.M. Essential fatty acid transfer and fetal development. Placenta 2005, 26, S70–S75. [Google Scholar] [CrossRef]
- Ostrea, E.M., Jr.; Hernandez, J.D.; Bielawski, D.M.; Kan, J.M.; Leonardo, G.M.; Abela, M.B.; Church, M.W.; Hannigan, J.H.; Janisse, J.J.; Ager, J.W.; et al. Fatty acid ethyl esters in meconium: Are they biomarkers of fetal alcohol exposure and effect? Alcohol Clin. Exp. Res. 2006, 30, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Beblo, S.; Murty, M.; Whitty, J.E.; Buda-Abela, M.; Janisse, J.; Rockett, H.; Martier, S.S.; Sokol, R.J.; Hannigan, J.H.; et al. Alcohol consumption in pregnant, black women is associated with decreased plasma and erythrocyte docosahexaenoic acid. Alcohol. Clin. Exp. Res. 2005, 29, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.E.; Huang, Y.S.; Mills, D.E.; Ward, G.R.; Ward, R.P.; McCutcheon, D. Interactive effects of prenatal ethanol and N-3 fatty acid supplementation on brain development in mice. Lipids 1989, 24, 989–997. [Google Scholar] [CrossRef]
- Wainwright, P.E.; Huang, Y.S.; Simmons, V.; Mills, D.E.; Ward, R.P.; Ward, G.R.; Winfield, D.; McCutcheon, D. Effects of prenatal ethanol and long-chain n-3 fatty acid supplementation on development in mice. 2. Fatty acid composition of brain membrane phospholipids. Alcohol. Clin. Exp. Res. 1990, 14, 413–420. [Google Scholar] [CrossRef]
- Moriguchi, T.; Greiner, R.S.; Salem, N., Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000, 75, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Salem, N., Jr.; Moriguchi, T.; Greiner, R.S.; McBride, K.; Ahmad, A.; Catalan, J.N.; Slotnick, B. Alterations in brain function after loss of docosahexaenoate due to dietary restriction of n-3 fatty acids. J. Mol. Neuros MN 2001, 16, 299–307. [Google Scholar] [CrossRef]
- Fedorova, I.; Hussein, N.; Baumann, M.H.; Di Martino, C.; Salem, N., Jr. An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behav. Neuros 2009, 123, 196–205. [Google Scholar] [CrossRef]
- Lozada, L.E.; Desai, A.; Kevala, K.; Lee, J.W.; Kim, H.Y. Perinatal brain docosahexaenoic acid concentration has a lasting impact on cognition in mice. J. Nutr. 2017, 147, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Mark, P.J.; Keelan, J.A.; Barden, A.; Mas, E.; Mori, T.A.; Waddell, B.J. Maternal dietary omega-3 fatty acid intake increases resolvin and protectin levels in the rat placenta. J. Lipid Res. 2013, 54, 2247–2254. [Google Scholar] [CrossRef]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef]
- Patten, A.R.; Brocardo, P.S.; Christie, B.R. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J. Nutr. Biochem. 2013, 24, 760–769. [Google Scholar] [CrossRef]
- Patten, A.R.; Sickmann, H.M.; Dyer, R.A.; Innis, S.M.; Christie, B.R. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neuros Lett. 2013, 551, 7–11. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Navas, M.D.; Lopez-Villodres, J.A.; Trujillo, M.; Espartero, J.L.; De La Cruz, J.P. Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia-reoxygenation. Neuros Lett. 2008, 446, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Donato-Trancoso, A.; Correa Atella, G.; Romana-Souza, B. Dietary olive oil intake aggravates psoriatic skin inflammation in mice via Nrf2 activation and polyunsaturated fatty acid imbalance. Int. Immunopharmacol. 2022, 108, 108851. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Liu, J.; Feng, Z. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015, 26, 190–199. [Google Scholar] [CrossRef]
- Vazquez-Gomez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gomez-Fidalgo, E.; Sanchez-Sanchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, e0177593. [Google Scholar] [CrossRef]
- Dunn, B.R.; Olguin, S.L.; Davies, S.; Pavlik, N.G.; Brigman, J.L.; Hamilton, D.; Savage, D.D.; Maxwell, J.R. Sex-specific alterations in cognitive control following moderate prenatal alcohol exposure and transient systemic hypoxia ischemia in the rat. Alcohol. Clin. Exp. Res. 2024, 48, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.E.; Dart, E.; Kaiser, D. Prenatal alcohol exposure disrupts performance in a differential reinforcement of low rates task specifically in adolescent male rats. Dev. Psychobiol. 2024, 66, e22555. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.J.; Boschen, K.E.; Roth, T.L.; Klintsova, A.Y. Sex differences in early postnatal microglial colonization of the developing rat hippocampus following a single-day alcohol exposure. J. Neuroimmune Pharmacol. 2018, 13, 189–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).