High-Dose Shilajit Enhances Xenograft-Mediated Bone Regeneration in a Rat Tibial Defect Model: An In Vivo Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Dosage of Shilajit
2.2. Study Design
2.3. Chemical Analysis of Shilajit Extract
2.4. Measurement of Serum TOS/TAS and TNF-α
2.5. Histological and Immunohistochemical Analyses
2.6. Statistical Analysis
3. Results
3.1. LC–MS/MS Analysis Reveals Potent Antioxidant Activity of Shilajit Extract
3.2. High-Dose Shilajit Enhances Antioxidant Capacity and Suppresses Oxidative and Inflammatory Markers
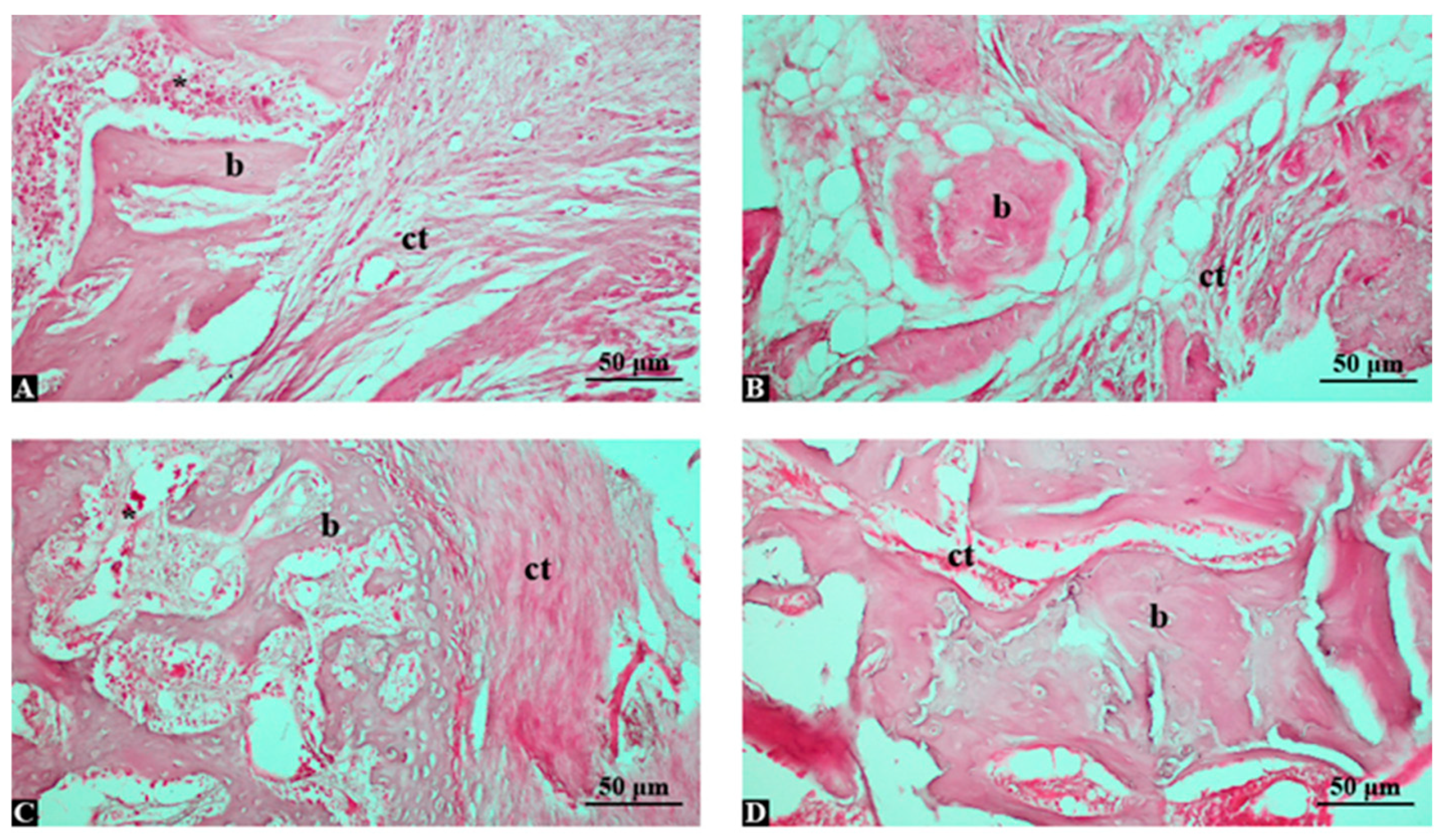
3.3. High-Dose Shilajit with Grafting Maximizes Bone Regeneration and Histological Quality
3.4. Shilajit Extract Increases New Bone Area and Reduces Fibrous Tissue in a Dose-Dependent Manner
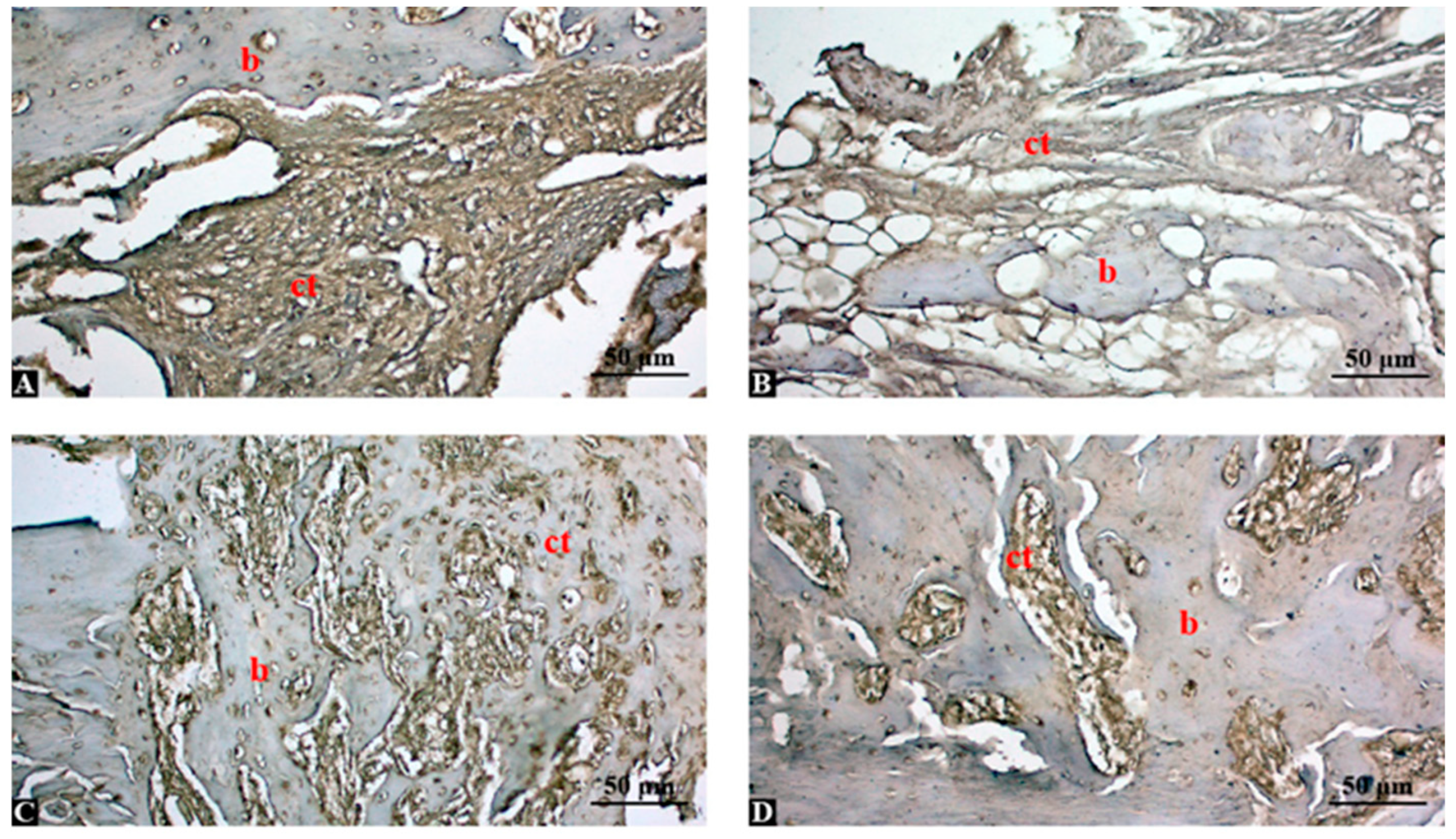
3.5. High-Dose Shilajit Markedly Reduces Local TNF-α Expression During Bone Healing
3.6. Semi-Quantitative Analysis Confirms Dose-Dependent Suppression of TNF-α by Shilajit
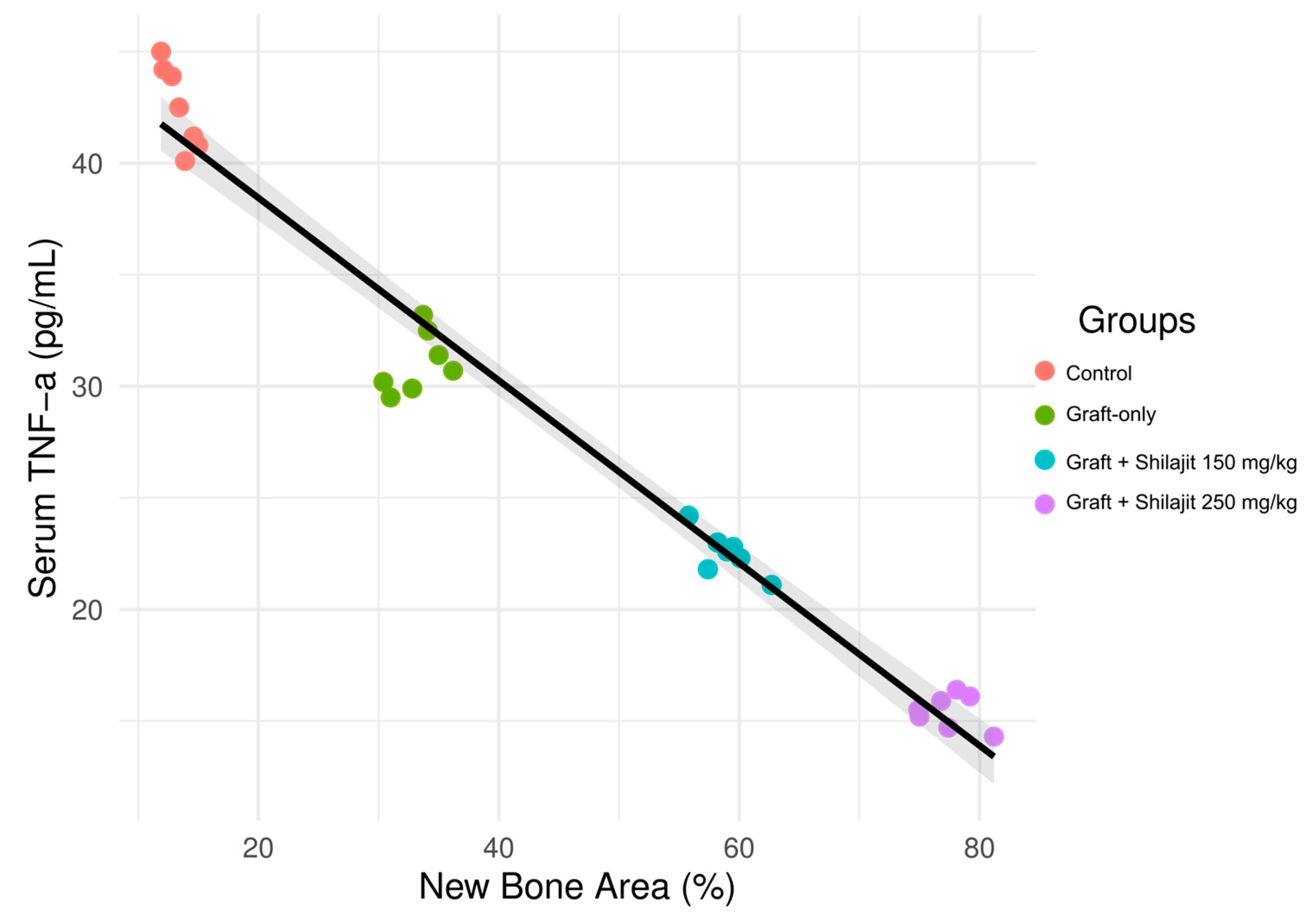
3.7. Inverse Association Between New Bone Formation and Serum TNF-α Levels Across Experimental Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TOS | Total oxidant status |
| TAS | Total antioxidant status |
| TNF | Tumor necrosis factor |
| H-E | Hematoxylin and eosin |
| DAB | 3,3′-diaminobenzidine |
| DPB | Dibenzo-α-pyrones |
| TPC | Total phenolic content |
| GAE | Gallic acid equivalent |
| ABTS | 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid |
| DPPH | 1,1-Diphenyl-2-picryl-hydrazyl |
| DA | Defect area |
| NBA | New bone area |
| RGA | Residual graft area |
| FTA | Fibrous tissue area |
| SD | Standart Deviation |
| EDTA | Ethylenediaminetetraacetic acid |
References
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, 20–27. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Ozden, E.; Kaya, B.; Guler, R. Investigation of the Effects of Thymoquinone and Dental Pulp-Derived Mesenchymal Stem Cells on Tibial Bone Defect Models. J. Craniofac. Surg. 2024, 35, 1958–1963. [Google Scholar] [CrossRef]
- Ciszyński, M.; Dominiak, S.; Dominiak, M.; Gedrange, T.; Hadzik, J. Allogenic Bone Graft in Dentistry: A Review of Current Trends and Developments. Int. J. Mol. Sci. 2023, 24, 16598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Osypko, K.F.; Ciszyński, M.P.; Kubasiewicz-Ross, P.; Hadzik, J. Bone tissue 3D bioprinting in regenerative dentistry through the perspective of the diamond concept of healing: A narrative review. Adv. Clin. Exp. Med. 2023, 32, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Sapoznikov, L.; Haim, D.; Zavan, B.; Scortecci, G.; Humphrey, M.F. A novel porcine dentin-derived bone graft material provides effective site stability for implant placement after tooth extraction: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 2899–2911. [Google Scholar] [CrossRef]
- Moraschini, V.; de Almeida, D.C.F.; Calasans-Maia, M.D.; Kischinhevsky, I.C.C.; Louro, R.S.; Granjeiro, J.M. Immunological response of allogeneic bone grafting: A systematic review of prospective studies. J. Oral Pathol. Med. 2020, 49, 395–403. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L.; Gunsolley, J.C. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann. Periodontol. 2003, 8, 227–265. [Google Scholar] [CrossRef]
- Timothius, C.J.C.; Kilic, H.N.; Gandhi, K.K.; Kakar, A.; John, V. Particulate bone graft materials for periodontal and implant surgery: A narrative review and case series. Dent. Rev. 2023, 3, 100068. [Google Scholar] [CrossRef]
- Kloskowski, T.; Szeliski, K.; Krzeszowiak, K.; Fekner, Z.; Kazimierski, Ł.; Jundziłł, A.; Drewa, T.; Pokrywczyńska, M. Mumio (Shilajit) as a potential chemotherapeutic for the urinary bladder cancer treatment. Sci. Rep. 2021, 11, 22614. [Google Scholar] [CrossRef]
- Kangari, P.; Roshangar, L.; Iraji, A.; Talaei-Khozani, T.; Razmkhah, M. Accelerating effect of Shilajit on osteogenic property of adipose-derived mesenchymal stem cells (ASCs). J. Orthop. Surg. Res. 2022, 17, 424. [Google Scholar] [CrossRef]
- Ghasemkhani, N.; Tabrizi, A.S.; Namazi, F.; Nazifi, S. Treatment effects of Shilajit on aspirin-induced gastric lesions in rats. Physiol. Rep. 2021, 9, e14822. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.; Hosmani, J.; Meer, R.M.; Alqarni, A.; Alumudh, A.; Perumal, E.; Karobari, M.I. The effects of Shilajit on periodontal ligament cells in wound healing: A comprehensive in vitro study. BMC Complement. Med. Ther. 2025, 25, 98. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S. Free radicals, oxidative stress and antioxidant defense. Phytomedica 2000, 21, 19. [Google Scholar]
- Elahi, S.; Mohamadi Sani, A.; Sarabi-Jamab, M. Physicochemical, antioxidant and antimicrobial characteristics of two types of mumies (shilajit). J. Food Meas. Charact. 2024, 18, 4137–4146. [Google Scholar] [CrossRef]
- Sadeghi, S.M.H.; Hosseini Khameneh, S.M.; Khodadoost, M.; Hosseini Kasnavieh, S.M.; Kamalinejad, M.; Gachkar, L.; Rampp, T.; Pasalar, M. Efficacy of Momiai in Tibia Fracture Repair: A Randomized Double-Blinded Placebo-Controlled Clinical Trial. J. Altern. Complement. Med. 2020, 26, 521–528. [Google Scholar] [CrossRef]
- Alshubaily, F.A.; Jambi, E.J. Correlation between Antioxidant and Anti-Osteoporotic Activities of Shilajit Loaded into Chitosan Nanoparticles and Their Effects on Osteoporosis in Rats. Polymers 2022, 14, 3972. [Google Scholar] [CrossRef] [PubMed]
- Pingali, U.; Nutalapati, C. Shilajit extract reduces oxidative stress, inflammation, and bone loss to dose-dependently preserve bone mineral density in postmenopausal women with osteopenia: A randomized, double-blind, placebo-controlled trial. Phytomedicine 2022, 105, 154334. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Sadan, M.; Almundarij, R.; Haridy, M.; EL-Khodery, S.; Elzarei, M.F.; Abo-Aziza, F.A.M.; Ibrahim, Z.H.; Abdellatif, A.A.H. Can Silver nanoparticles stabilized by Shilajit (Asphaltum punjabianum) accelerate tibial bone defects repair in rabbits: A preliminary study. Int. J. Vet. Sci. 2025, 14, 512–519. [Google Scholar]
- Çetin, E.; Samcak, T.; Keleş, Ö.F.; Ünlü, İ.; Akyol, M.E.; Arabacı, Ö. Histopathological and immunohistochemical investigation of the effect of Shilajit in rats with experimental spinal cord injury. Ulus. Travma Acil Cerrahi Derg. 2023, 29, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Agin, H.D.; Gunes, N.; Guler, R. A comparative investigation of the effects of Resveratrol and dental pulp delivered mesenchimal stem cells on rat tibia bone defect healing. Rev. Cient.-Fac. Cienc. Vet. 2024, 34, e34372. [Google Scholar] [CrossRef]
- Kamgar, E.; Kaykhaii, M.; Zembrzuska, J. A Comprehensive Review on Shilajit: What We Know about Its Chemical Composition. Crit. Rev. Anal. Chem. 2023, 55, 461–473. [Google Scholar] [CrossRef]
- Ege, S.; Akduman, H.; Aşır, A.; Korak, T. Excessive Weight Gain During Pregnancy Increased Ponoxarase 1 Level in Neonatal Cord Blood. Antioxidants 2025, 14, 105. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Aşır, F.; Erdemci, F.; Çankırı, Z.; Korak, T.; Başaran, S.Ö.; Kaplan, Ö.; Yükselmiş, Ö.; Dönmezdil, N.; Ayaz, H.; Kaplan, Ş.; et al. Zonisamide Ameliorated the Apoptosis and Inflammation in Cerebellar Tissue of Induced Alcohol Addiction Animal Model. Life 2024, 14, 795. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Keşim, D.A.; Aşır, F.; Ayaz, H.; Korak, T. The Effects of Ellagic Acid on Experimental Corrosive Esophageal Burn Injury. Curr. Issues Mol. Biol. 2024, 46, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, Y.B.; Singh, V.P. Role of Tamra bhasma, an Ayurvedic preparation, in the management of lipid peroxidation in liver of albino rats. Indian J. Exp. Biol. 1996, 34, 66–70. [Google Scholar] [PubMed]
- Dashnyam, K.; Bayaraa, O.; Mandakhbayar, N.; Park, J.H.; Lee, J.H.; Jang, T.S.; Luvsan, K.; Kim, H.W. Nanoscale Calcium Salt-Based Formulations as Potential Therapeutics for Osteoporosis. ACS Biomater. Sci. Eng. 2020, 6, 4604–4613. [Google Scholar] [CrossRef]
| Parameter | Result | Unit/Expression |
|---|---|---|
| Fulvic acid | 215.4 ± 4.8 | mg/g extract |
| Dibenzo-α-pyrones | 12.7 ± 0.9 | mg/g extract |
| Total phenolic content | 35.6 ± 1.1 | mg GAE/g extract |
| ABTS radical scavenging | 48.2 ± 1.6 | IC50 µg/mL |
| DPPH radical scavenging | 62.5 ± 2.0 | IC50 µg/mL |
| Group (n = 7) | TAS (mmol Trolox equiv/L) | TOS (µmol H2O2 equiv/L) | TNF-α (pg/mL) |
|---|---|---|---|
| Control | 0.53 ± 0.09 | 68.8 ± 1.48 | 42.5 (40.1–45.0) |
| Graft-only | 0.95 ± 0.13 * | 50.2 ± 4.69 * | 31.4 (29.5–33.2) * |
| Graft + Shilajit 150 mg/kg | 1.09 ± 0.23 ** | 38.6 ± 1.61 ** | 22.8 (21.1–24.2) ** |
| Graft + Shilajit 250 mg/kg | 1.34 ± 0.27 *** | 20.7 ± 3.01 *** | 15.7 (14.3–17.1) *** |
| Group (n = 7) | DA (mm2) | NBA (mm2) | NBA% | RGA% |
|---|---|---|---|---|
| Control | 7.05 (6.90–7.18) | 0.95 (0.82–1.08) | 13.5 (11.9–15.2) | 0.0 |
| Graft-only | 7.01 (6.88–7.12) | 2.35 (2.10–2.58) | 33.6 (30.4–36.9) | 28.4 (25.1–31.6) |
| Graft + Shilajit 150 mg/kg | 7.03 (6.92–7.16) | 4.15 (3.88–4.41) | 59.1 (55.8–62.7) | 17.6 (15.2–20.1) |
| Graft + Shilajit 250 mg/kg | 7.02 (6.89–7.15) | 5.48 (5.25–5.72) | 78.1 (74.9–81.2) | 8.9 (7.1–10.6) |
| Group | Positive Cell Rate (%) | Mean ± SD | Statistical Comparison |
|---|---|---|---|
| Control | 68.2%, 70.4%, 66.9%, 69.1%, 67.5%, 71.0%, 68.8% | 68.8 ± 1.5 | |
| Graft-only | 52.0%, 50.6%, 48.9%, 51.3%, 53.1%, 49.7%, 50.5% | 50.9 ± 1.5 | p < 0.0001 (vs. control) |
| Graft + Shilajit 150 mg/kg | 38.7%, 36.9%, 35.2%, 37.8%, 36.5%, 38.3%, 37.0% | 37.2 ± 1.1 | p < 0.0001 (vs. graft-only) |
| Graft + Shilajit 250 mg/kg | 22.1%, 23.8%, 21.4%, 22.9%, 23.5%, 20.7%, 22.6% | 22.4 ± 1.0 | p < 0.0001 (vs. graft-only and 150 mg/kg shilajit) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guler, R.; Ozden, E.; Asır, F.; Gulsun, B. High-Dose Shilajit Enhances Xenograft-Mediated Bone Regeneration in a Rat Tibial Defect Model: An In Vivo Experimental Study. Life 2025, 15, 1528. https://doi.org/10.3390/life15101528
Guler R, Ozden E, Asır F, Gulsun B. High-Dose Shilajit Enhances Xenograft-Mediated Bone Regeneration in a Rat Tibial Defect Model: An In Vivo Experimental Study. Life. 2025; 15(10):1528. https://doi.org/10.3390/life15101528
Chicago/Turabian StyleGuler, Ridvan, Ersin Ozden, Firat Asır, and Belgin Gulsun. 2025. "High-Dose Shilajit Enhances Xenograft-Mediated Bone Regeneration in a Rat Tibial Defect Model: An In Vivo Experimental Study" Life 15, no. 10: 1528. https://doi.org/10.3390/life15101528
APA StyleGuler, R., Ozden, E., Asır, F., & Gulsun, B. (2025). High-Dose Shilajit Enhances Xenograft-Mediated Bone Regeneration in a Rat Tibial Defect Model: An In Vivo Experimental Study. Life, 15(10), 1528. https://doi.org/10.3390/life15101528







