Inter-Organ Crosstalk in Neurodegenerative Disease
Abstract
1. Introduction
2. Data Collection Methodology
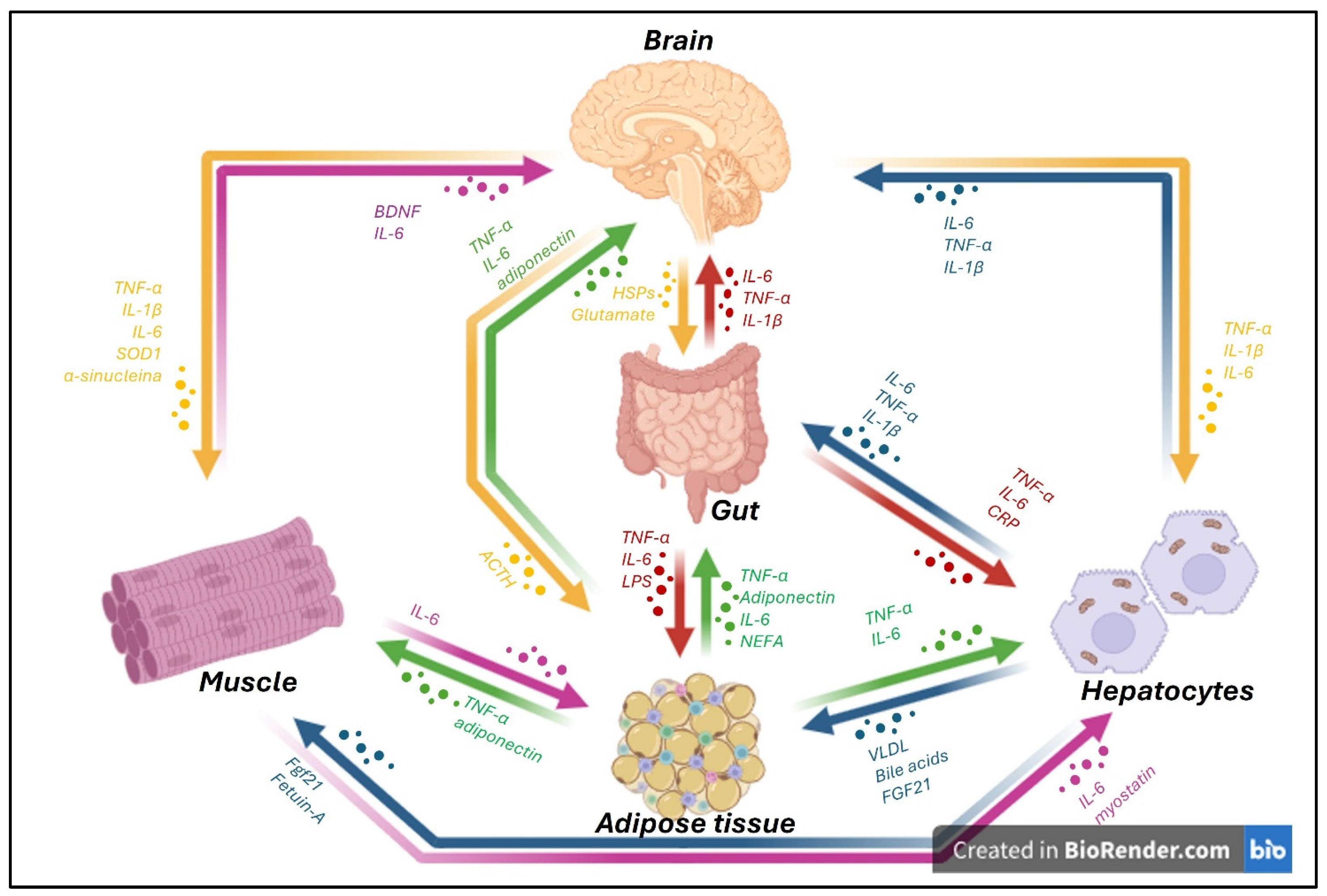
3. Gut–Brain Axis
3.1. Microbiota–Gut–Brain Axis
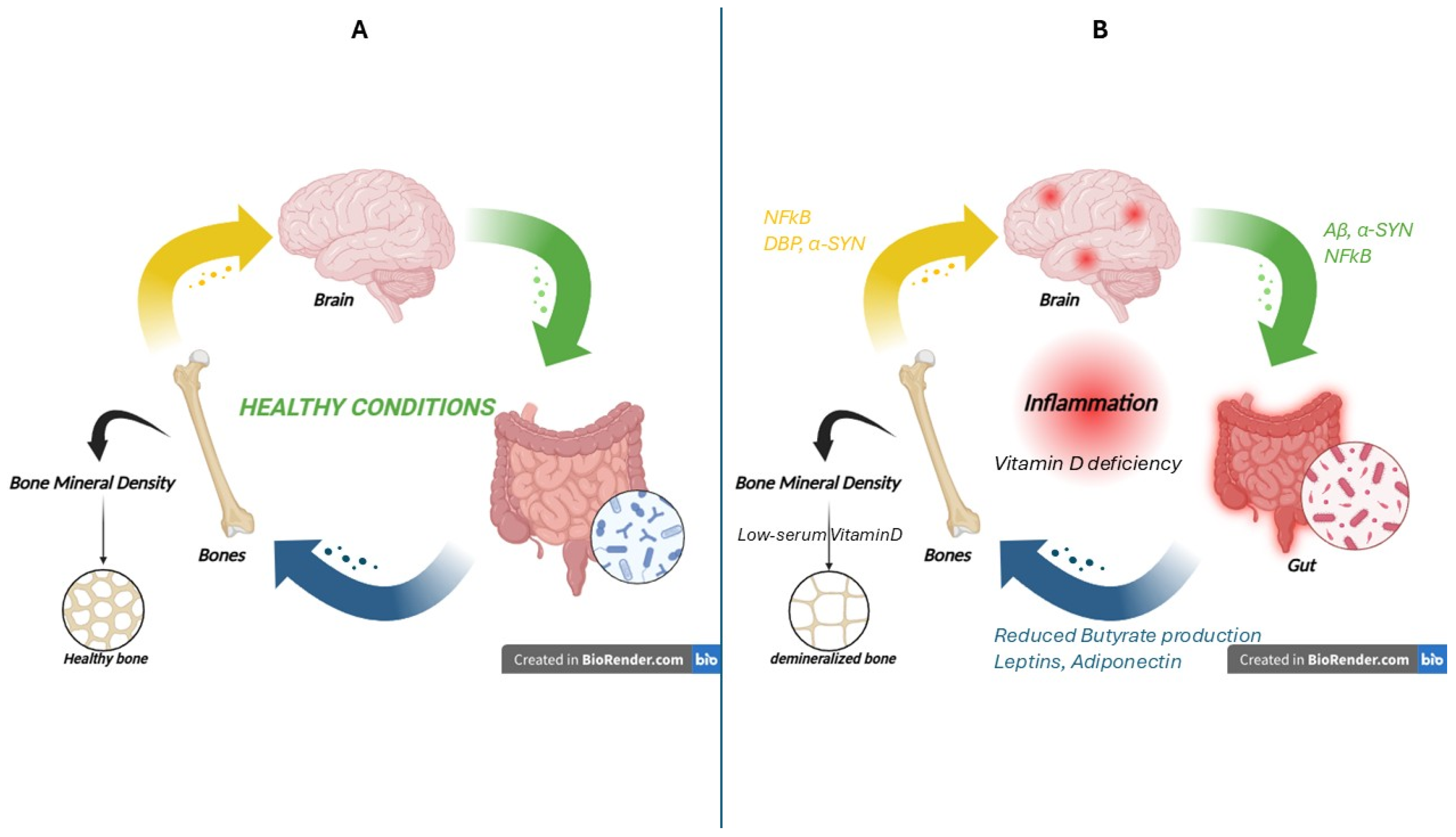
3.2. Brain–Gut–Bone Axis
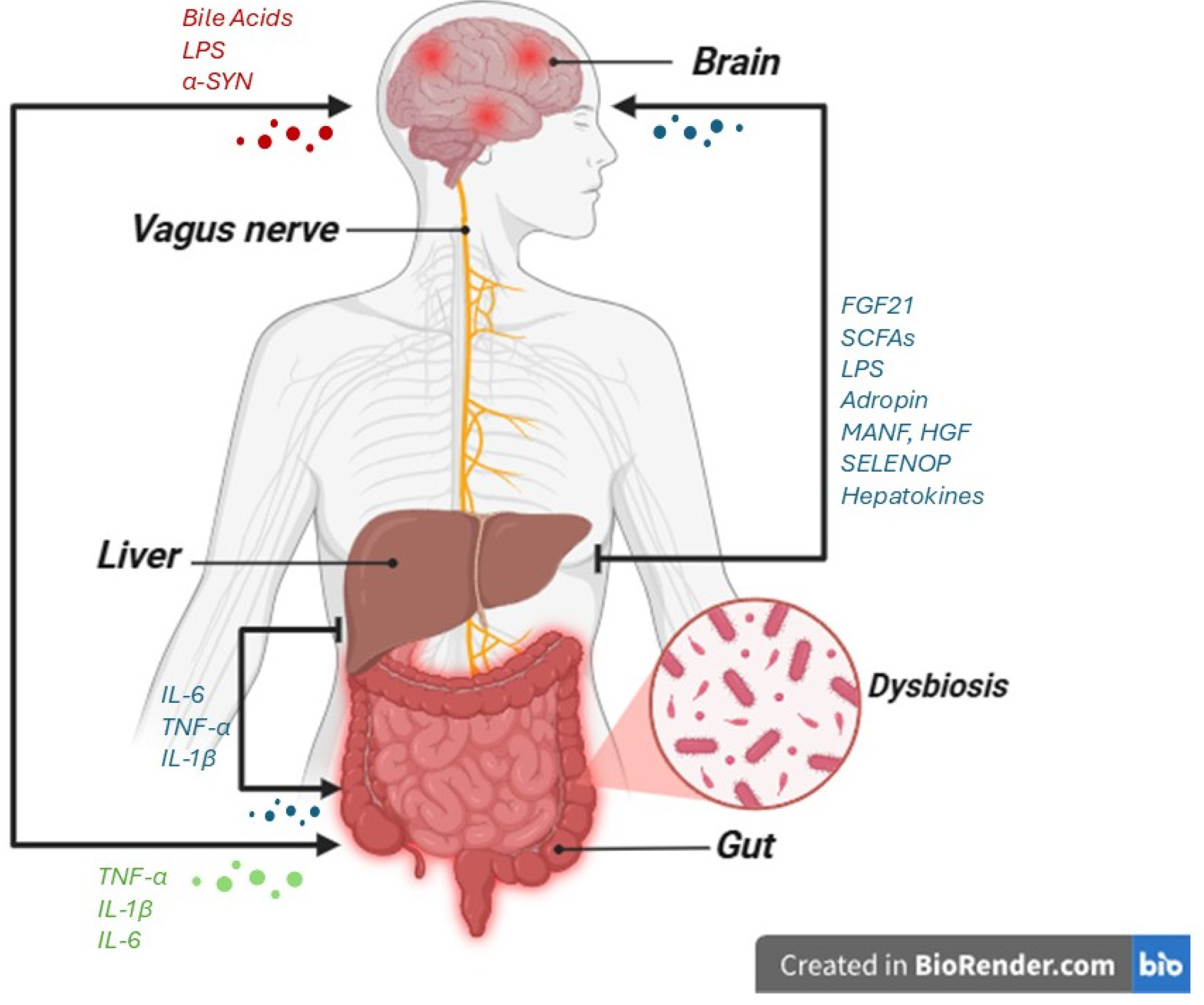
4. Liver–Brain Axis
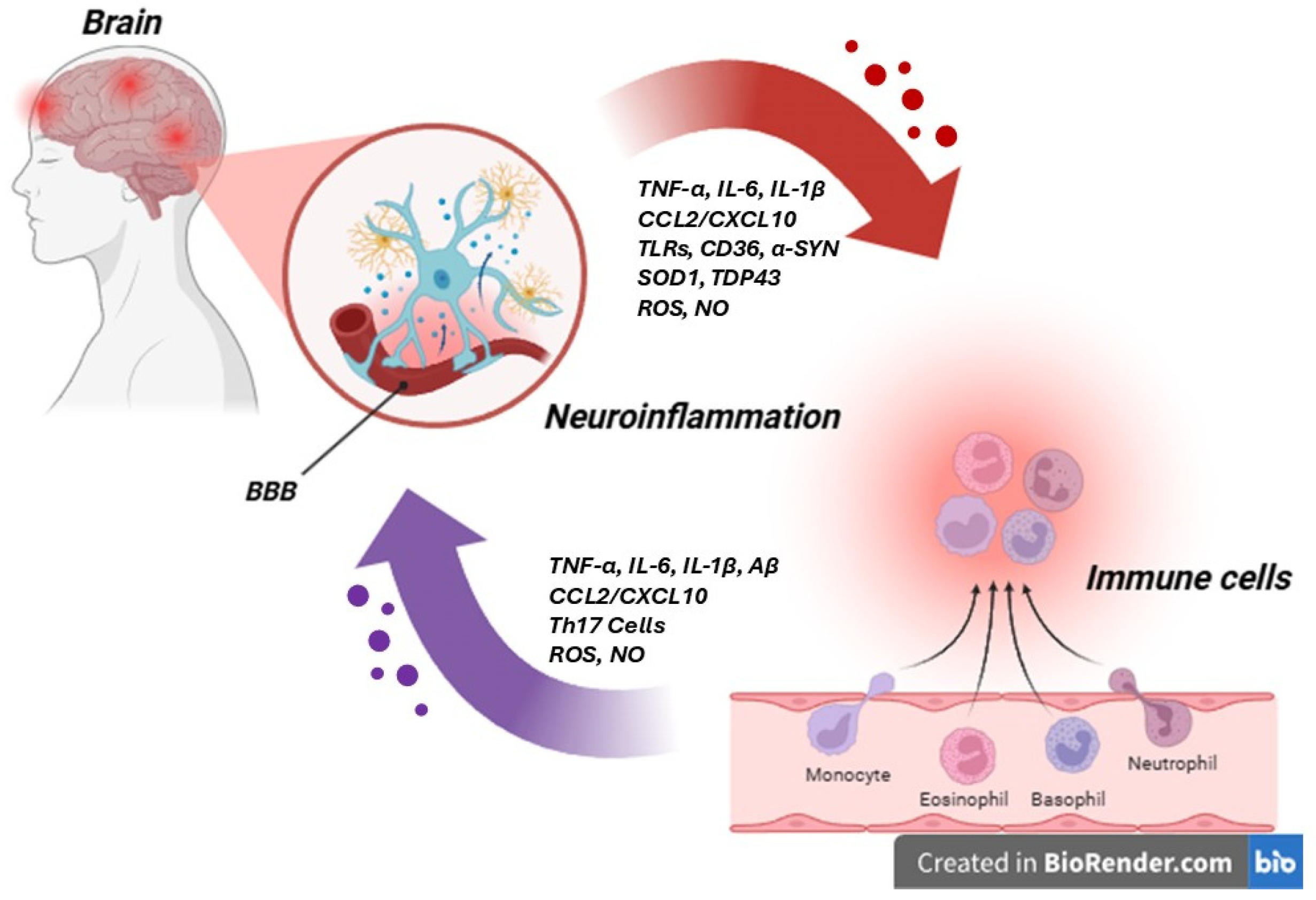
5. Neuro-Immune Axis
6. Brain–Adipose Tissue Axis
7. Muscle–Brain Axis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ND | Neurodegenerative diseases |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| FGF21 | Fibroblast growth factor 21 |
| BDNF | Brain-derived neurotrophic factor |
| HSPs | Heat shock proteins |
| ACTH | Adrenocorticotropic Hormone |
| CNS | Central nervous system |
| ENS | Enteric nervous system |
| ANS | Autonomic nervous system |
| NTS | Nucleus of the solitary tract |
| GM | Gut microbiota |
| LPS | Lipopolysaccharide |
| TLRs | Toll-like receptors |
| SOD1 | Superoxide dismutase 1 |
| BMD | Bone mineral density |
| BBB | Blood–brain barrier |
| UPS | Ubiquitin-proteasome system |
| ROS | Reactive oxygen species |
| vWAT | Visceral white adipose tissue |
| BAT | Brown adipose tissue |
References
- Sheikh, S.; Safia; Haque, E.; Mir, S.S. Neurodegenerative Diseases: Multifactorial Conformational Diseases and Their Therapeutic Interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef]
- Delpech, J.C.; Herron, S.; Botros, M.B.; Ikezu, T. Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci. 2019, 42, 361–372. [Google Scholar] [CrossRef]
- D’Amico, G.; Carista, A.; Manna, O.M.; Paladino, L.; Picone, D.; Sarullo, S.; Sausa, M.; Cappello, F.; Vitale, A.M.; Caruso Bavisotto, C. Brain–Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs. Biology 2024, 13, 1056. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Benea, S.-N.; Căruntu, C.; Nancoff, A.-S.; Homentcovschi, C.; Bucurica, S. Rewiring the Brain Through the Gut: Insights into Microbiota–Nervous System Interactions. Curr. Issues Mol. Biol. 2025, 47, 489. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Khlevner, J.; Park, Y.; Margolis, K.G. Brain-Gut Axis: Clinical Implications. Gastroenterol. Clin. N. Am. 2018, 47, 727–739. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 18, 101982. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bonfili, L.; Wei, T.; Eleuteri, A.M. Understanding the Gut-Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders. Nutrients 2023, 15, 4631. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Konturek, J.W.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 2004, 55 Pt 2, 137–154. [Google Scholar]
- Petrut, S.M.; Bragaru, A.M.; Munteanu, A.E.; Moldovan, A.D.; Moldovan, C.A.; Rusu, E. Gut over Mind: Exploring the Powerful Gut-Brain Axis. Nutrients 2025, 17, 842. [Google Scholar] [CrossRef]
- Holland, A.M.; Bon-Frauches, A.C.; Keszthelyi, D.; Melotte, V.; Boesmans, W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol. Life Sci. 2021, 78, 4713–4733. [Google Scholar] [CrossRef]
- Ma, L.; Wang, H.B.; Hashimoto, K. The vagus nerve: An old but new player in brain-body communication. Brain Behav. Immun. 2025, 124, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Ehrström, M.; Levin, F.; Kirchgessner, A.L.; Schmidt, P.T.; Hilsted, L.M.; Grybäck, P.; Jacobsson, H.; Hellström, P.M.; Näslund, E. Stimulatory effect of endogenous orexin A on gastric emptying and acid secretion independent of gastrin. Regul. Pept. 2005, 132, 9–16. [Google Scholar] [CrossRef]
- Tunisi, L.; Forte, N.; Fernández-Rilo, A.C.; Mavaro, I.; Capasso, R.; D’Angelo, L.; Milić, N.; Cristino, L.; Di Marzo, V.; Palomba, L. Orexin-A Prevents Lipopolysaccharide-Induced Neuroinflammation at the Level of the Intestinal Barrier. Front. Endocrinol. 2019, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, A.L. Orexins in the brain-gut axis. Endocr. Rev. 2002, 23, 1–15. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Bosch, T.C.G.; McFall-Ngai, M.J. Metaorganisms as the New Frontier. Zoology 2011, 114, 185–190. [Google Scholar] [CrossRef]
- Intili, G.; Paladino, L.; Rappa, F.; Alberti, G.; Plicato, A.; Calabrò, F.; Fucarino, A.; Cappello, F.; Bucchieri, F.; Tomasello, G.; et al. From Dysbiosis to Neurodegenerative Diseases through Different Communication Pathways: An Overview. Biology 2023, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Fucarino, A.; Burgio, S.; Paladino, L.; Bavisotto, C.; Pitruzzella, A.; Bucchieri, F.; Cappello, F. The Microbiota Is Not an Organ: Introducing the Muco-Microbiotic Layer as a Novel Morphofunctional Structure. Anatomia 2022, 1, 186–203. [Google Scholar] [CrossRef]
- Carranza-Naval, M.J.; Del Marco, A.; Hierro-Bujalance, C.; Alves-Martinez, P.; Infante-Garcia, C.; Vargas-Soria, M.; Herrera, M.; Barba-Cordoba, B.; Atienza-Navarro, I.; Lubian-Lopez, S.; et al. Liraglutide Reduces Vascular Damage, Neuronal Loss, and Cognitive Impairment in a Mixed Murine Model of Alzheimer’s Disease and Type 2 Diabetes. Front. Aging Neurosci. 2021, 16, 741923. [Google Scholar] [CrossRef] [PubMed]
- Kaiyrlykyzy, A.; Kozhakhmetov, S.; Babenko, D.; Zholdasbekova, G.; Alzhanova, D.; Olzhayev, F.; Baibulatova, A.; Kushugulova, A.R.; Askarova, S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022, 6, 15115. [Google Scholar] [CrossRef]
- Khedr, E.M.; Omeran, N.; Karam-Allah Ramadan, H.; Ahmed, G.K.; Abdelwarith, A.M. Alteration of Gut Microbiota in Alzheimer’s Disease and Their Relation to the Cognitive Impairment. J. Alzheimers Dis. 2022, 88, 1103–1114. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, S.; He, Y.; Xu, M.; Qiao, X.; Zhu, Y.; Wu, W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Markers 2022, 19, 9484217. [Google Scholar] [CrossRef]
- Wanapaisan, P.; Chuansangeam, M.; Nopnipa, S.; Mathuranyanon, R.; Nonthabenjawan, N.; Ngamsombat, C.; Thientunyakit, T.; Muangpaisan, W. Association between Gut Microbiota with Mild Cognitive Impairment and Alzheimer’s Disease in a Thai Population. Neurodegener. Dis. 2022, 22, 43–54. [Google Scholar] [CrossRef]
- Yıldırım, S.; Nalbantoğlu, Ö.U.; Bayraktar, A.; Ercan, F.B.; Gündoğdu, A.; Velioğlu, H.A.; Göl, M.F.; Soylu, A.E.; Koç, F.; Gülpınar, E.A.; et al. Stratification of the Gut Microbiota Composition Landscape across the Alzheimer’s Disease Continuum in a Turkish Cohort. mSystems 2022, 2022 22, e0000422. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer’s and Parkinson’s Diseases. Cold Spring Harb. Perspect. Biol. 2016, 8, a023630. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wan, Z.Y.; Roybo, L.; Melk, R.; Li, J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014, 128, 805–820. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198. [Google Scholar] [CrossRef]
- O’Hara, J.R.; Ho, W.; Linden, D.R.; Mawe, G.M.; Sharkey, K.A. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G998–G1007. [Google Scholar] [CrossRef]
- Goehler, L.E.; Gaykema, R.P.; Opitz, N.; Reddaway, R.; Badr, N.; Lyte, M. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005, 19, 334–344. [Google Scholar] [CrossRef]
- Li, R.; Miao, Z.; Liu, Y.; Chen, X.; Wang, H.; Su, J.; Chen, J. The Brain-Gut-Bone Axis in Neurodegenerative Diseases: Insights, Challenges, and Future Prospects. Adv. Sci. 2024, 11, e2307971. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lo, R.Y. Alzheimer’s disease and osteoporosis. Tzu Chi Med. J. 2017, 29, 138–142. [Google Scholar]
- Zemanova, N.; Omelka, R.; Mondockova, V.; Kovacova, V.; Martiniakova, M. Roles of Gut Microbiome in Bone Homeostasis and Its Relationship with Bone-Related Diseases. Biology 2022, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.A.; Rosen, C.J. Parkinson’s disease and osteoporosis: Basic and clinical implications. Expert. Rev. Endocrinol. Metab. 2020, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Hu, Y.; Guo, Y.; Liu, D. Modulation of bone remodeling by the gut microbiota: A new therapy for osteoporosis. Bone Res. 2023, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Morini, E.; Portaro, S.; Leonetti, D.; De Cola, M.C.; De Luca, R.; Bonanno, M.; Quartarone, A.; Calabrò, R.S. Bone Health Status in Individuals with Amyotrophic Lateral Sclerosis: A Cross-Sectional Study on the Role of the Trabecular Bone Score and Its Implications in Neurorehabilitation. Int. J. Environ. Res. Public Health 2023, 20, 2923. [Google Scholar] [CrossRef]
- Zhao, R. Irisin at the crossroads of inter-organ communications: Challenge and implications. Front. Endocrinol. 2022, 13, 989135. [Google Scholar] [CrossRef] [PubMed]
- Lunetta, C.; Lizio, A.; Tremolizzo, L.; Ruscica, M.; Macchi, C.; Riva, N.; Weydt, P.; Corradi, E.; Magni, P.; Sansone, V. Serum irisin is upregulated in patients affected by amyotrophic lateral sclerosis and correlates with functional and metabolic status. J. Neurol. 2018, 265, 3001–3008. [Google Scholar] [CrossRef]
- Mazzini, L.; De Marchi, F.; Niccolai, E.; Mandrioli, J.; Amedei, A. Gastrointestinal Status and Microbiota Shaping in Amyotrophic Lateral Sclerosis: A New Frontier for Targeting? In Amyotrophic Lateral Sclerosis [Internet]; Araki, T., Ed.; Exon Publications: Brisbane, AU, USA, 2021; Chapter 8. [Google Scholar]
- Chen, S.; Cai, X.; Lao, L.; Wang, Y.; Su, H.; Sun, H. Brain-Gut-Microbiota Axis in Amyotrophic Lateral Sclerosis: A Historical Overview and Future Directions. Aging Dis. 2024, 15, 74–95. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, M.; Kaminska, M.; Oluwasegun, A.K.; Mosig, A.S.; Wilmes, P. Emulating the gut-liver axis: Dissecting the microbiome’s effect on drug metabolism using multiorgan-on-chip models. Curr. Opin. Endocr. Metab. Res. 2021, 18, 94–101. [Google Scholar] [CrossRef]
- Pan, L.; Xie, L.; Yang, W.; Feng, S.; Mao, W.; Ye, L.; Cheng, H.; Wu, X.; Mao, X. The role of brain-liver-gut Axis in neurological disorders. Burns Trauma 2025, 13, tkaf011. [Google Scholar] [CrossRef]
- Aghara, H.; Patel, M.; Chadha, P.; Parwani, K.; Chaturvedi, R.; Mandal, P. Unraveling the Gut-Liver-Brain Axis: Microbiome, Inflammation, and Emerging Therapeutic Approaches. Mediat. Inflamm. 2025, 2025, 6733477. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, K.; Jiang, Y.; Huang, Y.; Zhang, Y.; Liao, Y. Metabolic Crosstalk between Liver and Brain: From Diseases to Mechanisms. Int. J. Mol. Sci. 2024, 25, 7621. [Google Scholar] [CrossRef]
- Song, D.; Li, Y.; Yang, L.L.; Luo, Y.X.; Yao, X.Q. Bridging systemic metabolic dysfunction and Alzheimer’s disease: The liver interface. Mol. Neurodegener. 2025, 20, 61. [Google Scholar] [CrossRef]
- Reitz, C. Dyslipidemia and the risk of Alzheimer’s disease. Curr. Atheroscler. Rep. 2013, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Forsyth, C.B.; Shaikh, M.; Voigt, R.M.; Engen, P.A.; Ramirez, V.; Keshavarzian, A. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front. Neurol. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Boktor, J.C.; Mahmoudian Dehkordi, S.; Kaddurah-Daouk, R.; Mazmanian, S.K. α-synuclein overexpression and the microbiome shape the gut and brain metabolome in mice. NPJ Park. Dis. 2024, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Rojo, N.; Blanco Benítez, M.; Cava, R.; Fuentes, J.M.; Canales Cortés, S.; González Polo, R.A. Convergence of Neuroinflammation, Microbiota, and Parkinson’s Disease: Therapeutic Insights and Prospects. Int. J. Mol. Sci. 2024, 25, 11629. [Google Scholar] [CrossRef]
- Koutzoumis, D.N.; Vergara, M.; Pino, J.; Buddendorff, J.; Khoshbouei, H.; Mandel, R.J.; Torres, G.E. Alterations of the gut microbiota with antibiotics protect dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp. Neurol. 2020, 325, 113159. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Yu, X.; Chen, Y.; Zhang, J.; Pang, C.; Xie, J.; Gao, L.; Du, L.; Cao, W.; et al. Fighting amyotrophic lateral sclerosis by protecting the liver? A prospective cohort study. Ann. Neurol. 2024, 97, 270–280. [Google Scholar] [CrossRef]
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.-H. Disorders of the enteric nervous system—A holistic view. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 393–410. [Google Scholar] [CrossRef]
- Kurlawala, Z.; McMillan, J.D.; Singhal, R.A.; Morehouse, J.; Burke, D.A.; Sears, S.M.; Duregon, E.; Beverly, L.J.; Siskind, L.J.; Friedland, R.P. Mutant and curli-producing E. coli enhance the disease phenotype in a hSOD1-G93A mouse model of ALS. Sci. Rep. 2023, 13, 5945. [Google Scholar] [CrossRef]
- Yang, L.; Mao, K.; Yu, H.; Chen, J. Neuroinflammatory responses and parkinson’ disease: Pathogenic mechanisms and therapeutic targets. J. Neuroimmune Pharmacol. 2020, 15, 830–837. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef]
- Vida, H.; Sahar, M.; Nikdouz, A.; Arezoo, H. Chemokines in neurodegenerative diseases. Immunol. Cell Biol. 2025, 103, 275–292. [Google Scholar] [CrossRef]
- Liu, T.; Wu, H.; Wei, J. Bridging the Gap: The Neuro-immune Axis as a Key Player in Neurodegenerative Disorders. Neurosci. Bull. 2025. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- González, H.; Elgueta, D.; Montoya, A.; Pacheco, R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 2014, 274, 1–13. [Google Scholar] [CrossRef]
- Grassivaro, F.; Menon, R.; Acquaviva, M.; Ottoboni, L.; Ruffini, F.; Bergamaschi, A. Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J. Neurosci. 2020, 40, 784–795. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.I.; Lin, L.; Makin, A.M.; Zhang, X.F.; Zhou, L.X.; Liao, X.Y.; Zhao, L.; Wang, F.; Luo, D.S. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor regulate the interaction between astrocytes and Schwann cells at the trigeminal root entry zone. Neural Regen. Res. 2023, 18, 1364–1370. [Google Scholar] [PubMed]
- Firdous, S.M.; Khan, S.A.; Maity, A. Oxidative stress-mediated neuroinflammation in Alzheimer’s disease. Naunyn Schmiedeberg Arch. Pharmacol. 2024, 397, 8189–8209. [Google Scholar] [CrossRef]
- Bhatia, V.; Sharma, S. Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer’s disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef]
- de Vrij, F.S.; Fischer, D.F.; van Leeuwen, F.W.; Hol, E.M. Protein quality control in Alzheimer’s disease by the ubiquitin proteasome system. Prog. Neurobiol. 2004, 74, 249–270. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.I.; Dawson, V.L.; Dawson, T.M. α-Synuclein pathology as a target in neurodegenerative diseases. Nat. Rev. Neurol. 2025, 21, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Wu, H. Ubiquitination-proteasome system (UPS) and autophagy two main protein degradation machineries in response to cell stress. Cells 2022, 11, 851. [Google Scholar] [CrossRef]
- Nixon, R.A.; Rubinsztein, D.C. Mechanisms of autophagy-lysosome dysfunction in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2024, 25, 926–946. [Google Scholar] [CrossRef] [PubMed]
- Xiromerisiou, G.; Marogianni, C.; Lampropoulos, I.C.; Dardiotis, E.; Speletas, M.; Ntavaroukas, P.; Androutsopoulou, A.; Kalala, F.; Grigoriadis, N.; Papoutsopoulou, S. Peripheral inflammatory markers TNF-α and CCL2 revisited: Association with Parkinson’s disease severity. Int. J. Mol. Sci. 2022, 24, 264. [Google Scholar] [CrossRef]
- Carata, E.; Muci, M.; Di Giulio, S.; Mariano, S.; Panzarini, E. Looking to the Future of the Role of Macrophages and Extracellular Vesicles in Neuroinflammation in ALS. Int. J. Mol. Sci. 2023, 24, 11251. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Carata, E.; Muci, M.; Di Giulio, S.; Di Giulio, T.; Mariano, S.; Panzarini, E. The Neuromuscular Disorder Mediated by Extracellular Vesicles in Amyotrophic Lateral Sclerosis. Curr. Issues Mol. Biol. 2024, 46, 5999–6017. [Google Scholar] [CrossRef]
- Carata, E.; Muci, M.; Mariano, S.; Panzarini, E. BV2 Microglial Cell Activation/Polarization Is Influenced by Extracellular Vesicles Released from Mutated SOD1 NSC-34 Motoneuron-like Cells. Biomedicines 2024, 12, 2069. [Google Scholar] [CrossRef]
- Jhanji, R.; Behl, T.; Sehgal, A.; Bungau, S. Mitochondrial dysfunction and traffic Jams in amyotrophic lateral sclerosis. Mitochondrion 2021, 58, 102–110. [Google Scholar] [CrossRef]
- Riku, Y.; Iwasaki, Y.; Ishigaki, S.; Akagi, A.; Hasegawa, M.; Nishioka, K.; Li, Y.; Riku, M.; Ikeuchi, T.; Fujioka, Y.; et al. Motor neuron TDP-43 proteinopathy in progressive supranuclear palsy and corticobasal degeneration. Brain 2022, 145, 2769–2784. [Google Scholar] [CrossRef]
- Rao, S.D.; Yin, H.Z.; Weiss, J.H. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J. Neurosci. 2003, 23, 2627–2633. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.J.; Xiang, Y. Bidirectional communication between brain and visceral white adipose tissue: Its potential impact on Alzheimer’s disease. EBioMedicine 2022, 84, 104263. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hulme, J.; Vo, T.K.; Van Vo, G. The Potential Crosstalk Between the Brain and Visceral Adipose Tissue in Alzheimer’s Development. Neurochem. Res. 2022, 47, 1503–1512. [Google Scholar] [CrossRef]
- Regensburger, M.; Kinfe, T.M. Role of the adipocyte immune brain axis in Parkinson’s disease: Friend or foe? Neural Regen. Res. 2023, 18, 2399–2400. [Google Scholar] [CrossRef]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J. Clin. Investig. 2020, 130, 1961–1976. [Google Scholar] [CrossRef]
- Trayhurn, P. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2022, 127, 161–164. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Kullmann, S.; Valenta, V.; Wagner, R.; Tschritter, O.; Machann, J.; Häring, H.U.; Preissl, H.; Fritsche, A.; Heni, M. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat. Commun. 2020, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Heni, M.; Veit, R.; Scheffler, K.; Machann, J.; Häring, H.U.; Fritsche, A.; Preissl, H. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care 2015, 38, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yang, G.; Bae, D.K.; Lee, S.H.; Yang, Y.H.; Kyung, J.; Kim, D.; Choi, E.K.; Choi, K.C.; Kim, S.U.; et al. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J. Neurosci. Res. 2013, 91, 660–670. [Google Scholar] [CrossRef]
- Chen, B.; Schneeberger, M. Neuro-Adipokine Crosstalk in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5932. [Google Scholar] [CrossRef]
- Liu, M.; Jiao, Q.; Du, X.; Bi, M.; Chen, X.; Jiang, H. Potential Crosstalk Between Parkinson’s Disease and Energy Metabolism. Aging Dis. 2021, 12, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.F.; Wang, Z.Y.; Li, X.F.; Song, J.; Hong, F.; Lian, H.; Wang, Q.; Feng, X.Y.; Tang, Y.Y.; Zhang, Y.; et al. Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-OHDA rat model of Parkinson’s disease. Brain Res. 2011, 1420, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Martin-Jiménez, C.A.; Gaitán-Vaca, D.M.; Echeverria, V.; González, J.; Barreto, G.E. Relationship Between Obesity, Alzheimer’s Disease, and Parkinson’s Disease: An Astrocentric View. Mol. Neurobiol. 2017, 54, 7096–7115. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, E.; Méquinion, M.; Leghay, C.; Sibran, W.; Stievenard, A.; Sarchione, A.; Bonte, M.; Vanbesien-Mailliot, C.; Viltart, O.; Saitoski, K.; et al. Overexpression of Wild-Type Human Alpha-Synuclein Causes Metabolism Abnormalities in Thy1-aSYN Transgenic Mice. Front. Mol. Neurosci. 2018, 11, 321. [Google Scholar] [CrossRef]
- Markaki, E.; Ellul, J.; Kefalopoulou, Z.; Trachani, E.; Theodoropoulou, A.; Kyriazopoulou, V. The role of ghrelin, neuropeptide Y and leptin peptides in weight gain after deep brain stimulation for Parkinson’s disease. Stereotact. Funct. Neurosurg. 2012, 90, 104–112. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef]
- Vaisman, N.; Lusaus, M.; Nefussy, B.; Niv, E.; Comaneshter, D.; Hallack, R.; Drory, V.E. Do Patients with Amyotrophic Lateral Sclerosis (ALS) Have Increased Energy Needs? J. Neurol. Sci. 2009, 279, 26–29. [Google Scholar] [CrossRef]
- Lindauer, E.; Dupuis, L.; Müller, H.-P.; Neumann, H.; Ludolph, A.C.; Kassubek, J. Adipose Tissue Distribution Predicts Survival in Amyotrophic Lateral Sclerosis. PLoS ONE 2013, 8, e67783. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Pinto, I.F.D.; Dantas, L.S.; Xavier, A.M.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; Glezer, I.; Yoshinaga, M.Y.; Miyamoto, S. Alterations in Lipid Metabolism of Spinal Cord Linked to Amyotrophic Lateral Sclerosis. Sci. Rep. 2019, 9, 11642. [Google Scholar] [CrossRef]
- Duranti, E.; Villa, C. From Brain to Muscle: The Role of Muscle Tissue in Neurodegenerative Disorders. Biology 2024, 13, 719. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Furrer, R.; Handschin, C. Molecular aspects of the exercise response and training adaptation in skeletal muscle. Free Radic. Biol. Med. 2024, 223, 53–68. [Google Scholar] [CrossRef]
- Liu, T.; Wu, H.; Wei, J. Beyond the Brain: Exploring the multi-organ axes in Parkinson’s disease pathogenesis. J. Adv. Res. 2025, 16. [Google Scholar] [CrossRef]
- Zare, N.; Bishop, D.J.; Levinger, I.; Febbraio, M.A.; Broatch, J.R. Exercise intensity matters: A review on evaluating the effects of aerobic exercise intensity on muscle-derived neuroprotective myokines. Alzheimers Dement. 2025, 11, e70056. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Serban, M.; Munteanu, O.; Covache-Busuioc, R.A.; Enyedi, M.; Ciurea, A.V.; Tataru, C.P. From Synaptic Plasticity to Neurodegeneration: BDNF as a Transformative Target in Medicine. Int. J. Mol. Sci. 2025, 26, 4271. [Google Scholar] [CrossRef] [PubMed]
- Minuti, A.; Raffaele, I.; Scuruchi, M.; Lui, M.; Muscarà, C.; Calabrò, M. Role and Functions of Irisin: A Perspective on Recent Developments and Neurodegenerative Diseases. Antioxidants 2025, 14, 554. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.T.; Sun, Y.; Xu, Z.; Wang, Y.; Yao, S.Y.; Yao, W.B.; Gao, X.D. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer’s disease. Redox Biol. 2019, 22, 101133. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, T.F.; Yu, L.; Jen, C.J.; Chuang, J.I.; Wu, F.S.; Wu, C.W.; Kuo, Y.M. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav. Immun. 2011, 25, 135–146. [Google Scholar] [CrossRef]
- Jones-Tabah, J.; He, K.; Karpilovsky, N.; Senkevich, K.; Deyab, G.; Pietrantonio, I.; Goiran, T.; Cousineau, Y.; Nikanorova, D.; Goldsmith, T.; et al. The Parkinson’s disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons. Mol. Neurodegener. 2024, 19, 88. [Google Scholar] [CrossRef]
- Duranti, E.; Villa, C. Muscle Involvement in Amyotrophic Lateral Sclerosis: Understanding the Pathogenesis and Advancing Therapeutics. Biomolecules 2023, 13, 1582. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Z.; Zhang, X.A.; Ning, K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise-A Narrative Review. Biomolecules 2024, 14, 1205. [Google Scholar] [CrossRef]

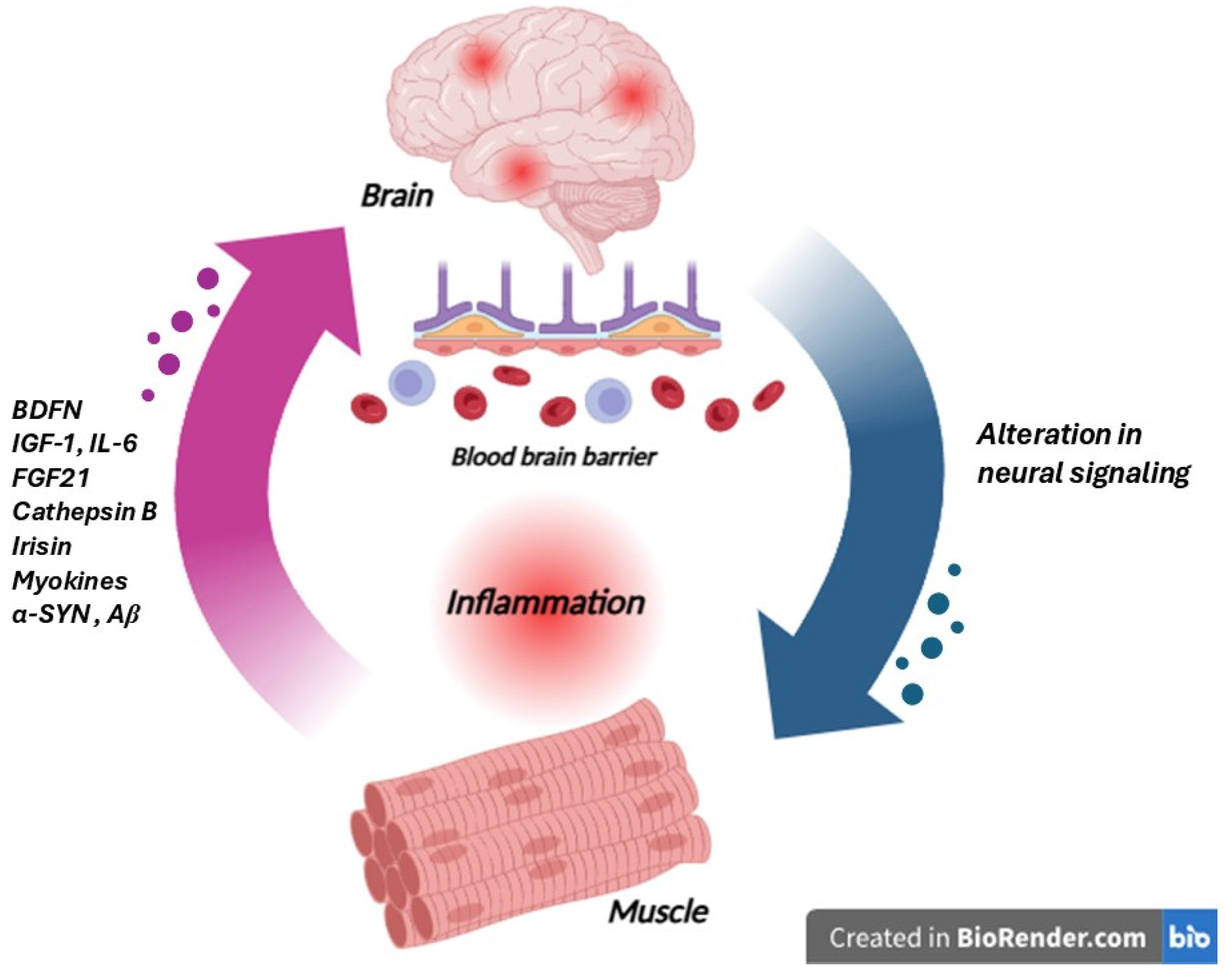
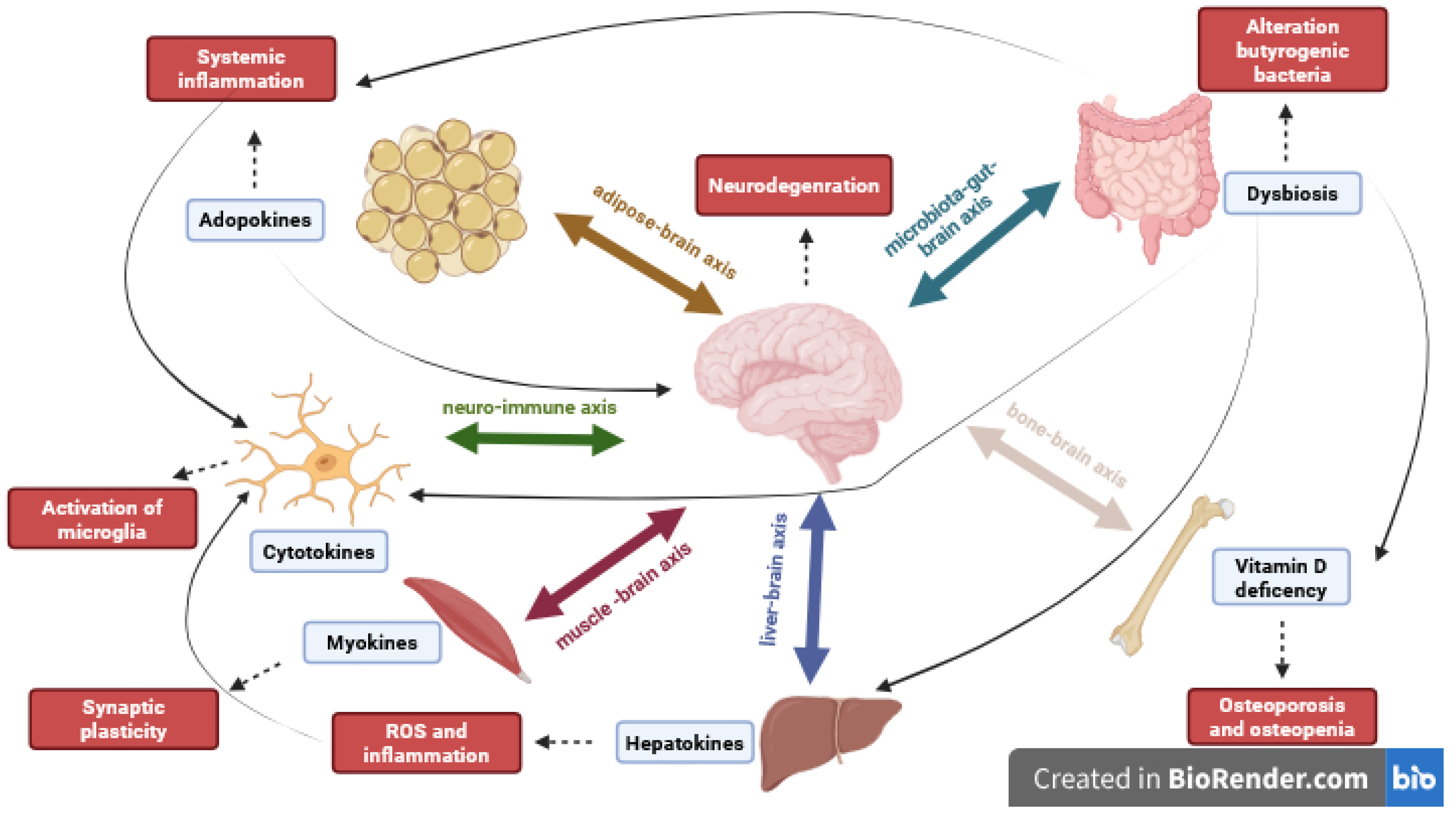
| Microbiota–Gut–Brain Axis | Brain–Gut–Bone Axis | Liver Brain Axis | Neuro-Immune Axis | Brain–Adipose Tissue Axis | Muscle–Brain Axis | |
|---|---|---|---|---|---|---|
| Gut–brain axis | LPS, amyloid, and other toxins mediate systemic inflammation [21,23,31,37] | LPS, amyloid, and other toxins mediate systemic inflammation [21,23,31,37] | ||||
| Microbiota–gut–brain axis | Dysbiosis causes misfolding or aggregation of protein [29,30,31] | Osteoporosis and osteopenia [38,39,40] | SCFAs, bile acids, and lipopolysaccharides impact neuroinflammation [48,49] | Dysbiosis activates microglia, promoting an inflammatory [29,30,31,32,33,34] | Dysbiosis reduces butyrate production and other adipokine secretion [88,89,90] | |
| Brain–gut–bone axis | Vitamin D deficiency reduces butyrate production and other adipokine secretion [38,39,40] | |||||
| Liver brain axis | SCFAs, bile acids, and lipopolysaccharides impact neuroinflammation [48,49] | Hepatic fatty acid metabolism and detoxification capacity may intensify systemic inflammation [50,51,52,53,54,55] | ||||
| Neuro-immune axis | Hepatokines promote oxidative stress and inflammation [48,49] | TNF-α, IL-1β, and IL-6, and chemokines like CCL2/MCP-1 and CXCL10, promoting neuroinflammation and recruiting peripheral immune cells [59,60,61,62,63] | Myokines modulate synaptic plasticity and cognitive function [103,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carata, E.; Destino, M.; Tenuzzo, B.A.; Panzarini, E. Inter-Organ Crosstalk in Neurodegenerative Disease. Life 2025, 15, 1499. https://doi.org/10.3390/life15101499
Carata E, Destino M, Tenuzzo BA, Panzarini E. Inter-Organ Crosstalk in Neurodegenerative Disease. Life. 2025; 15(10):1499. https://doi.org/10.3390/life15101499
Chicago/Turabian StyleCarata, Elisabetta, Moris Destino, Bernardetta Anna Tenuzzo, and Elisa Panzarini. 2025. "Inter-Organ Crosstalk in Neurodegenerative Disease" Life 15, no. 10: 1499. https://doi.org/10.3390/life15101499
APA StyleCarata, E., Destino, M., Tenuzzo, B. A., & Panzarini, E. (2025). Inter-Organ Crosstalk in Neurodegenerative Disease. Life, 15(10), 1499. https://doi.org/10.3390/life15101499







