Abstract
Aging is accompanied by complex cellular and molecular changes that compromise CNS function. Among these, glial cells (astrocytes, microglia, and oligodendrocytes) play a central role in maintaining neural homeostasis, modulating synaptic activity, and supporting metabolic demands. Emerging evidence indicates that aging disrupts glial cell physiology through processes including mitochondrial dysfunction, impaired proteostasis, chronic low-grade inflammation, and altered intercellular signaling. These alterations contribute to synaptic decline, myelin degeneration, and persistent, low-grade inflammation of the CNS. This review synthesizes current knowledge on the bidirectional relationship between aging and glial cell dysfunction, highlighting how age-related systemic and CNS-specific factors exacerbate glial impairments and, in turn, accelerate neural deterioration. Finally, this study discusses some potential therapeutic strategies aimed at preserving or restoring glial function to promote CNS resilience in aging populations. Understanding this interplay offers critical opportunities for mitigating cognitive decline and improving quality of life in older adults.
Keywords:
aging; glial cells; astrocytes; microglia; oligodendrocytes; neuroinflammation; inflammaging; senescence 1. Introduction
Aging is a multifactorial biological process characterized by a progressive decline in physiological integrity, reduced cellular resilience, and increased susceptibility to disease [1,2,3]. Within the CNS, aging not only affects neurons but also induces profound alterations in the function and homeostatic regulation of glial cells, including astrocytes, microglia, and oligodendrocytes [4]. These non-neuronal cell populations are now recognized as dynamic regulators of synaptic plasticity, neurovascular coupling, metabolic homeostasis, and neuroimmune surveillance [5,6]. The cumulative molecular and cellular alterations that accompany aging perturb these glial functions, thereby accelerating cognitive decline, increasing vulnerability to neurodegenerative disorders, and undermining brain health [7,8].
At the molecular level, aging induces extensive reprogramming of glial cell gene expression, driven by the cumulative impact of epigenetic drift (defined as stochastic alterations in the epigenome that accumulate over time) encompassing changes in DNA methylation patterns, histone modifications, and chromatin remodeling [9,10,11]. In aging glial cells, chromatin accessibility is often reduced at loci associated with neuroprotective and metabolic genes, while pro-inflammatory and stress-response genes might become more accessible, driving a maladaptive transcriptional shift [12,13]. Mitochondrial dysfunction, a well-established hallmark of aging, plays a central role in this process [14]. In glial cells, compromised electron transport chain efficiency reduces ATP production, impairing the high-energy-demanding functions of those cells [15]. This inefficiency also leads to excessive production of ROS, which induce oxidative damage on lipids, proteins, and nucleic acids [15].
Astrocytes, which play essential roles in maintaining CNS homeostasis, supporting neuronal function, and regulating the BBB [16], undergo a shift toward a reactive phenotype in response to aforementioned insults. Their reactive state is characterized by hypertrophy, increased expression of intermediate filament proteins like GFAP and vimentin, and the secretion of several pro-inflammatory mediators, such as IL-1β, TNF-α, and CCL2 [17,18]. Sustained activation of the NF-κB signaling pathway locks astrocytes into an inflammatory state, further impairing their neuroprotective roles [19]. One functional consequence is the reduction in glutamate clearance due to decreased expression of excitatory amino acid transporters EAAT1 and EAAT2, creating conditions favorable for excitotoxic neuronal damage [20].
Microglia, the resident immune sentinels of the CNS [21], undergo a parallel but distinct aging trajectory, a process often known as microglial priming [22]. With aging process, PRR pathways, particularly TLR4 signaling, become dysregulated, making microglia hyperresponsive to secondary insults including infections or trauma [23]. Primed microglia exhibit amplified and sustained inflammatory responses, but paradoxically show reduced phagocytic efficiency, compromising the clearance of myelin debris, apoptotic cells, and aggregated proteins such as amyloid-β [24,25,26]. Dysfunction in purinergic signaling, especially through P2X7 and P2Y12 receptors, further disrupts microglial chemotaxis and injury sensing [27,28]. Autophagic flux declines with age, leading to lysosomal dysfunction, which traps damaged organelles and undigested materials inside the cell [29]. This failure of clearance mechanisms sustains the presence of DAMPs in the CNS microenvironment, perpetuating a self-reinforcing cycle of inflammation and neuronal stress [30].
OPCs, the main source of new myelinating oligodendrocytes in the adult CNS [31], also exhibit significant age-related decline [32]. Aging OPCs show impaired proliferation and differentiation capacity, largely driven by epigenetic repression of the genes implied in myelin synthesis, such as MBP and PLP1 [33]. Furthermore, OPCs become less responsive to mitogenic growth factors, including PDGF-A and FGF2, which usually promote OPC expansion and maturation [34,35]. The loss of regenerative capacity impairs remyelination efficiency and contributes to the progressive degradation of white matter integrity, a crucial substrate for cognitive processing speed and executive function [36].
These issues are exacerbated by systemic aging factors, including chronic low-grade inflammation (known as inflammaging), characterized by increased levels of circulating pro-inflammatory cytokines, as well as alterations in metabolic hormones such as insulin and IGF-1 [37,38]. These systemic molecules facilitate glial senescence via activation of the cell cycle inhibitors p16 and p21, inducing an irreversible growth arrest that further impairs the CNS reparative and adaptive capacity [39]. Over time, these converging cellular and molecular deficits create a CNS environment more susceptible to neurodegenerative processes [40]. Furthermore, these glial modifications do not occur in isolation but rather within a complex and bidirectional interplay with aging neurons, vascular elements, and the immune system [41]. The concept of the Neuro-Glia-Vascular unit underscores the key role of glial cells in the regulation of CNS homeostasis, whereby age-related dysfunctions in a single cell type can propagate disturbances throughout the neural network [42]. Such perturbations can impair synaptic integrity and neurovascular coupling, ultimately contributing to and neurodegenerative processes [43].
Finally, this article offers an extensive analysis of glial cells and their involvement in the aging process, beginning with the cellular and molecular modifications that glial cells undergo during the aging process. Subsequent sections address the impact of these alterations on brain health, highlighting their contribution to cognitive deterioration. Finally, this article explores preventive and therapeutic strategies aimed at restoring glial function and enhancing neural resilience during brain aging.
2. Aging-Associated Changes in Glial Cells
Glial cells undergo progressive structural and functional modifications during aging that can profoundly influence CNS homeostasis. These changes are not merely secondary to neuronal decline but represent intrinsic alterations in glial biology, encompassing shifts in morphology, signaling dynamics, metabolic activity, and immune responsiveness. The following sections summarize the principal morphological and physiological alterations identified in glial populations during the aging process.
2.1. Morphological Alterations
During the aging process, glial cells orchestrate numerous morphological alterations driven by the interplay between cell-autonomous senescence-associated molecular mechanisms and the cumulative impact of chronic, low-grade neuroinflammatory stimuli sustained throughout the lifespan within the CNS microenvironment [44].
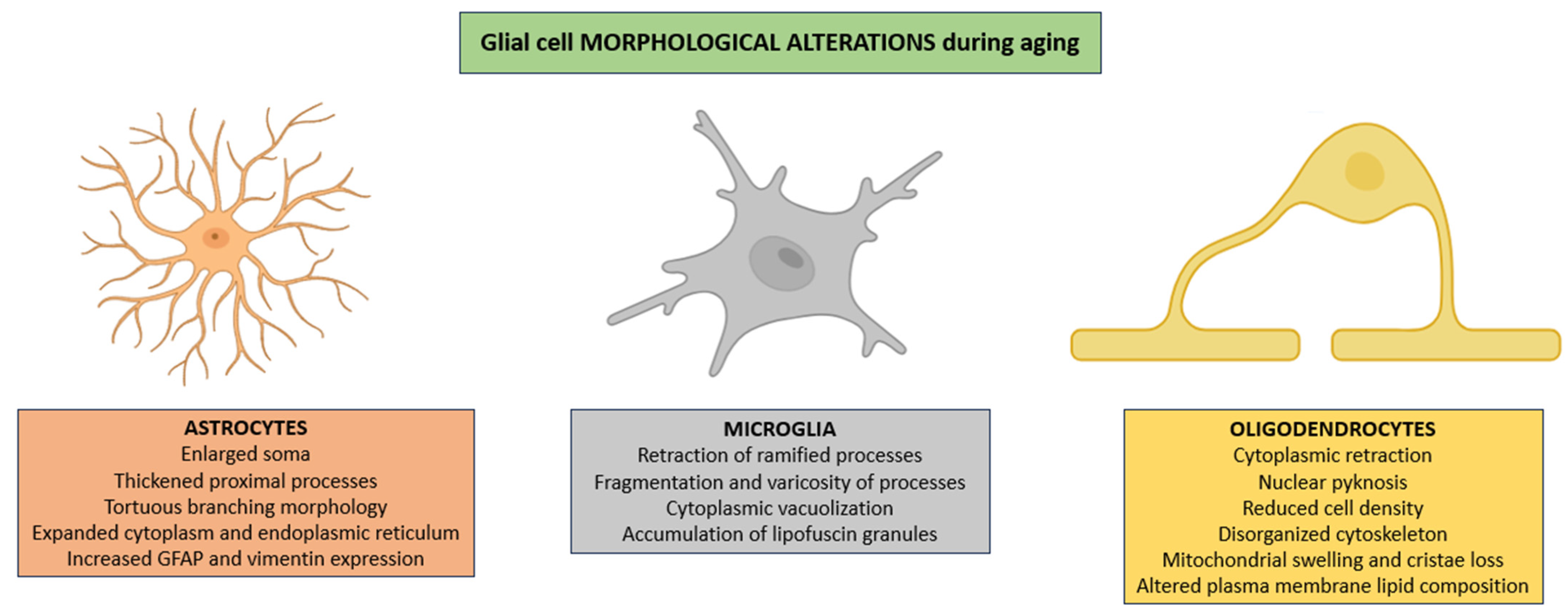
Astrocytes frequently exhibit pronounced hypertrophy characterized not only by enlargement of the soma and thickening of proximal processes but also by a notable increase in cytoplasmic volume, an expansion of the endoplasmic reticulum, and a redistribution of intermediate filaments that results in tortuous branching morphologies [45]. All of these changes are usually accompanied by enhanced expression of cytoskeletal proteins such as GFAP and vimentin, reflecting a shift toward a chronically reactive state that alters astrocytic interactions with both neuronal and vascular elements (Figure 1) [46].
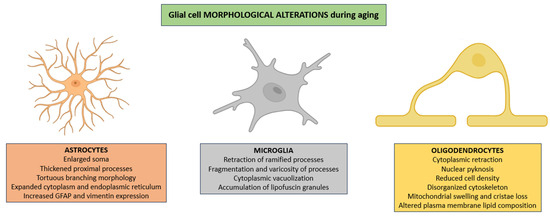
Figure 1.
Morphological alterations in glial populations across the aging process. In aged CNS, astrocytes show pronounced hypertrophy, irregular branching, cytoplasmic enlargement, and increased GFAP and vimentin expression, reflecting a chronic reactive state. Microglia display progressive dystrophic features, including process retraction and fragmentation, cytoplasmic vacuolization, and lipofuscin deposition. Oligodendrocytes show cytoplasmic shrinkage, nuclear pyknosis, reduced cell density, cytoskeletal disruption, mitochondrial structural defects, and altered plasma membrane lipid composition, collectively impairing white matter integrity and glial–neuronal signaling. Abbreviations: GFAP (glial fibrillary acidic protein).
In parallel, microglia exhibit progressive dystrophic changes characterized by retraction of ramified processes, which are essential for environmental surveillance; fragmentation and varicosity of the remaining processes, consistent with cytoskeletal disintegration; cytoplasmic vacuolization, suggestive of impaired autophagic flux; and intracellular accumulation of autofluorescent lipofuscin granules, representing morphological correlates of diminished lysosomal degradative capacity (Figure 1) [47,48,49].
Oligodendrocytes frequently exhibit cytoplasmic retraction, nuclear pyknosis reflecting chromatin condensation, and a marked reduction in cellular density within affected white matter regions [50]. These alterations promote the age-related thinning of the myelin sheath, patchy demyelination, and the disruption of axonal conduction fidelity, ultimately compromising the integrity of white matter tracts [51,52]. These modifications are frequently accompanied by profound disturbances in cytoskeletal architecture, including disorganization of intermediate filaments, disruption of actin filaments, and destabilization of microtubules, which together undermine intracellular transport and process stability [53,54]. Additionally, mitochondrial structural derangements (such as swelling, loss of cristae definition, and outer membrane rupture) disrupt bioenergetic homeostasis and exacerbate oxidative stress [55]. Finally, alterations in plasma membrane lipid composition, particularly reductions in polyunsaturated fatty acids and cholesterol distribution, further impair membrane fluidity, receptor clustering, and ion channel function, thus compounding deficits in glial–neuronal communication and metabolic support (Figure 1) [56].
2.2. Physiopathological Alterations
In the aging CNS, glial cells exhibit progressive dysfunction arising from interrelated pathological processes, like bioenergetic impairment due to mitochondrial dysfunction and oxidative stress, disrupted neuroglial communication, defective proteostasis and autophagy, diminished regenerative potential, and cellular senescence, collectively fostering neuroinflammation and heightened neural vulnerability.
2.2.1. Mitochondrial Dysfunction and Oxidative Stress
Mitochondrial dysfunction constitutes a critical driver of glial cell impairment within the aging CNS, where it is linked to the progressive accumulation of oxidative stress [57]. With aging, mitochondrial quality control mechanisms (PINK1/Parkin-mediated mitophagy) demonstrate reduced efficiency, enabling the persistence and accumulation of bioenergetically compromised mitochondria [58]. This is aggravated by an imbalance between ROS production and the antioxidant defense mechanisms of the CNS, as evidenced by the age-related downregulation of mitochondrial SOD2 activity [59].
In astrocytes, aging compromises mitochondrial electron transport chain efficiency, predominantly at complexes I (NADH:ubiquinone oxidoreductase) and IV (cytochrome c oxidase), leading to reduced ATP synthesis and increased electron leakage that increases reactive oxygen species production [60]. This leakage encourages excessive generation of ROS, such as superoxide anions (O2−) and hydrogen peroxide (H2O2) [61,62]. Elevated ROS levels drive the oxidation of membrane lipids [63], structural and enzymatic proteins [64], and nucleic acids [65], thereby disrupting astrocytic metabolic homeostasis and activating redox-sensitive transcription factors (like NF-κB), which contribute to neuroinflammatory signaling (Table 1) [66].
In microglia, mitochondrial respiratory dysfunction impairs oxidative phosphorylation (OXPHOS) capacity, inducing a compensatory metabolic shift toward aerobic glycolysis [67,68]. This metabolic shift, while sustaining basal energy levels, drives a prolonged pro-inflammatory phenotype (commonly identified as “M1-like” activation) and compromises microglial phagocytic clearance of cellular debris, protein aggregates, and apoptotic cells [69]. The persistence of this dysfunctional metabolic state exacerbates neuroinflammation and reduces the capacity for resolution of CNS injury (Table 1) [70].
Finally, oligodendrocytes, which depend extensively on mitochondrial ATP production for the synthesis and maintenance of myelin sheaths [71], are particularly susceptible to oxidative damage (targeting mtDNA) and to lipid peroxidation within myelin membranes, a process intensified by the high content of polyunsaturated fatty acids (Table 1) [55,72].
2.2.2. Impairment of Proteostasis and Autophagy
The impairment of proteostasis and autophagy in glial cells is a pivotal factor in the aging CNS, contributing significantly to neurodegeneration and cognitive decline [73,74]. Proteostasis, encompassing the intricate regulation of protein synthesis, folding, trafficking, and degradation, becomes increasingly compromised in glial cells, due to cumulative oxidative damage, mitochondrial dysfunction, and neuroinflammatory signaling characteristic of aging [75,76]. In contrast, autophagy, a conserved catabolic process responsible for the degradation and recycling of damaged organelles, protein aggregates, and other cytoplasmic components, is impaired in glial cells during the aging process [75,77].
In astroglia, the disruption of proteostasis manifests as impaired function of molecular chaperones including heat shock proteins (e.g., Hsp70), resulting in the accumulation of misfolded proteins and cytotoxic aggregates [78]. This proteotoxic stress interferes with regulation of glutamate and ion homeostasis, exacerbating neuronal excitotoxicity [79]. Furthermore, aging astrocytes exhibit a marked reduction in autophagic flux, evidenced by decreased expression of crucial autophagy-related genes such as Beclin-1 and LC3 [80], alongside lysosomal dysfunction characterized by impaired acidification and enzymatic activity, which impairs the removal of dysfunctional mitochondria and protein aggregates (Table 1) [81].
In microglia, proteasomal insufficiency combined with defective autophagy leads to intracellular accumulation of dysfunctional mitochondria and protein inclusions, which trigger sustained inflammasome activation (e.g., NLRP3) and release of some pro-inflammatory cytokines (e.g., IL-1β and TNF-α) [82,83,84]. This neuroinflammatory milieu further disrupts proteostasis by increasing oxidative damage and impairing chaperone-mediated autophagy (CMA), a selective pathway crucial for degradation of cytosolic proteins containing KFERQ-like motifs (Table 1) [85].
Oligodendrocytes experience pronounced impairments in proteostasis and autophagy as a consequence of aging [86,87]. Impaired UPS and autophagic clearance of myelin debris and damaged organelles compromises oligodendrocyte viability and myelin integrity, leading to demyelination and reduced nerve conduction [88,89]. Moreover, inhibition of the proteasome during aging induces DNA fragmentation and promotes oligodendrocyte degeneration (Table 1) [90].
2.2.3. Cellular Senescence
Cellular senescence in glial cells represents a key contributor to age-associated neurodegenerative decline, orchestrated via molecular networks that integrate DNA damage responses, epigenetic remodeling, and inflammatory signaling [91,92].
In astrocytes, chronic exposure to oxidative stress and mitochondrial dysfunction underlies the accumulation of both nuclear and mtDNA lesions, specifically double-strand breaks (DSBs) and oxidative base modifications such as 8-oxoguanine, which activate the DNA damage response (DDR) through ATM and ATR kinases [93,94,95]. These pathological processes phosphorylate downstream CHK1 and CHK2 effectors, stabilizing p53, which transcriptionally upregulates p21, inducing a stable cell cycle arrest at the G1 to S phase transition [96]. Parallel induction of the INK4a/ARF locus enhances p16 expression, which suppresses CDK4/6 kinase activity, thereby inhibiting phosphorylation of the retinoblastoma protein (Rb), resulting in the growth-suppressive conformation and enforcing a stable senescent cell cycle arrest [97]. These checkpoints converge to establish a robust proliferative arrest in astrocytes, thereby preventing uncontrolled cellular proliferation while simultaneously compromising their neurotrophic support functions [98]. Senescent astrocytes also develop a senescence-associated secretory phenotype (SASP), orchestrated at the transcriptional level by NF-κB [99,100]. This SASP includes the production and secretion of some pro-inflammatory cytokines (e.g., IL-6 and IL-8), chemokines (e.g., CXCL10), growth factors (e.g., TGF-β), and matrix metalloproteinases (e.g., MMP-3 and MMP-9) [101,102]. These factors modulate the microenvironment, disrupting BBB integrity, inducing astrogliosis, and impairing synaptic plasticity [103]. Epigenetic remodeling in astrocytes, defined by the accumulation of methylation marks like H3K4me3 and the loss of histone acetylation marks such as H3K27ac, reinforces SASP gene expression and stabilizes the senescent phenotype [104,105]. Finally, DNA methylation changes (e.g., hypermethylation of neuroprotective gene promoters alongside global hypomethylation) alter chromatin accessibility, further strengthening dysfunctional phenotypes (Table 1) [106].
In microglia, senescence is driven by telomere attrition, oxidative stress, and persistent low-grade inflammatory stimuli [107]. Telomere attrition activates DDR signaling (through ATM and ATR kinases), upregulating p16, p21 and p53, thereby strengthening cell cycle arrest [108]. Moreover, senescent microglia exhibit a pronounced pro-inflammatory SASP, with increased secretion of several cytokines (e.g., IL-1β, TNF-α, and IFN-γ), chemokines (e.g., CCL5 and CXCL10), and increased NO production [109,110]. Epigenetic alterations, such as increased expression of some HDACs (e.g., HDAC1) sustain transcriptional activation of the SASP microglia [111]. Finally, increased DNA demethylation patterns have been observed in the aged CNS, thereby contributing to a heightened activation of pro-inflammatory pathways (Table 1) [112].
OPCs and mature oligodendrocytes display unique senescence-associated alterations during aging [113]. In response to aging-induced demyelination, the p53/p21 and p16/Rb pathways are robustly activated [114]. On the other hand, senescent OPCs adopt an SASP phenotype that includes secretion of matrix metalloproteinases (e.g., MMP-3) and several pro-inflammatory cytokines (e.g., IL-6 and TNF-α), disrupting extracellular matrix integrity and impeding neuron-glia signaling, key process for remyelination [32,115]. Finally, during the aging process, a reduction in HDAC activity (e.g., HDAC1, HDAC2, HDAC3, and HDAC8) is observed, and a strong decrease in global DNA hypomethylation in aged oligodendrocytes correlates with reduced DNMT1 expression (Table 1) [116,117].
2.2.4. Alterations in Intercellular Signaling and Communication
Aging drives several profound changes in intercellular signaling and communication within the CNS, impacting glial cell function [118]. These alterations involve cellular, molecular, and network-scale modifications that perturb the precisely regulated crosstalk between neurons and glial cells, as well as interglial communication among glial subpopulations [119,120]. Such dysregulation of glial signaling pathways undermines the capacity of glial cells to maintain homeostasis, modulate synaptic activity, and orchestrate immune responses [121]. Consequently, these signaling perturbations contribute to the progressive decline in neural plasticity and cognitive function observed during aging [122,123].
Astrocytes, which normally support neuronal activity via Ca2+-dependent gliotransmitter release and regulation of extracellular neurotransmitter levels [124], exhibit dysregulated intracellular Ca2+ dynamics and reduced expression of connexins (e.g., Cx43), leading to impaired gap junctional communication [125]. This disruption impairs their ability to adequately propagate Ca2+ waves and release gliotransmitters (e.g., ATP and D-serine), essential modulators of synaptic transmission (Table 1) [126,127].
Conversely, the composition and molecular cargo of extracellular vesicles (EVs) released by aged microglia exhibit substantial alterations that markedly modulate intercellular signaling within the CNS [128]. These EVs, which include exosomes and microvesicles, serve as key mediators of cell-to-cell communication by transferring bioactive molecules such as proteins, lipids, and miRNAs to recipient neurons and glial cells [129]. In the context of aging, microglia-derived EVs show a modified proteomic and lipidomic profile characterized by increased contents of several pro-inflammatory cytokines (e.g., IL-1β and TNF-α), DAMPs, lipid peroxidation products, and miRNAs that regulate inflammatory pathways and immune responses (e.g., miR-192; Table 1) [130,131,132,133,134].
Finally, in the aging CNS, the accumulation of myelin debris resulting from impaired clearance mechanisms represents a key pathological feature that profoundly impacts oligodendrocyte function and remyelination capacity [135]. This impairment in the clearance of myelin debris promotes the recruitment and activation of peripheral and CNS resident inflammatory cells, accompanied by sustained microglial activation (Table 1) [136,137].

Table 1.
Table showing age-related alterations in glial cell function. Abbreviations: ATP (adenosine triphosphate), ROS (reactive oxygen species), CNS (central nervous system), mtDNA (mitochondrial DNA), DDR (DNA damage response), ATM (ataxia-telangiectasia mutated), ATR (ATM and Rad3-related), p16 (cyclin-dependent kinase inhibitor 2A), CDK4/6 (cyclin-dependent kinases 4 and 6), Rb (retinoblastoma protein), SASP (senescence-associated secretory phenotype), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), H3K4me3 (histone 3 lysine 4 trimethylation), H3K27ac (histone H3 lysine 27 acetylation), IL-1β (interleukin 1 beta), TNF-α (tumor necrosis factor alpha), IFN-γ (interferon gamma), CCL5 (C-C motif chemokine ligand 5), CXCL10 (C-X-C motif chemokine ligand 10), NO (nitric oxide), DNA (deoxyribonucleic acid), p53 (tumor protein p53), p21 (cyclin-dependent kinase inhibitor 1A), MMP-3 (matrix metalloproteinase 3), IL-6 (interleukin 6), ECM (extracellular matrix), HDAC (histone deacetylase), DNMT1 (DNA methyltransferase 1), Ca2+ (calcium ion), EV (extracellular vesicle), DAMP (damage-associated molecular pattern), and miRNA (microRNA).
Table 1.
Table showing age-related alterations in glial cell function. Abbreviations: ATP (adenosine triphosphate), ROS (reactive oxygen species), CNS (central nervous system), mtDNA (mitochondrial DNA), DDR (DNA damage response), ATM (ataxia-telangiectasia mutated), ATR (ATM and Rad3-related), p16 (cyclin-dependent kinase inhibitor 2A), CDK4/6 (cyclin-dependent kinases 4 and 6), Rb (retinoblastoma protein), SASP (senescence-associated secretory phenotype), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), H3K4me3 (histone 3 lysine 4 trimethylation), H3K27ac (histone H3 lysine 27 acetylation), IL-1β (interleukin 1 beta), TNF-α (tumor necrosis factor alpha), IFN-γ (interferon gamma), CCL5 (C-C motif chemokine ligand 5), CXCL10 (C-X-C motif chemokine ligand 10), NO (nitric oxide), DNA (deoxyribonucleic acid), p53 (tumor protein p53), p21 (cyclin-dependent kinase inhibitor 1A), MMP-3 (matrix metalloproteinase 3), IL-6 (interleukin 6), ECM (extracellular matrix), HDAC (histone deacetylase), DNMT1 (DNA methyltransferase 1), Ca2+ (calcium ion), EV (extracellular vesicle), DAMP (damage-associated molecular pattern), and miRNA (microRNA).
| Physiological Alteration | Cell Type | Consequences | References |
|---|---|---|---|
| Mitochondrial dysfunction and oxidative stress | Astroglia | Reduced efficiency of the mitochondrial electron transport chain Reduced ATP synthesis Enhanced electron leakage leading to elevated ROS production Oxidative damage to lipids, proteins, and nucleic acids Disruption of astrocytic metabolic homeostasis Activation of redox-sensitive transcription factors Amplification of neuroinflammatory signaling | [60,61,62,63,64,65,66] |
| Microglia | Impaired oxidative phosphorylation capacity Compensatory metabolic shift toward aerobic glycolysis Sustained pro-inflammatory phenotype Reduced phagocytic clearance of cellular debris Exacerbation of neuroinflammation Impaired resolution of CNS injury | [67,68,69,70] | |
| Oligodendroglia | High susceptibility to oxidative damage and lipid peroxidation mtDNA disruption Compromised synthesis and maintenance of myelin sheaths | [55,71,72] | |
| Impairment of proteostasis and autophagy | Astroglia | Impaired function of chaperones Accumulation of misfolded proteins and cytotoxic aggregates Disruption of glutamate and ion homeostasis Reduced autophagic flux Lysosomal dysfunction Impaired clearance of protein aggregates | [78,79,80,81] |
| Microglia | Proteasomal insufficiency combined with defective autophagy Accumulation of dysfunctional mitochondria and protein inclusions Sustained inflammasome activation Release of pro-inflammatory cytokines Neuroinflammatory milieu exacerbates oxidative damage Impairment of chaperone-mediated autophagy | [82,83,84,85] | |
| Oligodendroglia | Pronounced impairments in proteostasis and autophagy Impaired proteolytic and autophagic degradation pathways Reduced oligodendrocyte viability and compromised myelin integrity Proteasome inhibition promotes oligodendrocyte degeneration | [86,87,88,89,90] | |
| Cellular senescence | Astroglia | Accumulation of nuclear and mtDNA lesions Activation of DDR via ATM/ATR → stable G1/S arrest Activation of the p16-CDK4/6-Rb pathway reinforces G1/S arrest Development of SASP phenotype driven by NF-κB Epigenetic remodeling: accumulation of H3K4me3, loss of H3K27ac, promoter hypermethylation of neuroprotective genes, and global hypomethylation → stabilization of the SASP phenotype | [93,94,95,96,97,98,99,100,101,102,103,104,105,106] |
| Microglia | SASP induced by telomere attrition and oxidative stress, among others DDR activation via ATM/ATR → proliferative arrest Pro-inflammatory SASP with secretion of IL-1β, TNF-α, IFN-γ, CCL5, and CXCL10, along with increased NO production Epigenetic alterations → increased SASP phenotype Age-associated DNA demethylation patterns amplify pro-inflammatory gene activation | [107,108,109,110,111,112] | |
| Oligodendroglia | Activation of p53/p21 and p16/Rb pathways in response to demyelination SASP phenotype with secretion of MMP-3, IL-6, and TNF-α → ECM disruption and impaired neuron-glia signaling Epigenetic changes: reduced HDAC activity and global DNA hypomethylation associated with decreased DNMT1 expression → compromised oligodendrocyte function | [113,114,115,116,117] | |
| Alterations in intercellular signaling and communication | Astroglia | Dysregulated intracellular Ca2+ dynamics Reduced connexins expression Impaired gap junctional communication Deficient propagation of Ca2+ waves Reduced gliotransmitter release (e.g., ATP, D-serine) Disruption of synaptic transmission modulation | [124,125,126,127] |
| Microglia | Altered composition and molecular cargo of EVs Modified proteomic and lipidomic profiles Increased content of pro-inflammatory cytokines and DAMPs Dysregulated transfer of regulatory miRNAs Exacerbation of inflammatory signaling and immune responses | [128,129,130,131,132,133,134] | |
| Oligodendroglia | Accumulation of uncleared myelin debris Impaired oligodendrocyte function Reduced remyelination capacity Activation of peripheral and CNS-resident inflammatory cells Sustained microglial activation | [135,136,137] |
3. Consequences for CNS Health
These age-related glial alterations compromise the efficiency of neuron–glia metabolic coupling and reduce the fidelity of intercellular signaling pathways, ultimately undermining neuronal plasticity [138]. Maladaptive glial remodeling during aging drives neurodegeneration by chronic neuroinflammation, promoting synaptic dysfunction, facilitating myelin degradation, and increasing susceptibility to neurodegenerative diseases.
3.1. Synaptic Dysfunction
Synaptic dysfunction and cognitive decline are central features of brain aging, closely linked to both neuronal and glial alterations [139,140]. Glial cells, usually considered supportive, play key roles in maintaining CNS homeostasis and regulating synaptic plasticity, neurotransmitter clearance, and circuit-level signaling, fundamental processes for learning, memory, and executive functions [8,141].
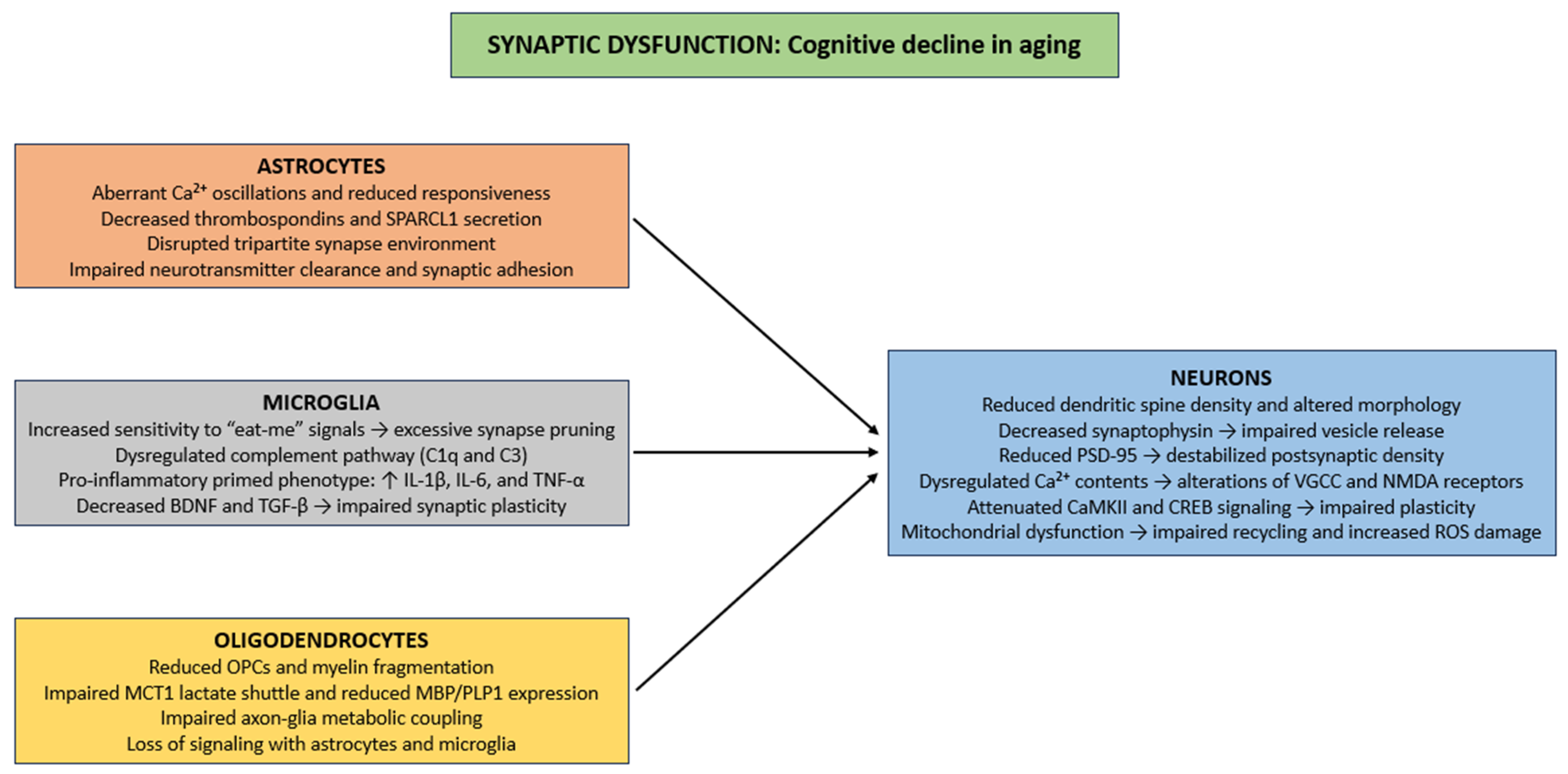
Aging induces several structural and functional synaptic deficits, including reduced dendritic spine density, alterations in spine morphology, and diminished synaptic contact area, principally in the hippocampus and prefrontal cortex (Figure 2) [142,143]. Presynaptically, reductions in synaptophysin impair synaptic vesicle release and decrease excitatory neurotransmission, thus compromising activity-dependent plasticity such as long-term potentiation (LTP) [144,145]. Postsynaptically, age-related changes in PSD-95, a major scaffolding protein, destabilize postsynaptic densities and disrupt clustering of NMDA and AMPA receptors, causing reduced synaptic efficacy, impaired Ca2+ signaling, and reduced activation of downstream effectors that support learning and memory [146]. Ca2+ homeostasis is exacerbated in aging neurons [147], as dysregulation of VGCC and NMDA receptors [148,149], thereby producing inappropriate or insufficient Ca2+ transients. These disturbances impair activation of Ca2+-dependent pathways, such as calmodulin, CaMKII, and CREB, which are necessary for spine stabilization, synaptic consolidation, and activity-dependent gene expression [150,151,152]. Finally, in aging neurons, mitochondrial impairment dysregulates vesicle recycling, neurotransmitter reuptake, and ionic balance, while oxidative damage from ROS to synaptic proteins and membrane lipids further impairs synaptic stability and contributes to neuronal degeneration (Figure 2) [153,154].
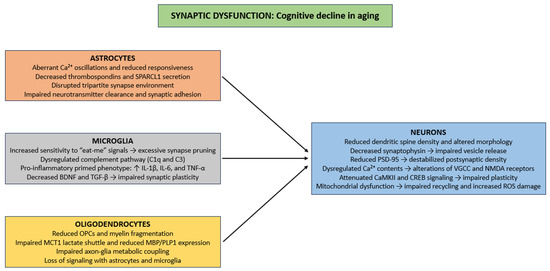
Figure 2.
Cellular mechanisms of synaptic dysfunction in CNS aging. Abbreviations: Ca2+ (calcium ion), SPARCL1 (secreted protein acidic and rich in cysteine-like 1), C1q (complement component 1q), C3 (complement component 3), IL-1β (interleukin 1 beta), IL-6 (Interleukin 6), TNF-α (tumor necrosis factor alpha), BDNF (brain-derived neurotrophic factor), TGF-β (Transforming growth factor beta), OPC (oligodendrocyte precursor cell), MCT1 (monocarboxylate transporter 1), MBP (myelin basic protein), PLP1 (proteolipid protein 1), PSD-95 (postsynaptic density protein 95), VGCC (voltage-gated calcium channel), NMDA (N-methyl-D-aspartate), CaMKII (calcium/calmodulin-dependent protein kinase II), CREB (cAMP response element-binding protein), and ROS (reactive oxygen species).
Within the framework of glial cell biology, aged astrocytes show marked dysregulation of intracellular Ca2+ signaling, characterized by aberrant Ca2+ oscillations and reduced responsiveness to neurotransmitter-induced activation [126,155]. These perturbations in Ca2+ homeostasis impair the exocytotic release of synaptogenic molecules. Specifically, the secretion of extracellular matrix and synapse-promoting proteins like thrombospondins, and SPARCL1 are markedly downregulated in senescent astrocytes [78,156,157,158]. Thrombospondins promote the initial formation of excitatory synapses through interactions with neuronal α2δ-1 receptors [159], whereas SPARCL1 mediates the bridging of presynaptic neurexins and postsynaptic neuroligins to stabilize nascent synaptic contacts [160]. The cumulative effect of these astrocytic dysfunctions is a disrupted tripartite synapse environment, characterized by diminished astrocyte–neuron crosstalk, weakened synaptic adhesion, and impaired neurotransmitter clearance (Figure 2) [161,162,163].
Microglia maintain synaptic architectural homeostasis through dynamic modulation of synapse number and functional properties [164]. This regulation is organized through two mechanisms: (i) synaptic activity-mediated phagocytic elimination of superfluous or functionally compromised synaptic elements, commonly initiated through recognition of “eat-me” signals, including externalized phosphatidylserine and deposited complement components [165]; (ii) paracrine signaling via the secretion of cytokines, chemokines, and neurotrophic factors, which modulate synaptic plasticity, spine morphology, and neurotransmitter receptor trafficking [166]. During the aging process, microglial regulation of synaptic homeostasis becomes impaired, giving rise to maladaptive changes in neural circuit function [167]. Augmented sensitivity to “eat-me” signals increase the efficiency of activity-dependent phagocytic clearance, an excess further exacerbated by age-related alterations in complement pathway regulation (such as dysregulated C1q and C3 expression) which can result in excessive or aberrant synapse elimination in vulnerable neuronal populations [168]. Simultaneously, paracrine signaling is impaired, as aged microglia adopt a pro-inflammatory “primed” phenotype, characterized by increased secretion of pro-inflammatory cytokines (e.g., IL-1β, TNF-α, and IL-6), alongside diminished release of neurotrophic factors (e.g., BDNF) and other synaptogenesis-inducing molecules (e.g., TGF-β) [169,170]. This shift in the secretory profile fosters chronic low-grade neuroinflammation, disrupts dendritic spine integrity, and perturbs neurotransmitter receptor trafficking (particularly the composition of AMPA and NMDA receptor subunits), thus compromising synaptic plasticity (Figure 2) [171,172].
Finally, aging is linked to decline in OPCs, myelin fragmentation, and impaired metabolic coupling between axons and glial cells, specifically through reduced lactate shuttling through monocarboxylate transporter MCT1 and decreased expression of some myelin proteins (e.g., MBP and PLP1) [113]. These impairments disrupt the timing of synaptic input integration and compromise the fidelity of high-frequency action potential conduction, thereby indirectly contributing to synaptic dysfunction [173]. Furthermore, oligodendrocytes engage in bidirectional signaling with astrocytes and microglia to maintain extracellular K+ and glutamate homeostasis, regulate neurotrophic factor availability, and modulate local synaptic microenvironments [174,175]. Age-related oligodendrocyte loss disrupts these interactions, exacerbating excitotoxic stress, impairing Ca2+ buffering, and amplifying the detrimental impact of aging on synaptic structure (Figure 2) [55,176].
3.2. Myelin Degradation and White Matter Loss
Aging induces pronounced structural and functional reorganization of the CNS, with white matter being especially affected, mainly mediated by the regulatory and supportive activities of glial cells [41]. Oligodendrocytes, the myelinating glia of the CNS, are central to axonal insulation and rapid action potential conduction, yet they become increasingly vulnerable to age-related stressors [55]. Oligodendrocytes, the principal myelinating cells of the CNS, play a central role in white matter deterioration observed during aging [177]. With advancing age, oligodendrocytes exhibit both functional decline and structural vulnerability, leading to myelin degradation and impaired axonal support [178].
At the molecular level, mitochondrial dysfunction constitutes a critical driver of age-related myelin deterioration. The accumulation of mtDNA mutations, impairment of electron transport chain activity, and consequent reductions in ATP production compromise the energy-dependent processes essential for myelin maintenance, including the synthesis, trafficking, and integration of key myelin proteins like MBP, PLP1, and MAG [72,179]. Oxidative stress represents a key contributor, characterized by the accumulation of ROS and RNS in aging oligodendrocytes, resulting from mitochondrial dysfunction and a decline in intrinsic antioxidant capacity [72,180]. These reactive species induce lipid peroxidation of myelin sheaths, protein carbonylation, and DNA damage, triggering oligodendrocyte apoptosis through intrinsic mitochondrial pathways, involving Bcl-2 family regulation, Bax/Bak-dependent release of cytochrome c, and subsequent activation of caspases 3 and 9 [181,182].
On the other hand, the endoplasmic reticulum stress response is chronically activated in aged oligodendrocytes, culminating in the accumulation of misfolded myelin proteins [183,184]. Finally, aging compromises OPC differentiation [185], with disruptions in key signaling pathways (e.g., Wnt/β-catenin) impeding the maturation of OPCs into myelinating oligodendrocytes [186]. At the same time, reduced PI3K-Akt-mTOR signaling in aged oligodendrocytes compromises cell survival and suppresses lipid biosynthesis, a process essential for myelin membrane expansion [187,188].
3.3. Inflammaging: The Role of Chronic Low-Grade Inflammation in the Aging Process
Inflammaging, a chronic low-grade inflammatory state that progressively develops with advancing age, arises from a multifactorial interplay involving immune dysregulation, the accumulation of senescent cells, mitochondrial dysfunction, and defective resolution of inflammation [189]. Within the CNS, its impact is particularly pronounced, manifesting through the sustained activation of glial populations, most prominently microglia and astrocytes [190].
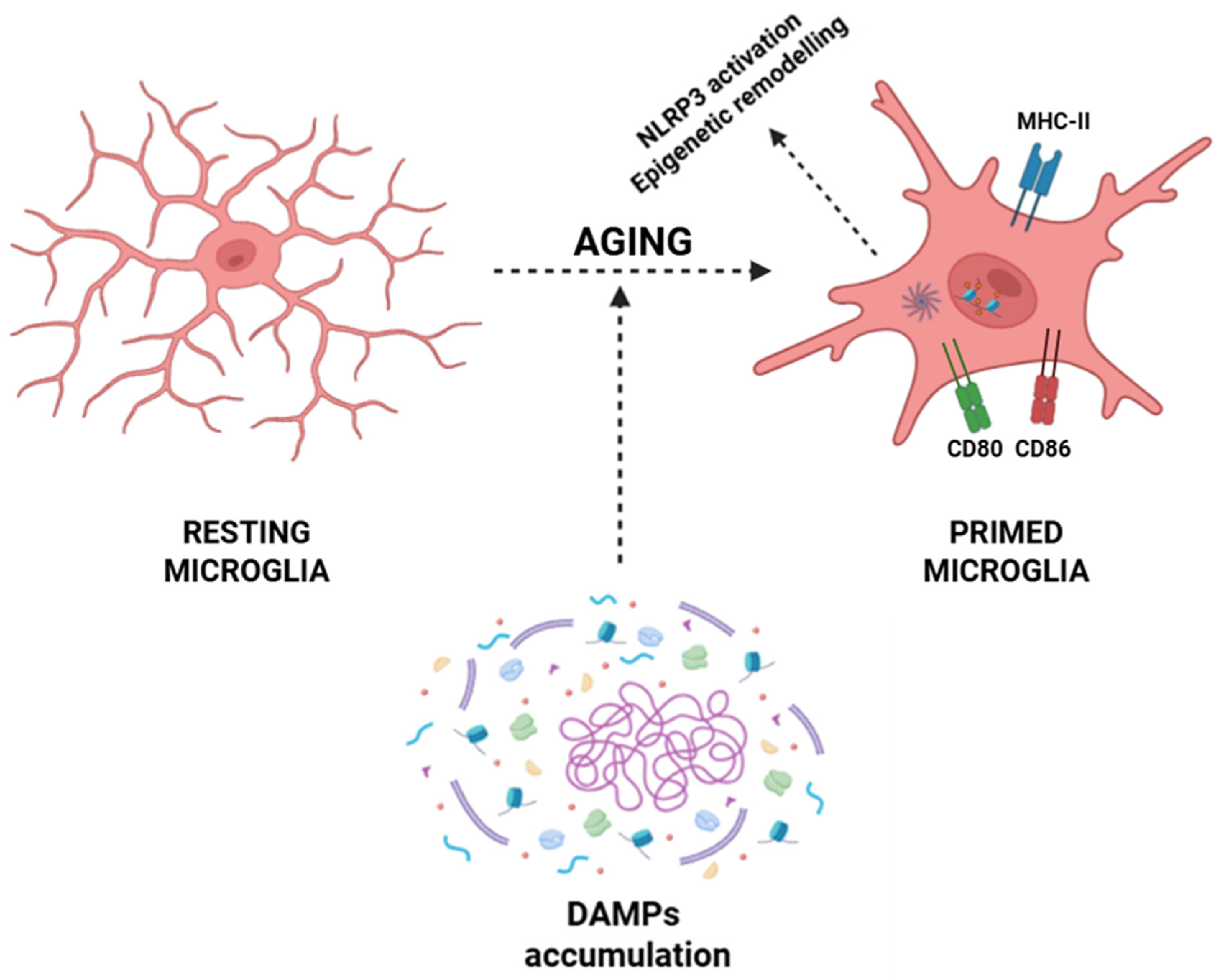
Within the CNS, aging microglia undergo a phenotypic conversion toward a primed state characterized by exaggerated pro-inflammatory cytokine production (e.g., IL-1β, IL-6, and TNF-α), heightened expression of MHC-II and costimulatory molecules (CD80 and CD86), and sustained activation of pro-inflammatory transcriptional programs via NF-κB, AP-1, and IRF pathways [22,23,49]. This transition is driven by the progressive accumulation of DAMPs arising during inflammaging, including mitochondrial dysfunction with excessive production of ROS and release of oxidized mitochondrial DNA into the cytosol, which activates sensors such as cGAS and NLRP3 inflammasomes [191,192,193]. Aggregates of β-amyloid, hyperphosphorylated τ, and other misfolded proteins further activate the NLRP3 inflammasome, inducing its oligomerization, recruitment of the adaptor protein ASC, and caspase-1-mediated proteolytic processing of pro-IL-1β and pro-IL-18 into their functional cytokines [194,195,196,197,198,199]. Simultaneously, chronic exposure to low-grade inflammatory cues induces epigenetic remodeling that stabilizes microglia in a primed pro-inflammatory phenotype, thus sustaining a self-amplifying neuroinflammatory loop that contributes to age-associated CNS dysfunction and neurodegeneration (Figure 3) [111,112].
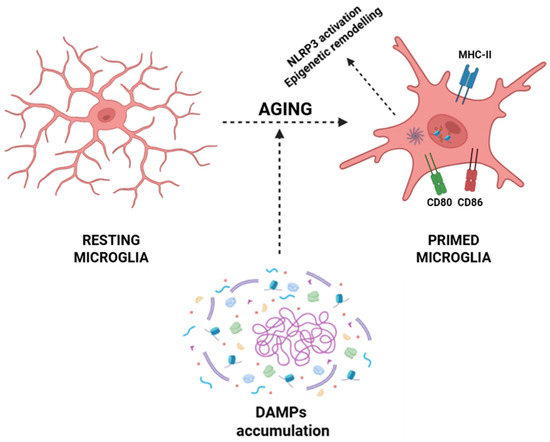
Figure 3.
Aging-associated priming of microglia. Resting microglia exhibit a ramified morphology under homeostatic conditions. During aging, the accumulation of DAMPs, together with processes such as NLRP3 inflammasome activation and epigenetic remodeling, promotes the transition of microglia into a primed state. Primed microglia display altered morphology and upregulation of immune-related molecules, like MHC-II, CD80, and CD86, contributing to a heightened inflammatory responsivity. Abbreviations: DAMP (damage-associated molecular pattern), NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3), MHC-II (major histocompatibility complex class II), CD80 (cluster of differentiation 80), and CD86 (cluster of differentiation 86).
In aging, astrocytes acquire a reactive astrogliosis phenotype, characterized by upregulation of GFAP, activation of the JAK/STAT3 pathway, secretion of chemokines such as CCL2 and CXCL10, and release of complement components, including C3, which can opsonize synapses and govern their microglia-mediated elimination [17,18,46,78,200]. The aforementioned changes are further amplified by the dissemination of many SASP factors from senescent endothelial cells, OPCs, and peripheral immune cells infiltrating through a BBB rendered increasingly permeable by chronic cytokine exposure and oxidative stress [201,202]. SASP mediators are transcriptionally driven by DNA damage response signaling through ATM/ATR kinases, cGAS-STING-dependent NF-κB and STAT3 activation, and mTORC1-mediated translation, creating a paracrine loop that induces secondary senescence in glia and neurons [93,94,95,203,204].
Dysregulated synthesis of specialized pro-resolving lipid mediators (SPMs) such as resolvins, protectins, and maresins in aged glia impairs the termination of inflammatory responses, locking neural tissues into a state of chronic neuroinflammation that disrupts synaptic homeostasis, impairs neurogenesis in the hippocampal niche, accelerates myelin degradation by oligodendrocyte loss, and propagates several neurodegenerative cascades characteristic of Alzheimer’s, Parkinson’s, and other age-associated CNS diseases [205,206].
Finally, the inflammaging process is maintained through integrated feedback loops (DAMP accumulation, glial senescence, SASP secretion, mitochondrial ROS production, and inflammasome activation) that not only promote neuronal susceptibility and cognitive decline but also integrate systemic and brain-specific aging mechanisms into a unified pathological process, rendering glial cells both targets and amplifiers of the molecular circuitry that underlies age-related chronic inflammation [207].
4. Age-Related Disorders Driven by Glial Cell Dysfunction
Age-related neurodegenerative disorders are increasingly recognized as arising not only from neuronal pathology but also from the cumulative dysfunction of glial cells, which are critical for maintaining CNS homeostasis [208]. In Alzheimer’s disease, microglial cells adopt a persistently activated phenotype, characterized by increased expression of MHC-II molecules and pro-inflammatory cytokines, which exacerbate oxidative stress and neurotoxicity [209]. Dysfunctional microglia show reduced phagocytic activity and impaired lysosomal processing, which compromises the clearance of Aβ plaques and facilitates extracellular plaque deposition [210]. Simultaneously, astrocytes exhibit impaired glutamate uptake due to downregulation of excitatory amino acid transporters (EAAT1/2), metabolic insufficiency through decreased lactate shuttle support, and Ca2+ signaling dysregulation, collectively leading to synaptic dysfunction and dendritic spine loss [211]. Dysregulated astrocyte–microglia crosstalk further amplifies inflammatory signaling via the NF-κB and NLRP3 inflammasome pathways, accelerating neurodegeneration and cognitive decline [212].
In Parkinson’s disease, astrocytic and microglial dysregulation similarly contributes to dopaminergic neurons susceptibility in the substantia nigra [213]. Microglia adopt a reactive, pro-inflammatory phenotype in response to α-synuclein aggregates, releasing ROS and NO [214], while astrocytes fail to adequately buffer glutamate and detoxify ROS [215]. Furthermore, glial production of neurotrophic factors, like GDNF and BDNF, is reduced, decreasing neuronal survival signaling [216]. Some experimental models indicate that microglial-mediated inflammasome activation (via NLRP3) and impaired mitophagy synergistically accelerate dopaminergic cell death [217,218,219,220].
Age-related demyelinating pathologies, such as multiple sclerosis and age-associated white matter degeneration, are associated with oligodendrocyte dysfunction [221,222]. Senescent oligodendrocytes exhibit reduced myelin production and repair capacity, leading to axonal conduction deficits and increased risk of secondary degeneration [223]. Astrocytes contribute to the development of multiple sclerosis through the secretion of inhibitory extracellular matrix proteins, including chondroitin sulfate proteoglycans, which restrict remyelination, while microglial senescence reduces the clearance of myelin debris, further compromising regeneration [224].
Growing evidence also implicates glial dysfunction in vascular cognitive impairment and age-related cognitive decline, in which astrocytes and microglia fail to maintain neurovascular coupling, contributing to cerebral hypoperfusion, BBB breakdown, and synaptic loss [225,226]. In aging-associated affective disorders and later-life depression, glial senescence is increasingly recognized as a fundamental contributor [227]. Postmortem studies revealed reduced astrocyte density, altered astrocytic morphology, and microglial hyperactivation in regions such as the prefrontal cortex and hippocampus [228,229,230].
Finally, emerging evidence indicates a role for glial dysfunction in chronic pain syndromes and neuroimmune disorders [231]. Age-related microglial priming enhances sensitivity to peripheral injury, strengthening central sensitization pathways, while astrocytic dysregulation of cytokine and neurotransmitter release contributes to chronic pain states [232,233].
5. Promoting Glial Health: Therapeutic Options for Aging Brains
The integrity and functionality of glial cells are pivotal for maintaining neural homeostasis, synaptic plasticity, and metabolic maintenance of CNS function. During the aging process, glial cells demonstrate phenotypic and functional alterations, including reactive astrogliosis, microglial priming, and oligodendrocyte loss, which mutually contribute to neuroinflammation, impaired synaptic function, and cognitive decline. Therapeutic strategies aimed at preserving or restoring glial homeostasis are therefore critical for mitigating age-associated neurodegeneration and promoting healthy brain aging.
5.1. Lifestyle and Nutritional Interventions
Dietary interventions represent a potent modulatory axis for glial cell function, particularly in the context of aging. Caloric restriction (CR) and intermittent fasting (IF) have been extensively demonstrated to induce hormetic stress responses that confer neuroprotective benefits [234,235]. Mechanistically, these interventions accentuate autophagic flux, strengthen mitochondrial quality control, and mitigate oxidative stress in glial cells [236,237]. Beyond their systemic metabolic effects, CR and IF can modulate glial inflammatory states by suppressing pro-inflammatory cytokine production and promoting an anti-inflammatory or homeostatic glial phenotype [238,239]. Furthermore, nutrient composition critically influences glial health, as long-chain ω-3 polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), incorporate into neuronal and glial membranes, enhancing membrane fluidity and receptor function [240]. These PUFAs have been demonstrated to reduce microglial activation and promote oligodendrocyte maturation and myelination [241]. On the other hand, dietary polyphenols (such as resveratrol, EGCG, and curcumin) show potent anti-inflammatory activities [242]. These compounds modulate ROS production, inhibit NLRP3 inflammasome activation, and modulate astrocytic and microglial senescence, thus mitigating age-associated neuroinflammation and supporting synaptic integrity [243,244,245].
On the other hand, diets enriched in bioactive compounds, such as polyphenols (e.g., resveratrol, epigallocatechin-3-gallate, and curcumin) and PUFAs exert significant effects on epigenetic regulation in glial cells [245,246]. However, the mechanisms underlying epigenetic regulation in glial cells remain understood and are currently the subject of ongoing research.
Regular physical exercise constitutes a potent, non-pharmacological strategy for preserving CNS homeostasis through modulation of glial function [247]. Aerobic exercise improves neurovascular coupling, boosts cerebral blood flow, and strengthens endothelial function, thus facilitating efficient delivery of O2 and nutrients to neurons and glial cells [248,249,250]. Exercise promotes the expression of several neurotrophic factors, most notably BDNF, which exerts pleiotropic effects on glial cells [251]. Enhanced BDNF signaling has been associated with a shift toward a neuroprotective microglial phenotype, reduced pro-inflammatory cytokine production, and strengthened astrocytic support of synaptic function [252,253]. Exercise also modulates astrocytic glycogen metabolism, increasing glycogen storage and mobilization, which supports sustained neuronal activity during periods of high energetic demand [254]. Moreover, physical activity stimulates oligodendrogenesis and myelin remodeling, which are essential for maintaining conduction velocity and network efficiency, mainly in aging brains [255,256,257]. Various animal studies have shown that voluntary wheel running and treadmill exercise strengthen myelin sheath thickness, promote the maturation of OPCs, and improve cognitive resilience under age-associated stress [258,259]. Ultimately, exercise-mediated promotion of antioxidant defenses in glial cells ameliorates oxidative damage and suppresses chronic neuroinflammatory signaling, thereby supporting the maintenance of CNS health [260].
5.2. Pharmacological Interventions
CNS aging is marked by sustained neuroinflammatory activity, disrupted metabolic regulation, and reduced neurotrophic support, all of which contribute to glial dysfunction and cognitive decline [261]. Pharmacological approaches are being explored to counteract these processes by targeting inflammation, trophic signaling, and metabolic homeostasis.
5.2.1. Anti-Inflammatory Agents
Ibuprofen, a widely used NSAID, has been shown to counteract age-related inflammatory processes in the CNS by modulating glial cell activation [262]. Research in animal models indicates that chronic ibuprofen administration reduces reactive astrogliosis and microglial activation [263,264,265,266]. The reduction in glial reactivity correlates with decreased gliosis and improved cognitive outcomes, mainly in executive function [267,268]. Furthermore, mechanistic studies also reveal that ibuprofen influences the expression of NMDA receptor subunits and splice variants, with the most prominent effects evident in age-affected regions of the brain [267].
MCC950, a selective inhibitor of the NLRP3 inflammasome, has been shown to attenuate age-related neuroinflammatory responses through the modulation of glial cell activity [269]. Experimental data show that MCC950 inhibits microglial and astrocytic activation, thereby preventing microglial polarization toward the pro-inflammatory M1 phenotype [270]. This modulation exerts neuroprotective effects, evidenced by attenuated neuronal injury, demyelination, and oligodendrocyte loss [269]. In aged mice, MCC950 treatment has been linked to enhanced myelin integrity and increased densities of OPCs, supporting the notion that targeting NLRP3-mediated glial activation can relieve age-related and pathological alterations in the CNS [271].
5.2.2. Neurotrophic Support
Exogenous delivery or endogenous enhancement of neurotrophic factors, including BDNF, GDNF, and IGF-1, has been identified as a promising therapeutic approach to mitigate age-associated impairments in glial cell functionality and to preserve neural homeostasis [272,273,274]. Mechanistically, BDNF predominantly engages the TrkB receptor, triggering downstream signaling cascades (like PI3K/Akt, MAPK/ERK, and PLCγ pathways) that converge to regulate glial cell survival, metabolic activity, and homeostatic function [275]. Exogenous administration of BDNF has been shown to influence aging glial cells by promoting the proliferation and maturation of oligodendrocytes, enhancing white matter repair and myelin integrity [276,277].
Similarly, IGF-1 interacts with the IGF-1R to enhance mitochondrial integrity, regulate lipid metabolism, and maintain proteostasis, processes that are markedly impaired in senescent astrocytes and oligodendrocytes [278,279]. Exogenous administration of IGF-1 exerts pronounced effects on aging glial cells, primarily by modulating neuroinflammatory responses and augmenting glial cell functional capacity [280,281]. Other studies show that IGF-1 acts as an anti-inflammatory agent by inhibiting astrocytic responses to inflammatory stimuli and by decreasing the pro-inflammatory M1 phenotype [282,283]. Moreover, IGF-1 supports oligodendrocyte survival and differentiation, helping to maintain myelin integrity in the aging CNS [284].
Finally, GDNF, via its interaction with GFRα1/RET receptor complexes, further promotes glial resilience by modulating redox homeostasis and providing trophic support to dopaminergic neural circuits [285]. Exogenous administration of GDNF in aged rodents constitutes a promising therapeutic strategy to ameliorate glial age-related dysfunction; however, further studies are required to validate this approach [286,287].
From another standpoint, small-molecule TrkB agonists and pharmacological modulators of IGF-1 signaling are under investigation as targeted regulators of glial survival, synaptic plasticity, and neurovascular interactions [288,289]. These interventions have the potential not only to attenuate age-related glial dysfunction and neuroinflammatory processes but also to promote healthy brain aging by preserving white matter integrity, cognitive resilience, and overall neural plasticity [288,289].
5.2.3. Metabolic Modulators
Age-related mitochondrial dysfunction in glial cells is a crucial contributor to the disruption of cellular homeostasis during aging [14], and accordingly, pharmacological strategies targeting mitochondrial preservation have demonstrated promising efficacy in both preclinical models and early-phase clinical studies [290]. NAD+ precursors, including nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), strengthen intracellular NAD+ levels, thereby enhancing mitochondrial sirtuin activity, optimizing oxidative phosphorylation efficiency, and facilitating DNA repair processes [291,292,293]. In the same way, AMPK activators, including metformin, promote mitochondrial biogenesis, enhance fatty acid oxidation, and regulate cellular energy-sensing pathways, thus collectively restoring metabolic homeostasis in aged glial cells [294,295,296]. Finally, mitochondria-targeted agents, including MitoQ and SS-31, might be considered as potential therapeutic strategies to counteract the aging process in glial cells, given their protective effects against several neurodegenerative pathologies [297,298].
5.3. Emerging Biotechnological Approaches
Glial dysfunction is increasingly recognized as an essential driver of CNS aging, contributing to metabolic imbalance, impaired synaptic support, among others. To counteract these processes, several emerging biotechnological strategies are under investigation.
Adoptive transfer of glial progenitor cells seeks to replenish astrocytic and oligodendroglial populations, thus restoring trophic support and remyelination in aged circuits. In parallel, gene therapy and epigenetic modulation offer tools to enhance neuroprotective pathways or suppress pro-inflammatory programs within glial cells. Together with these approaches, nanotechnology-based delivery systems enable controlled delivery of therapeutic agents to glial populations, improving efficacy while minimizing side effects.
Together, these strategies represent a convergent effort to preserve CNS homeostasis and delay neurodegenerative progression by directly targeting the glial compartment.
5.3.1. Adoptive Transfer of Glial Progenitor Cells
The implantation of glial progenitor cells, as well as astrocytes and oligodendrocytes derived from induced pluripotent stem cells (iPSCs), represents a highly promising therapeutic strategy for counteracting age-associated glial depletion [299,300]. These interventions aim not only to replenish depleted glial populations but also to restore essential metabolic, trophic, and homeostatic support to neurons, while regulating local neuroimmune responses in the aging CNS [301,302].
Mechanistically, engrafted astrocytes have been demonstrated to secrete an array of neurotrophic factors (e.g., BDNF, GDNF, and CNTF) that promote neuronal survival, dendritic arborization, and synaptic plasticity [303,304]. Moreover, engrafted astrocytes facilitate neuronal metabolic support by promoting lactate transport via monocarboxylate transporters (MCT1/4), regulating extracellular K+ and glutamate levels, and reinforcing antioxidant defenses [305,306]. Oligodendrocytes derived from iPSCs can facilitate remyelination through the expression of MBP and PLP, restoring saltatory conduction and improving axonal integrity in demyelinated or aging neural circuits [300,307].
In addition to providing trophic and metabolic support, transplanted glia modulates neuroinflammation, as engrafted astrocytes and oligodendrocytes communicate with microglia through cytokine and chemokine signaling (such as IL-10, TGF-β, and CX3CL1), thereby attenuating microglial hyperactivation and the secretion of pro-inflammatory mediators [304,308].
Preclinical studies further demonstrate that these transplanted glial populations are capable of integrating into existing neural circuits, forming functional gap junctions, and contributing to synaptic modulation as well as neurotransmitter recycling [309,310,311,312,313].
5.3.2. Gene Therapy and Epigenetic Modulation
Targeted gene therapy aimed at modulating glial cell function represents a cutting-edge strategy for mitigating age-related neurodegenerative processes and preserving CNS homeostasis [314]. By enhancing the expression of several neuroprotective genes or selectively silencing pro-inflammatory and senescence-associated pathways within glial cells, gene therapy can immediately influence neuronal survival, synaptic function, and metabolic support [315]. For instance, viral vector-driven overexpression of neurotrophic factors like BDNF, GDNF, and IGF-1 in glial populations has been shown to improve synaptic plasticity, reduce oxidative stress, and enhance neuronal resilience in preclinical models of aging and neurodegeneration [316,317,318]. On the other hand, RNAi, CRISPR-Cas-mediated gene editing, or antisense oligonucleotides can be employed to downregulate the expression of pro-inflammatory cytokines, NLRP3 inflammasome components, and SASP mediators, thereby mitigating the chronic neuroinflammation that typifies the aged CNS [319,320,321].
Complementing gene therapy, epigenetic modulation provides a parallel and synergistic approach to restoring glial functionality [322]. Age-related transcriptional dysregulation in glial populations frequently results from changes in chromatin accessibility, alterations in histone post-translational modifications, and changes of DNA methylation patterns [323]. Histone deacetylase (HDAC) inhibitors, like compounds targeting HDACs (e.g., MS-275 and SAHA) can reverse transcriptional repression of neuroprotective genes, encouraging antioxidant defenses, anti-inflammatory signaling, and metabolic homeostasis [324].
5.3.3. Nanotechnology and Drug Delivery Systems
Innovative nanocarrier platforms and BBB-penetrant delivery systems are emerging as transformative strategies for the modulation of glial cell activity within the aged CNS [325,326]. These methods harness the specific physicochemical properties of nanoparticles (such as size, surface charge, and functionalization with targeting ligands) to achieve cell-specific uptake by glial cells, thus enabling the localized delivery of neuroprotective compounds, anti-inflammatory agents, or gene-editing tools [327]. Surface modifications with antibodies (e.g., anti-TfR and anti-IR), other peptides (e.g., angiopep-2 and TAT), and/or small molecules allow nanoparticles to traverse the BBB via receptor-mediated transcytosis, circumventing the restrictive tight junctions that typically impede CNS drug delivery [328,329,330,331].
Mechanistically, nanocarriers can be engineered to release their cargo in a spatiotemporally controlled manner, responding to intracellular cues such as acidic pH within endosomes/lysosomes, elevated ROS, or specific enzymatic activity (e.g., matrix metalloproteinases or cathepsins), thereby strengthening intracellular bioavailability in target glial populations [332,333]. Following cellular uptake, these carriers release various small-molecule neuroprotective drugs that activate several antioxidant pathways (e.g., Nrf2, SOD2, and HO-1), which collectively counteract oxidative stress and mitochondrial dysfunction [334,335,336].
Moreover, advanced formulations such as liposomes, polymeric nanoparticles, dendrimers, and exosome-mimetic vesicles are being optimized for long circulation, minimal immunogenicity, and high BBB permeability [337,338,339,340]. Targeted delivery to glial cells also mitigates off-target effects and reduces systemic toxicity, which is critical when administering potent anti-inflammatory or gene-modifying agents in aged individuals [327].
6. Conclusions
The aging process exerts a profound and multifaceted impact on CNS integrity, with glial cells (astrocytes, microglia, and oligodendrocytes) emerging as critical mediators of age-related neural vulnerability. Emerging evidence indicates that these glial populations experience significant structural, functional, and molecular alterations during aging. Astrocytes show reduced metabolic support and impaired neurotransmitter recycling, compromising neuronal energy homeostasis and synaptic function. On the other hand, microglia adopt a pro-inflammatory and senescent phenotype, characterized by persistent low-grade activation, altered phagocytic capacity, and dysregulated pro-inflammatory cytokine production, which collectively propagate a neuroinflammatory milieu. Oligodendrocytes and their progenitor cells display diminished myelination efficiency and regenerative potential, contributing to deficits in axonal conduction and neuronal network stability.
The convergence of these glial abnormalities contributes to increased oxidative stress, disrupts synaptic plasticity, and undermines the capacity of the CNS to respond to injury or pathological insult. Notably, glial aging is not simply a passive outcome of chronological progression but represents some molecular and cellular adaptations, including modifications in gene expression, mitochondrial dysfunction, and disrupted intercellular signaling. This underscores the concept that glial cells are active determinants of CNS aging, rather than secondary responders to neuronal decline.
Therapeutically, targeting glial dysfunction represents a promising avenue to preserve CNS health and mitigate age-associated neurological disorders. Interventions aimed at restoring glial metabolic competence, modulating neuroinflammatory signaling pathways, promoting oligodendrocyte regeneration, or rejuvenating senescent glial populations hold substantial potential to enhance neural resilience. Moreover, the identification of robust biomarkers of glial senescence and dysfunction might enable early detection of CNS vulnerability and inform the development of precision therapeutics.
In summary, the interplay of aging and glial cell dysfunction constitutes a key determinant of CNS homeostasis, cognitive integrity, and susceptibility to neurodegenerative processes. Future research should prioritize a mechanistic understanding of glial senescence, explore interventional strategies that restore glial functionality, and integrate glia-centric approaches into broader frameworks for healthy brain aging. Such efforts are critical for translating fundamental insights into clinically meaningful strategies to promote longevity and neural health across the lifespan.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I gratefully acknowledge BioRender for providing a professional and scientifically rigorous platform that enabled the creation of high-quality graphical illustrations presented in this review.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Akt | Protein kinase B (PKB) |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPK | AMP-activated protein kinase |
| AP-1 | Activator protein 1 |
| ARF | Alternative reading frame protein |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| ATM | Ataxia-telangiectasia mutated |
| ATP | Adenosine triphosphate |
| ATR | ATM and Rad3-related |
| Bak | Bcl-2 homologous antagonist/killer |
| Bax | Bcl-2–associated X protein |
| BBB | Blood–brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| C3 | Complement component 3 |
| Ca2+ | Calcium ion |
| CaMKII | Calcium/calmodulin-dependent protein kinase II |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCL5 | C-C motif chemokine ligand 5 |
| CD80 | Cluster of differentiation 80 |
| CD86 | Cluster of differentiation 86 |
| CDK4/6 | Cyclin-dependent kinases 4 and 6 |
| cGAS | Cyclic GMP-AMP synthase |
| CHK1 | Checkpoint kinase 1 |
| CHK2 | Checkpoint kinase 2 |
| CMA | Chaperone-mediated autophagy |
| CNS | Central nervous system |
| CNTF | Ciliary neurotrophic factor |
| CR | Caloric restriction |
| CREB | cAMP response element-binding protein |
| CRISPR-Cas | Clustered regularly interspaced short palindromic repeats-CRISPR-associated protein |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| Cx43 | Connexin 43 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DAMP | Damage-associated molecular pattern |
| DDR | DNA damage response |
| DHA | Docosahexaenoic acid |
| DNA | Deoxyribonucleic acid |
| DNMT1 | DNA methyltransferase 1 |
| DSB | Double-strand break |
| EAAT1 | Excitatory amino acid transporter 1 |
| EAAT2 | Excitatory amino acid transporter 2 |
| ECM | Extracellular matrix |
| EGCG | Epigallocatechin-3-gallate |
| EPA | Eicosapentaenoic acid |
| ERK | Extracellular signal-regulated kinase |
| EV | Extracellular vesicle |
| FGF2 | Fibroblast growth factor 2 |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GFRα1 | GDNF family receptor alpha 1 |
| H2O2 | Hydrogen peroxide |
| H3K27ac | Histone 3 lysine 27 acetylation |
| H3K4me3 | Histone 3 lysine 4 trimethylation |
| HDAC | Histone deacetylase |
| HDAC1 | Histone deacetylase 1 |
| HDAC2 | Histone deacetylase 2 |
| HDAC3 | Histone deacetylase 3 |
| HDAC8 | Histone deacetylase 8 |
| HO-1 | Heme oxigenase 1 |
| Hsp70 | Heat shock protein 70 |
| IF | Intermittent fasting |
| IFN-γ | Interferon gamma |
| IGF-1 | Insulin-like growth factor 1 |
| IGF-1R | Insulin-like growth factor 1 receptor |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| INK4a | Inhibitor of cyclin-dependent kinase 4a (p16 protein) |
| iPSCs | Induced pluripotent stem cells |
| IR | Insulin receptor |
| IRF | Interferon regulatory factor |
| JAK | Janus kinase |
| K+ | Potassium ion |
| KFERQ | Lys-Phe-Glu-Arg-Gln peptide |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| LTP | Long-term potentiation |
| M1 | Pro-inflammatory microglial phenotype |
| MAG | Myelin-associated glycoprotein |
| MAPK | Mitogen-activated protein kinase |
| MBP | Myelin basic protein |
| MCT1 | Monocarboxylate transporter 1 |
| MCT1/4 | Monocarboxylate transporter 1 and 4 |
| MHC-II | Major histocompatibility complex class II |
| miRNA | MicroRNA |
| MMP-3 | Matrix metalloproteinase 3 |
| MMP-9 | Matrix metalloproteinase 9 |
| MS-275 | N-(2-aminophenyl)-4-[N-(pyridine-3ylmethoxy-carbonyl)aminomethyl]benzamide |
| mtDNA | Mitochondrial DNA |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADH | Nicotinamide adenine dinucleotide (reduced form) |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NMDA | N-methyl-D-aspartate |
| NMN | Nicotinamide mononucleotide |
| NO | Nitric oxide |
| NR | Nicotinamide riboside |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSAID | Non-steroidal anti-inflammatory drug |
| O2− | Superoxide anion |
| OPC | Oligodendrocyte precursor cell |
| OXPHOS | Oxidative phosphorylation |
| p16 | Cyclin-dependent kinase inhibitor 2A (CDKN2A) |
| p21 | Cyclin-dependent kinase inhibitor 1 (CDKN1A) |
| P2X7 | Purinergic receptor P2X ligand-gated ion channel 7 |
| P2Y12 | Purinergic receptor P2Y, G-protein coupled, 12 |
| p53 | Tumor protein p53 |
| PDGF-A | Platelet-derived growth factor A |
| PI3K | Phosphoinositide 3-kinase |
| PINK1 | PTEN-induced kinase 1 |
| PLCγ | Phospholipase C gamma |
| PLP1 | Proteolipid protein 1 |
| pro-IL-18 | Pro-interleukin 18 |
| pro-IL-1β | Pro-interleukin 1 beta |
| PRR | Pattern recognition receptor |
| PSD-95 | Postsynaptic density protein 95 |
| PUFA | Polyunsaturated fatty acid |
| Rb | Retinoblastoma protein |
| RET | Rearranged during transfection |
| RNAi | RNA interference |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SAHA | Suberoylanilide hydroxamic acid |
| SASP | Senescence-associated secretory phenotype |
| SOD2 | Superoxide dismutase 2 |
| SPARCL1 | Secreted protein acidic and rich in cysteine-like 1 |
| SPM | Specialized pro-resolving lipid mediator |
| SS-31 | Elamipretide |
| STAT3 | Signal transducer and activator of transcription 3 |
| STING | Stimulator of interferon genes |
| TAT | Trans-activator of transcription |
| TfR | Transferrin receptor |
| TGF-β | Transforming growth factor beta |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| TrkB | Tropomyosin receptor kinase B |
| UPS | Ubiquitin–proteasome system |
| VGCC | Voltage-gated calcium channel |
| Wnt | Wingless/integrated |
| α2δ-1 | Alpha-2-delta-1 subunit of VGCC |
| ω-3 | Omega 3 |
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- García-Domínguez, M. Chronic pain in the elderly: Exploring cellular and molecular mechanisms and therapeutic perspectives. Front. Aging 2024, 5, 1477017. [Google Scholar] [CrossRef]
- Salas, I.H.; Burgado, J.; Allen, N.J. Glia: Victims or villains of the aging brain? Neurobiol. Dis. 2020, 143, 105008. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Zorec, R.; Rodriguez-Arellano, J.J.; Parpura, V. Neuroglia in Ageing. Adv. Exp. Med. Biol. 2019, 1175, 181–197. [Google Scholar]
- La Sala, G.; Farini, D. Glial Cells and Aging: From the CNS to the Cerebellum. Int. J. Mol. Sci. 2025, 26, 7553. [Google Scholar] [CrossRef]
- Pellegrini, C.; Pirazzini, C.; Sala, C.; Sambati, L.; Yusipov, I.; Kalyakulina, A.; Ravaioli, F.; Kwiatkowska, K.M.; Durso, D.F.; Ivanchenko, M.; et al. A Meta-Analysis of Brain DNA Methylation Across Sex, Age, and Alzheimer’s Disease Points for Accelerated Epigenetic Aging in Neurodegeneration. Front. Aging Neurosci. 2021, 13, 639428. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, C.; Zhang, Y. Aging-related histone modification changes in brain function. Ibrain 2023, 9, 205–213. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Alterations in Chromatin Structure and Function in the Microglia. Front. Cell Dev. Biol. 2021, 8, 626541. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.W.; Zhou, O.Y.; Zhong, B.L.; Sun, E.D.; Navarro Negredo, P.; Nair, S.; Sharmin, M.; Ruetz, T.J.; Wilson, M.; Kundaje, A.; et al. Chromatin accessibility dynamics of neurogenic niche cells reveal defects in neural stem cell adhesion and migration during aging. Nat. Aging 2023, 3, 866–893. [Google Scholar] [CrossRef]
- Sun, E.D.; Nagvekar, R.; Pogson, A.N.; Brunet, A. Brain aging and rejuvenation at single-cell resolution. Neuron 2025, 113, 82–108. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Ferri, E.; Calvani, R.; Coelho-Júnior, H.J.; Marzetti, E.; Arosio, B. Age-Associated Glia Remodeling and Mitochondrial Dysfunction in Neurodegeneration: Antioxidant Supplementation as a Possible Intervention. Nutrients 2022, 14, 2406. [Google Scholar] [CrossRef]
- Byrns, C.N.; Perlegos, A.E.; Miller, K.N.; Jin, Z.; Carranza, F.R.; Manchandra, P.; Beveridge, C.H.; Randolph, C.E.; Chaluvadi, V.S.; Zhang, S.L.; et al. Senescent glia link mitochondrial dysfunction and lipid accumulation. Nature 2024, 630, 475–483. [Google Scholar] [CrossRef]
- Gradisnik, L.; Velnar, T. Astrocytes in the central nervous system and their functions in health and disease: A review. World J. Clin. Cases 2023, 11, 3385–3394. [Google Scholar] [CrossRef]
- Palmer, A.L.; Ousman, S.S. Astrocytes and Aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Labarta-Bajo, L.; Allen, N.J. Astrocytes in aging. Neuron 2025, 113, 109–126. [Google Scholar] [CrossRef]
- Haidet-Phillips, A.M.; Hester, M.E.; Miranda, C.J.; Meyer, K.; Braun, L.; Frakes, A.; Song, S.; Likhite, S.; Murtha, M.J.; Foust, K.D.; et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 2011, 29, 824–828. [Google Scholar] [CrossRef]
- Sharan, S.; Tewari, B.P.; Joshi, P.G. Aging-Related Changes in Expression and Function of Glutamate Transporters in Rat Spinal Cord Astrocytes. Neuroglia 2023, 4, 290–306. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.; Mustafa, S.; Collins-Praino, L.E. The Hallmarks of Ageing in Microglia. Cell. Mol. Neurobiol. 2025, 45, 45. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Jin, Y.; Zhang, Y.; Wu, J.; Xu, Z.; Huang, Y.; Cai, L.; Gao, S.; Liu, T.; et al. Transcriptional and epigenetic decoding of the microglial aging process. Nat. Aging 2023, 3, 1288–1311. [Google Scholar] [CrossRef]
- Thériault, P.; Rivest, S. Microglia: Senescence Impairs Clearance of Myelin Debris. Curr. Biol. 2016, 26, R772–R775. [Google Scholar] [CrossRef][Green Version]
- Liu, P.; Wang, Q.; Wang, S.; Liu, Y.; Chen, Q.; Qin, W.; Liu, X.; Ye, X.; Jiao, Y.; Yuan, H.; et al. Single-Cell RNA-Seq Reveals Aging-Related Impairment of Microglial Efferocytosis Contributing to Apoptotic Cells Accumulation After Retinal Injury. Aging Cell 2025, 24, e70097. [Google Scholar] [CrossRef]
- Caldeira, C.; Cunha, C.; Vaz, A.R.; Falcão, A.S.; Barateiro, A.; Seixas, E.; Fernandes, A.; Brites, D. Key Aging-Associated Alterations in Primary Microglia Response to Beta-Amyloid Stimulation. Front. Aging Neurosci. 2017, 9, 277. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, G.; Huang, Z.; Ding, X.; Sun, Q.; Zhang, Y.; Zhu, R.; Guan, H.; Ji, M. ATP-P2X7R-mediated microglia senescence aggravates retinal ganglion cell injury in chronic ocular hypertension. J. Neuroinflamm. 2023, 20, 180. [Google Scholar] [CrossRef]
- Damani, M.R.; Zhao, L.; Fontainhas, A.M.; Amaral, J.; Fariss, R.N.; Wong, W.T. Age-related alterations in the dynamic behavior of microglia. Aging Cell 2011, 10, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Wu, Z. Microglia-aging: Roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav. Brain Res. 2009, 201, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thundyil, J.; Lim, K.L. DAMPs and neurodegeneration. Ageing Res. Rev. 2015, 24, 17–28. [Google Scholar] [CrossRef]
- Butt, A.M.; Papanikolaou, M.; Rivera, A. Physiology of Oligodendroglia. Adv. Exp. Med. Biol. 2019, 1175, 117–128. [Google Scholar] [PubMed]
- Gomez, P.T.; Carver, C.M.; Rodriguez, S.L.; Wang, L.; Zhang, X.; Schafer, M.J. Aging and senescent fates of oligodendrocyte precursor cells in the mouse brain. NPJ Aging 2024, 10, 47. [Google Scholar] [CrossRef]
- Dansu, D.K.; Sauma, S.; Huang, D.; Li, M.; Moyon, S.; Casaccia, P. The epigenetic landscape of oligodendrocyte progenitors changes with time. bioRxiv 2024. [Google Scholar] [CrossRef]
- Woodruff, R.H.; Fruttiger, M.; Richardson, W.D.; Franklin, R.J. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 2004, 25, 252–262. [Google Scholar] [CrossRef]
- Guardiola-Diaz, H.M.; DiBenedictis, B.T.; Prendaj, E.; Bansal, R. Diverse Responses of Oligodendrocytes to Different FGF-Family Members: Uncoupling Structure-Function Relationship Within FGF Subfamilies. ASN Neuro 2024, 16, 2371163. [Google Scholar] [CrossRef]
- Dimovasili, C.; Fair, A.E.; Garza, I.R.; Batterman, K.V.; Mortazavi, F.; Moore, T.L.; Rosene, D.L. Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain. Geroscience 2023, 45, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Greco, A.; Teliti, M.; Croce, L.; Chytiris, S.; Magri, F.; Gaetano, C.; Rotondi, M. Inflamm-ageing: How cytokines and nutrition shape the trajectory of ageing. Cytokine Growth Factor Rev. 2025, 82, 31–42. [Google Scholar] [CrossRef]
- Witkowska-Sędek, E.; Pyrżak, B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Cent. Eur. J. Immunol. 2020, 45, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Villaseñor, I.; Garwood, C.J.; Heath, P.R.; Simpson, J.E.; Ince, P.G.; Wharton, S.B. Expression of p16 and p21 in the frontal association cortex of ALS/MND brains suggests neuronal cell cycle dysregulation and astrocyte senescence in early stages of the disease. Neuropathol. Appl. Neurobiol. 2020, 46, 171–185. [Google Scholar] [CrossRef]
- Jurcau, M.C.; Jurcau, A.; Cristian, A.; Hogea, V.O.; Diaconu, R.G.; Nunkoo, V.S. Inflammaging and Brain Aging. Int. J. Mol. Sci. 2024, 25, 10535. [Google Scholar] [CrossRef]
- Groh, J.; Simons, M. White matter aging and its impact on brain function. Neuron 2025, 113, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, Y.; Wang, S.; Huang, Z.; Jin, F.; Zou, Q.; Li, J.; Pu, Y.; Cai, Z. Bioenergetic Impairment in the Neuro-Glia-Vascular Unit: An Emerging Physiopathology during Aging. Aging Dis. 2021, 12, 2080–2095. [Google Scholar] [CrossRef]
- Gleichman, A.J.; Carmichael, S.T. Glia in neurodegeneration: Drivers of disease or along for the ride? Neurobiol. Dis. 2020, 142, 104957. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E. Neuroglial cells: Morphological changes during normal aging. Rend. Fis. Acc. Lincei 2013, 24, 101–106. [Google Scholar] [CrossRef]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Davoli, M.A.; Belliveau, C.; Tanti, A.; Ma, J.C.; Farmer, W.T.; Turecki, G.; Murai, K.K.; Mechawar, N. Characterization of Vimentin-Immunoreactive Astrocytes in the Human Brain. Front. Neuroanat. 2020, 14, 31. [Google Scholar] [CrossRef]
- Hart, A.D.; Wyttenbach, A.; Perry, V.H.; Teeling, J.L. Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain Behav. Immun. 2012, 26, 754–765. [Google Scholar] [CrossRef]
- Davies, D.S.; Ma, J.; Jegathees, T.; Goldsbury, C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. 2017, 27, 795–808. [Google Scholar] [CrossRef]
- Ana, B. Aged-Related Changes in Microglia and Neurodegenerative Diseases: Exploring the Connection. Biomedicines 2024, 12, 1737. [Google Scholar] [CrossRef]
- Radwan, D.; El-Kattan, A.; Zoair, M.; Ibrahim, N. Developmental and Aging-Related Changes of the Optic Nerve in the Albino Rat: Histological, Histomorphometric and Immunohistochemical study. Egypt. J. Histol. 2022, 46, 1149–1164. [Google Scholar] [CrossRef]
- Peters, A. The effects of normal aging on myelin and nerve fibers: A review. J. Neurocytol. 2002, 31, 581–593. [Google Scholar] [CrossRef]
- Hampton, D.W.; Innes, N.; Merkler, D.; Zhao, C.; Franklin, R.J.; Chandran, S. Focal immune-mediated white matter demyelination reveals an age-associated increase in axonal vulnerability and decreased remyelination efficiency. Am. J. Pathol. 2012, 180, 1897–1905. [Google Scholar] [CrossRef]
- Richter-Landsberg, C. The cytoskeleton in oligodendrocytes. Microtubule dynamics in health and disease. J. Mol. Neurosci. 2008, 35, 55–63. [Google Scholar] [CrossRef]
- Seixas, A.I.; Azevedo, M.M.; Paes de Faria, J.; Fernandes, D.; Mendes Pinto, I.; Relvas, J.B. Evolvability of the actin cytoskeleton in oligodendrocytes during central nervous system development and aging. Cell. Mol. Life Sci. 2019, 76, 1–11. [Google Scholar] [CrossRef]
- Sams, E.C. Oligodendrocytes in the aging brain. Neuronal Signal. 2021, 5, NS20210008. [Google Scholar] [CrossRef]
- Montani, L. Lipids in regulating oligodendrocyte structure and function. Semin. Cell Dev. Biol. 2021, 112, 114–122. [Google Scholar] [CrossRef]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M.; Melov, S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Brazhe, N.; Morozova, K.; Yashin, K.; Bychkov, M.; Nosova, O.; Sutyagina, O.; Brazhe, A.; Parshina, E.; Li, L.; et al. Mitochondrial malfunction and atrophy of astrocytes in the aged human cerebral cortex. Nat. Commun. 2023, 14, 8380. [Google Scholar] [CrossRef]
- Jiang, T.; Cadenas, E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 2014, 13, 1059–1067. [Google Scholar] [CrossRef]
- Tsesmelis, K.; Maity-Kumar, G.; Croner, D.; Sprissler, J.; Tsesmelis, M.; Hein, T.; Baumann, B.; Wirth, T. Accelerated aging in mice with astrocytic redox imbalance as a consequence of SOD2 deletion. Aging Cell 2023, 22, e13911. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Feng, A.; Rock, R.B. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem. Res. 2013, 38, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; Schreibelt, G.; Drexhage, J.; Hazes, T.; Dijkstra, C.D.; van der Valk, P.; de Vries, H.E. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- MohanKumar, S.M.J.; Murugan, A.; Palaniyappan, A.; MohanKumar, P.S. Role of cytokines and reactive oxygen species in brain aging. Mech. Ageing Dev. 2023, 214, 111855. [Google Scholar] [CrossRef]
- Mela, V.; Mota, B.C.; Milner, M.; McGinley, A.; Mills, K.H.G.; Kelly, Á.M.; Lynch, M.A. Exercise-induced re-programming of age-related metabolic changes in microglia is accompanied by a reduction in senescent cells. Brain Behav. Immun. 2020, 87, 413–428. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, K.; Wang, F.; Wu, W.; Mai, W.; Qiu, L.; Luo, Y.; Ge, W.P.; Sun, B.; Shi, L.; et al. Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity. Nat. Metab. 2022, 4, 1756–1774. [Google Scholar] [CrossRef]
- Stojiljkovic, M.R.; Schmeer, C.; Witte, O.W. Senescence and aging differentially alter key metabolic pathways in murine brain microglia. Neurosci. Lett. 2024, 828, 137751. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kwon, M.S. Aged Microglia in Neurodegenerative Diseases: Microglia Lifespan and Culture Methods. Front. Aging Neurosci. 2022, 13, 766267. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Rinholm, J.E. Mitochondria in Myelinating Oligodendrocytes: Slow and Out of Breath? Metabolites 2021, 11, 359. [Google Scholar] [CrossRef]
- Tse, K.H.; Herrup, K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech. Ageing Dev. 2017, 161, 37–50. [Google Scholar] [CrossRef]
- Hetz, C. Adapting the proteostasis capacity to sustain brain healthspan. Cell 2021, 184, 1545–1560. [Google Scholar] [CrossRef]
- Palmer, J.E.; Wilson, N.; Son, S.M.; Obrocki, P.; Wrobel, L.; Rob, M.; Takla, M.; Korolchuk, V.I.; Rubinsztein, D.C. Autophagy, aging, and age-related neurodegeneration. Neuron 2025, 113, 29–48. [Google Scholar] [CrossRef]
- Bar-Ziv, R.; Dutta, N.; Hruby, A.; Sukarto, E.; Averbukh, M.; Alcala, A.; Henderson, H.R.; Durieux, J.; Tronnes, S.U.; Ahmad, Q.; et al. Glial-derived mitochondrial signals affect neuronal proteostasis and aging. Sci. Adv. 2023, 9, eadi1411. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Chowdhury, S.; Ray, R.; Karmakar, P. Relating aging and autophagy: A new perspective towards the welfare of human health. EXCLI J. 2023, 22, 732–748. [Google Scholar] [PubMed]
- Boisvert, M.M.; Erikson, G.A.; Shokhirev, M.N.; Allen, N.J. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018, 22, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Vazquez, S.K.; Massieu, L.; Rincón-Heredia, R.; García-de la Torre, P.; Quiroz-Baez, R.; Gomez-Verjan, J.C.; Rivero-Segura, N.A. Glutamatergic Neurotransmission in Aging and Neurodegenerative Diseases: A Potential Target to Improve Cognitive Impairment in Aging. Arch. Med. Res. 2024, 55, 103039. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, C.J. Astrocytes autophagy in aging and neurodegenerative disorders. Biomed. Pharmacother. 2020, 122, 109691. [Google Scholar] [CrossRef]
- Lee, E.; Jung, Y.J.; Park, Y.R.; Lim, S.; Choi, Y.J.; Lee, S.Y.; Kim, C.H.; Mun, J.Y.; Chung, W.S. A distinct astrocyte subtype in the aging mouse brain characterized by impaired protein homeostasis. Nat. Aging 2022, 2, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Flowers, A.; Bell-Temin, H.; Jalloh, A.; Stevens, S.M., Jr.; Bickford, P.C. Proteomic anaysis of aged microglia: Shifts in transcription, bioenergetics, and nutrient response. J. Neuroinflamm. 2017, 14, 96. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Yu, H.; Xue, C.; Pei, X.; Chen, Y.; Guan, Y. The regulation of microglia by aging and autophagy in multiple sclerosis. Pharmacol. Res. 2025, 216, 107786. [Google Scholar] [CrossRef]
- Jin, C.; Shao, Y.; Zhang, X.; Xiang, J.; Zhang, R.; Sun, Z.; Mei, S.; Zhou, J.; Zhang, J.; Shi, L. A Unique Type of Highly-Activated Microglia Evoking Brain Inflammation via Mif/Cd74 Signaling Axis in Aged Mice. Aging Dis. 2021, 12, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Valdor, R.; Martinez-Vicente, M. The Role of Chaperone-Mediated Autophagy in Tissue Homeostasis and Disease Pathogenesis. Biomedicines 2024, 12, 257. [Google Scholar] [CrossRef]
- de la Fuente, A.G.; Queiroz, R.M.L.; Ghosh, T.; McMurran, C.E.; Cubillos, J.F.; Bergles, D.E.; Fitzgerald, D.C.; Jones, C.A.; Lilley, K.S.; Glover, C.P.; et al. Changes in the Oligodendrocyte Progenitor Cell Proteome with Ageing. Mol. Cell. Proteom. 2020, 19, 1281–1302. [Google Scholar] [CrossRef]
- Ktena, N.; Spyridakos, D.; Georgilis, A.; Kalafatakis, I.; Thomoglou, E.; Kolaxi, A.; Nikoletopoulou, V.; Savvaki, M.; Karagogeos, D. Disruption of Oligodendroglial Autophagy Leads to Myelin Morphological Deficits, Neuronal Apoptosis, and Cognitive Decline in Aged Mice. Glia 2025, 73, 1383–1397. [Google Scholar] [CrossRef]
- Sloane, J.A.; Hinman, J.D.; Lubonia, M.; Hollander, W.; Abraham, C.R. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J. Neurochem. 2003, 84, 157–168. [Google Scholar] [CrossRef]
- Chen, H.; Yang, G.; Xu, D.E.; Du, Y.T.; Zhu, C.; Hu, H.; Luo, L.; Feng, L.; Huang, W.; Sun, Y.Y.; et al. Autophagy in Oligodendrocyte Lineage Cells Controls Oligodendrocyte Numbers and Myelin Integrity in an Age-dependent Manner. Neurosci. Bull. 2025, 41, 374–390. [Google Scholar] [CrossRef]
- Goldbaum, O.; Vollmer, G.; Richter-Landsberg, C. Proteasome inhibition by MG-132 induces apoptotic cell death and mitochondrial dysfunction in cultured rat brain oligodendrocytes but not in astrocytes. Glia 2006, 53, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Branen, L.; Sivasubramanian, M.K.; Monteiro, R.; Subramanian, M. Aging is associated with glial senescence in the brainstem—Implications for age-related sympathetic overactivity. Aging 2021, 13, 13460–13473. [Google Scholar] [CrossRef]
- Pao, P.C.; Patnaik, D.; Watson, L.A.; Gao, F.; Pan, L.; Wang, J.; Adaikkan, C.; Penney, J.; Cam, H.P.; Huang, W.C.; et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat. Commun. 2020, 11, 2484. [Google Scholar] [CrossRef]
- Araujo, A.P.B.; Vargas, G.; Hayashide, L.S.; Matias, I.; Andrade, C.B.V.; de Carvalho, J.J.; Gomes, F.C.A.; Diniz, L.P. Aging promotes an increase in mitochondrial fragmentation in astrocytes. Front. Cell. Neurosci. 2024, 18, 1496163. [Google Scholar] [CrossRef]
- Reyes-Ábalos, A.L.; Álvarez-Zabaleta, M.; Olivera-Bravo, S.; Di Tomaso, M.V. Astrocyte DNA damage and response upon acute exposure to ethanol and corticosterone. Front. Toxicol. 2024, 5, 1277047. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, J.; Sultana, R.; Parveen, A.; Kim, S.Y. Recent Advances in Anti-Aging Therapeutic Strategies Targeting DNA Damage Response and Senescence-Associated Secretory Phenotype-Linked Signaling Cascade. Cell Biochem. Funct. 2025, 43, e70046. [Google Scholar] [CrossRef] [PubMed]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Cohen, J.; Torres, C. Astrocyte senescence: Evidence and significance. Aging Cell 2019, 18, e12937. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Haapasalo, A.; Hiltunen, M.; Soininen, H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur. J. Neurosci. 2011, 34, 3–11. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 2012, 7, e45069. [Google Scholar] [CrossRef]
- Stamouli, E.C.; Politis, A.M. Pro-inflammatory cytokines in Alzheimer’s disease. Psychiatriki 2016, 27, 264–275. [Google Scholar] [CrossRef]
- Preininger, M.K.; Kaufer, D. Blood-Brain Barrier Dysfunction and Astrocyte Senescence as Reciprocal Drivers of Neuropathology in Aging. Int. J. Mol. Sci. 2022, 23, 6217. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, N.C.; Henderson, M.L.; Selvamani, A.; Park, M.J.; Dindot, S.; Miranda, R.C.; Sohrabji, F. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 2015, 10, 142–152. [Google Scholar] [CrossRef]
- Ling, E.; Nemesh, J.; Goldman, M.; Kamitaki, N.; Reed, N.; Handsaker, R.E.; Genovese, G.; Vogelgsang, J.S.; Gerges, S.; Kashin, S.; et al. A concerted neuron-astrocyte program declines in ageing and schizophrenia. Nature 2024, 627, 604–611. [Google Scholar] [CrossRef]
- Acevedo, A.; Torres, F.; Kiwi, M.; Baeza-Lehnert, F.; Barros, L.F.; Lee-Liu, D.; González-Billault, C. Metabolic switch in the aging astrocyte supported via integrative approach comprising network and transcriptome analyses. Aging 2023, 15, 9896–9912. [Google Scholar] [CrossRef]
- Rim, C.; You, M.J.; Nahm, M.; Kwon, M.S. Emerging role of senescent microglia in brain aging-related neurodegenerative diseases. Transl. Neurodegener. 2024, 13, 10. [Google Scholar] [CrossRef]
- Stojiljkovic, M.R.; Ain, Q.; Bondeva, T.; Heller, R.; Schmeer, C.; Witte, O.W. Phenotypic and functional differences between senescent and aged murine microglia. Neurobiol. Aging 2019, 74, 56–69. [Google Scholar] [CrossRef]
- Han, T.; Xu, Y.; Sun, L.; Hashimoto, M.; Wei, J. Microglial response to aging and neuroinflammation in the development of neurodegenerative diseases. Neural Regen. Res. 2024, 19, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Malvaso, A.; Gatti, A.; Negro, G.; Calatozzolo, C.; Medici, V.; Poloni, T.E. Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring. Cells 2023, 12, 2824. [Google Scholar] [CrossRef] [PubMed]
- Auzmendi-Iriarte, J.; Moreno-Cugnon, L.; Saenz-Antoñanzas, A.; Grassi, D.; de Pancorbo, M.M.; Arevalo, M.A.; Wood, I.C.; Matheu, A. High levels of HDAC expression correlate with microglial aging. Expert Opin. Ther. Targets 2022, 26, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.M.; Lawson, M.A.; Johnson, R.W. Aging and peripheral lipopolysaccharide can modulate epigenetic regulators and decrease IL-1β promoter DNA methylation in microglia. Neurobiol. Aging 2016, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sainz, A.; Rojas, R.; Ruiz, A.; Matute, C. Oligodendrocytes and myelin in aging and disease. Ageing Longev. 2025, 6, 51–58. [Google Scholar] [CrossRef]
- Parandavar, E.; Shafizadeh, M.; Ahmadian, S.; Javan, M. Long-term demyelination and aging-associated changes in mice corpus callosum; evidence for the role of accelerated aging in remyelination failure in a mouse model of multiple sclerosis. Aging Cell 2024, 23, e14211. [Google Scholar] [CrossRef]
- Windener, F.; Grewing, L.; Thomas, C.; Dorion, M.F.; Otteken, M.; Kular, L.; Jagodic, M.; Antel, J.; Albrecht, S.; Kuhlmann, T. Physiological aging and inflammation-induced cellular senescence may contribute to oligodendroglial dysfunction in MS. Acta Neuropathol. 2024, 147, 82. [Google Scholar] [CrossRef]
- Shen, S.; Liu, A.; Li, J.; Wolubah, C.; Casaccia-Bonnefil, P. Epigenetic memory loss in aging oligodendrocytes in the corpus callosum. Neurobiol. Aging 2008, 29, 452–463. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.C.; Xiao, B.J.; Guo, X.D.; Zheng, Q.X.; Wu, B. Age-related Changes in the Global DNA Methylation Profile of Oligodendrocyte Progenitor Cells Derived from Rat Spinal Cords. Curr. Med. Sci. 2019, 39, 67–74. [Google Scholar] [CrossRef]
- Urushihata, T.; Satoh, A. Role of the central nervous system in cell non-autonomous signaling mechanisms of aging and longevity in mammals. J. Physiol. Sci. 2024, 74, 40. [Google Scholar] [CrossRef]
- Mayorga-Weber, G.; Rivera, F.J.; Castro, M.A. Neuron-glia (mis)interactions in brain energy metabolism during aging. J. Neurosci. Res. 2022, 100, 835–854. [Google Scholar] [CrossRef]
- Hanslik, K.L.; Marino, K.M.; Ulland, T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell. Neurosci. 2021, 15, 718324. [Google Scholar] [CrossRef]
- Latham, A.S.; Moreno, J.A.; Geer, C.E. Biological agents and the aging brain: Glial inflammation and neurotoxic signaling. Front. Aging 2023, 4, 1244149. [Google Scholar] [CrossRef]
- Dickstein, D.L.; Kabaso, D.; Rocher, A.B.; Luebke, J.I.; Wearne, S.L.; Hof, P.R. Changes in the structural complexity of the aged brain. Aging Cell 2007, 6, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Lazareva, N.; Semyanov, A. Glial decline and loss of homeostatic support rather than inflammation defines cognitive aging. Neural Regen. Res. 2022, 17, 565–566. [Google Scholar] [CrossRef]
- Goenaga, J.; Araque, A.; Kofuji, P.; Herrera Moro Chao, D. Calcium signaling in astrocytes and gliotransmitter release. Front. Synaptic Neurosci. 2023, 15, 1138577. [Google Scholar] [CrossRef]
- Orellana, J.A.; von Bernhardi, R.; Giaume, C.; Sáez, J.C. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev. Neurosci. 2012, 23, 163–177. [Google Scholar] [CrossRef]
- Verkhratsky, A. Astroglial Calcium Signaling in Aging and Alzheimer’s Disease. Cold Spring Harb. Perspect. Biol. 2019, 11, a035188. [Google Scholar] [CrossRef]
- Lalo, U.; Bogdanov, A.; Pankratov, Y. Diversity of Astroglial Effects on Aging- and Experience-Related Cortical Metaplasticity. Front. Mol. Neurosci. 2018, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Arvanitaki, E.S.; Goulielmaki, E.; Gkirtzimanaki, K.; Niotis, G.; Tsakani, E.; Nenedaki, E.; Rouska, I.; Kefalogianni, M.; Xydias, D.; Kalafatakis, I.; et al. Microglia-derived extracellular vesicles trigger age-related neurodegeneration upon DNA damage. Proc. Natl. Acad. Sci. USA 2024, 121, e2317402121. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Raffaele, S.; Fumagalli, M.; Verderio, C. The multiple faces of extracellular vesicles released by microglia: Where are we 10 years after? Front. Cell. Neurosci. 2022, 16, 984690. [Google Scholar] [CrossRef]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef]
- Raffaele, S.; Lombardi, M.; Verderio, C.; Fumagalli, M. TNF Production and Release from Microglia via Extracellular Vesicles: Impact on Brain Functions. Cells 2020, 9, 2145. [Google Scholar] [CrossRef]
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Kouwaki, T.; Oshiumi, H. Aging-Associated Extracellular Vesicles Contain Immune Regulatory microRNAs Alleviating Hyperinflammatory State and Immune Dysfunction in the Elderly. iScience 2020, 23, 101520. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Li, A.M.; Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 2018, 21, 683–695. [Google Scholar] [CrossRef]
- Gross, P.S.; Durán-Laforet, V.; Ho, L.T.; Melchor, G.S.; Zia, S.; Manavi, Z.; Barclay, W.E.; Lee, S.H.; Shults, N.; Selva, S.; et al. Senescent-like microglia limit remyelination through the senescence associated secretory phenotype. Nat. Commun. 2025, 16, 2283. [Google Scholar] [CrossRef]
- Xu, T.; Liu, C.; Deng, S.; Gan, L.; Zhang, Z.; Yang, G.Y.; Tian, H.; Tang, Y. The roles of microglia and astrocytes in myelin phagocytosis in the central nervous system. J. Cereb. Blood Flow Metab. 2023, 43, 325–340. [Google Scholar] [CrossRef]
- Lynch, A.M.; Murphy, K.J.; Deighan, B.F.; O’Reilly, J.A.; Gun’ko, Y.K.; Cowley, T.R.; Gonzalez-Reyes, R.E.; Lynch, M.A. The impact of glial activation in the aging brain. Aging Dis. 2010, 1, 262–278. [Google Scholar] [PubMed]
- Navakkode, S.; Kennedy, B.K. Neural ageing and synaptic plasticity: Prioritizing brain health in healthy longevity. Front. Aging Neurosci. 2024, 16, 1428244. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A. Neuroglial decline defines cognitive ageing. Ageing Longev. 2025, 6, 7–26. [Google Scholar] [CrossRef]
- Nirzhor, S.S.R.; Khan, R.I.; Neelotpol, S. The Biology of Glial Cells and Their Complex Roles in Alzheimer’s Disease: New Opportunities in Therapy. Biomolecules 2018, 8, 93. [Google Scholar] [CrossRef]
- Petralia, R.S.; Mattson, M.P.; Yao, P.J. Communication breakdown: The impact of ageing on synapse structure. Ageing Res. Rev. 2014, 14, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Mullany, P.; Lynch, M.A. Changes in protein synthesis and synthesis of the synaptic vesicle protein, synaptophysin, in entorhinal cortex following induction of long-term potentiation in dentate gyrus: An age-related study in the rat. Neuropharmacology 1997, 36, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Ojo, B.; Rezaie, P.; Gabbott, P.L.; Davies, H.; Colyer, F.; Cowley, T.R.; Lynch, M.; Stewart, M.G. Age-related changes in the hippocampus (loss of synaptophysin and glial-synaptic interaction) are modified by systemic treatment with an NCAM-derived peptide, FGL. Brain Behav. Immun. 2012, 26, 778–788. [Google Scholar] [CrossRef]
- Savioz, A.; Leuba, G.; Vallet, P.G. A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease. Ageing Res. Rev. 2014, 18, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Tavernarakis, N. Calcium homeostasis in aging neurons. Front. Genet. 2012, 3, 200. [Google Scholar] [CrossRef]
- Michailidis, I.E.; Abele-Henckels, K.; Zhang, W.K.; Lin, B.; Yu, Y.; Geyman, L.S.; Ehlers, M.D.; Pnevmatikakis, E.A.; Yang, J. Age-related homeostatic midchannel proteolysis of neuronal L-type voltage-gated Ca2+ channels. Neuron 2014, 82, 1045–1057. [Google Scholar] [CrossRef]
- Gramuntell, Y.; Klimczak, P.; Coviello, S.; Perez-Rando, M.; Nacher, J. Effects of Aging on the Structure and Expression of NMDA Receptors of Somatostatin Expressing Neurons in the Mouse Hippocampus. Front. Aging Neurosci. 2021, 13, 782737. [Google Scholar] [CrossRef]
- Zhang, G.R.; Cheng, X.R.; Zhou, W.X.; Zhang, Y.X. Age-related expression of calcium/calmodulin-dependent protein kinase II A in the hippocampus and cerebral cortex of senescence accelerated mouse prone/8 mice is modulated by anti-Alzheimer’s disease drugs. Neuroscience 2009, 159, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Oh, M.M.; Disterhoft, J.F. CREB, cellular excitability, and cognition: Implications for aging. Behav. Brain Res. 2017, 322, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Rumian, N.L.; Freund, R.K.; Dell’Acqua, M.L.; Coultrap, S.J.; Bayer, K.U. Decreased nitrosylation of CaMKII causes aging-associated impairments in memory and synaptic plasticity in mice. Sci. Signal. 2023, 16, eade5892. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Zsurka, G.; Peeva, V.; Kotlyar, A.; Kunz, W.S. Is There Still Any Role for Oxidative Stress in Mitochondrial DNA-Dependent Aging? Genes 2018, 9, 175. [Google Scholar] [CrossRef]
- Popov, A.; Brazhe, A.; Denisov, P.; Sutyagina, O.; Li, L.; Lazareva, N.; Verkhratsky, A.; Semyanov, A. Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 2021, 20, e13334. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, N.; Yu, B.; Zhang, W.; Wan, J. Transcriptomic profiling of microglia and astrocytes throughout aging. J. Neuroinflamm. 2020, 17, 97. [Google Scholar] [CrossRef]
- Huber, R.E.; Babbitt, C.; Peyton, S.R. Heterogeneity of brain extracellular matrix and astrocyte activation. J. Neurosci. Res. 2024, 102, e25356. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Miranda, F.; Araujo, A.P.B.; Medinas, D.B.; Gomes, F.C.A. Astrocytic Hevin/SPARCL-1 Regulates Cognitive Decline in Pathological and Normal Brain Aging. Aging Cell 2025, 24, e14493. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef]
- Fan, S.; Gangwar, S.P.; Machius, M.; Rudenko, G. Interplay between hevin, SPARC, and MDGAs: Modulators of neurexin-neuroligin transsynaptic bridges. Structure 2021, 29, 664–678.e6. [Google Scholar] [CrossRef]
- Mora, F.; Segovia, G.; del Arco, A. Aging, plasticity and environmental enrichment: Structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev. 2007, 55, 78–88. [Google Scholar] [CrossRef]
- Orre, M.; Kamphuis, W.; Osborn, L.M.; Melief, J.; Kooijman, L.; Huitinga, I.; Klooster, J.; Bossers, K.; Hol, E.M. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol. Aging 2014, 35, 1–14. [Google Scholar] [CrossRef]
- Noriega-Prieto, J.A.; Araque, A. Sensing and Regulating Synaptic Activity by Astrocytes at Tripartite Synapse. Neurochem. Res. 2021, 46, 2580–2585. [Google Scholar] [CrossRef]
- Guedes, J.R.; Ferreira, P.A.; Costa, J.M.; Cardoso, A.L.; Peça, J. Microglia-dependent remodeling of neuronal circuits. J. Neurochem. 2022, 163, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Huo, A.; Wang, J.; Li, Q.; Li, M.; Qi, Y.; Yin, Q.; Luo, W.; Shi, J.; Cong, Q. Molecular mechanisms underlying microglial sensing and phagocytosis in synaptic pruning. Neural Regen. Res. 2024, 19, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar] [CrossRef]
- Heuer, S.E.; Bloss, E.B.; Howell, G.R. Strategies to dissect microglia-synaptic interactions during aging and in Alzheimer’s disease. Neuropharmacology 2024, 254, 109987. [Google Scholar] [CrossRef] [PubMed]
- Antignano, I.; Liu, Y.; Offermann, N.; Capasso, M. Aging microglia. Cell. Mol. Life Sci. 2023, 80, 126. [Google Scholar] [CrossRef]
- Brawek, B.; Skok, M.; Garaschuk, O. Changing Functional Signatures of Microglia along the Axis of Brain Aging. Int. J. Mol. Sci. 2021, 22, 1091. [Google Scholar] [CrossRef]
- Greenwood, E.K.; Brown, D.R. Senescent Microglia: The Key to the Ageing Brain? Int. J. Mol. Sci. 2021, 22, 4402. [Google Scholar] [CrossRef]
- Moca, E.N.; Lecca, D.; Hope, K.T.; Etienne, F.; Schaler, A.W.; Espinoza, K.; Chappell, M.S.; Gray, D.T.; Tweedie, D.; Sidhu, S.; et al. Microglia Drive Pockets of Neuroinflammation in Middle Age. J. Neurosci. 2022, 42, 3896–3918. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Wen, J.; Wang, S.; Yuan, M.; Sun, H.; Shu, L.; Yang, X.; Pu, Y.; Cai, Z. Microglia in brain aging: An overview of recent basic science and clinical research developments. J. Biomed. Res. 2024, 38, 122–136. [Google Scholar] [CrossRef]
- Rivera, A.; Vanzuli, I.; Arellano, J.J.; Butt, A. Decreased Regenerative Capacity of Oligodendrocyte Progenitor Cells (NG2-Glia) in the Ageing Brain: A Vicious Cycle of Synaptic Dysfunction, Myelin Loss and Neuronal Disruption? Curr. Alzheimer Res. 2016, 13, 413–418. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [PubMed]
- Bhusal, A.; Afridi, R.; Lee, W.H.; Suk, K. Bidirectional Communication Between Microglia and Astrocytes in Neuroinflammation. Curr. Neuropharmacol. 2023, 21, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, N.; Xiao, L.; Wang, F.; Li, T. Replenishing the Aged Brains: Targeting Oligodendrocytes and Myelination? Front. Aging Neurosci. 2021, 13, 760200. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Zhang, J.; Huang, L.; Niu, Y.; Chen, H.; Liu, Q.; Wang, R. Oligodendrocytes Play a Critical Role in White Matter Damage of Vascular Dementia. Neuroscience 2024, 538, 1–10. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Zou, P.; Zong, X.; Zhang, Q. Myelin dysfunction in aging and brain disorders: Mechanisms and therapeutic opportunities. Mol. Neurodegener. 2025, 20, 69. [Google Scholar] [CrossRef]
- Rao, V.T.S.; Khan, D.; Cui, Q.L.; Fuh, S.C.; Hossain, S.; Almazan, G.; Multhaup, G.; Healy, L.M.; Kennedy, T.E.; Antel, J.P. Distinct age and differentiation-state dependent metabolic profiles of oligodendrocytes under optimal and stress conditions. PLoS ONE 2017, 12, e0182372. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Kapoor, R.; Felts, P.A. Demyelination: The role of reactive oxygen and nitrogen species. Brain Pathol. 1999, 9, 69–92. [Google Scholar] [CrossRef]
- Fernandes, M.G.F.; Luo, J.X.X.; Cui, Q.L.; Perlman, K.; Pernin, F.; Yaqubi, M.; Hall, J.A.; Dudley, R.; Srour, M.; Couturier, C.P.; et al. Age-related injury responses of human oligodendrocytes to metabolic insults: Link to BCL-2 and autophagy pathways. Commun. Biol. 2021, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.W.; Kamen, Y.; Piedra, E.T.; Hill, R.A. Oligodendrocyte Maturation Alters the Cell Death Mechanisms That Cause Demyelination. J. Neurosci. 2024, 44, e1794232024. [Google Scholar] [CrossRef]
- Rivera, A.; Azim, K.; Butt, A. Oligodendroglial changes in ageing human white matter. Ageing Longev. 2025, 6, 144–151. [Google Scholar] [CrossRef]
- Lin, W.; Harding, H.P.; Ron, D.; Popko, B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J. Cell Biol. 2005, 169, 603–612. [Google Scholar] [CrossRef]
- Leenders, F.; Koole, L.; Slaets, H.; Tiane, A.; Hove, D.V.D.; Vanmierlo, T. Navigating oligodendrocyte precursor cell aging in brain health. Mech. Ageing Dev. 2024, 220, 111959. [Google Scholar] [CrossRef]
- Heo, D.; Kim, A.A.; Neumann, B.; Doze, V.N.; Xu, Y.K.T.; Mironova, Y.A.; Slosberg, J.; Goff, L.A.; Franklin, R.J.M.; Bergles, D.E. Transcriptional profiles of mouse oligodendrocyte precursor cells across the lifespan. Nat. Aging 2025, 5, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.A.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, F1000 Faculty Rev-998. [Google Scholar] [CrossRef]
- Fedder-Semmes, K.N.; Appel, B. The Akt-mTOR Pathway Drives Myelin Sheath Growth by Regulating Cap-Dependent Translation. J. Neurosci. 2021, 41, 8532–8544. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Pathological and Inflammatory Consequences of Aging. Biomolecules 2025, 15, 404. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakano, M.; Hisaoka-Nakashima, K.; Morioka, N. Aging-related dysregulation of energy metabolism and mitochondrial dynamics in microglia. J. Clin. Biochem. Nutr. 2025, 76, 239–244. [Google Scholar] [CrossRef]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef]
- Hanslik, K.L.; Ulland, T.K. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer’s Disease. Front. Neurol. 2020, 11, 570711. [Google Scholar] [CrossRef]
- Jagust, W.J.; Landau, S.M.; Alzheimer’s Disease Neuroimaging Initiative. Temporal Dynamics of β-Amyloid Accumulation in Aging and Alzheimer Disease. Neurology 2021, 96, e1347–e1357. [Google Scholar] [CrossRef] [PubMed]
- Alrouji, M.; Alshammari, M.S.; Tasqeeruddin, S.; Shamsi, A. Interplay Between Aging and Tau Pathology in Alzheimer’s Disease: Mechanisms and Translational Perspectives. Antioxidants 2025, 14, 774. [Google Scholar] [CrossRef] [PubMed]
- Hulse, J.; Bhaskar, K. Crosstalk Between the NLRP3 Inflammasome/ASC Speck and Amyloid Protein Aggregates Drives Disease Progression in Alzheimer’s and Parkinson’s Disease. Front. Mol. Neurosci. 2022, 15, 805169. [Google Scholar] [CrossRef] [PubMed]
- Friker, L.L.; Scheiblich, H.; Hochheiser, I.V.; Brinkschulte, R.; Riedel, D.; Latz, E.; Geyer, M.; Heneka, M.T. β-Amyloid Clustering around ASC Fibrils Boosts Its Toxicity in Microglia. Cell Rep. 2020, 30, 3743–3754.e6. [Google Scholar] [CrossRef]
- Wang, T.; Ruan, B.; Wang, J.; Zhou, Z.; Zhang, X.; Zhang, C.; Zhao, H.; Yang, Y.; Yuan, D. Activation of NLRP3-Caspase-1 pathway contributes to age-related impairments in cognitive function and synaptic plasticity. Neurochem. Int. 2022, 152, 105220. [Google Scholar] [CrossRef]
- Hu, M.Y.; Lin, Y.Y.; Zhang, B.J.; Lu, D.L.; Lu, Z.Q.; Cai, W. Update of inflammasome activation in microglia/macrophage in aging and aging-related disease. CNS Neurosci. Ther. 2019, 25, 1299–1307. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Sayed, W.M. Implication of JAK1/STAT3/SOCS3 Pathway in Aging of Cerebellum of Male Rat: Histological and Molecular study. Sci. Rep. 2020, 10, 8840. [Google Scholar] [CrossRef]
- Behfar, Q.; Ramirez Zuniga, A.; Martino-Adami, P.V. Aging, Senescence, and Dementia. J. Prev. Alzheimers Dis. 2022, 9, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.J.; Woods, G.; Rane, A.; Demaria, M.; Campisi, J.; Andersen, J.K. Cellular senescence and the aging brain. Exp. Gerontol. 2015, 68, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Petersen, R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018, 128, 1208–1216. [Google Scholar] [CrossRef]
- Meldolesi, J. Role of Senescent Astrocytes in Health and Disease. Int. J. Mol. Sci. 2023, 24, 8498. [Google Scholar] [CrossRef]
- Doyle, R.; Sadlier, D.M.; Godson, C. Pro-resolving lipid mediators: Agents of anti-ageing? Semin. Immunol. 2018, 40, 36–48. [Google Scholar] [CrossRef]
- Ponce, J.; Ulu, A.; Hanson, C.; Cameron-Smith, E.; Bertoni, J.; Wuebker, J.; Fisher, A.; Siu, K.C.; Marmelat, V.; Adamec, J.; et al. Role of Specialized Pro-resolving Mediators in Reducing Neuroinflammation in Neurodegenerative Disorders. Front. Aging Neurosci. 2022, 14, 780811. [Google Scholar] [CrossRef]
- Gaspar-Silva, F.; Trigo, D.; Magalhaes, J. Ageing in the brain: Mechanisms and rejuvenating strategies. Cell. Mol. Life Sci. 2023, 80, 190. [Google Scholar] [CrossRef]
- El Hiba, O.; Balzano, T.; Jayakumar, A.R. Editorial: Gliopathies in aging-related brain diseases: From understanding to therapy. Front. Neurosci. 2022, 16, 1017877. [Google Scholar] [CrossRef]
- Valiukas, Z.; Tangalakis, K.; Apostolopoulos, V.; Feehan, J. Microglial activation states and their implications for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2025, 12, 100013. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V.; Rodriguez-Arellano, J.J.; Zorec, R. Astroglia in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 273–324. [Google Scholar] [PubMed]
- Mallach, A.; Zielonka, M.; van Lieshout, V.; An, Y.; Khoo, J.H.; Vanheusden, M.; Chen, W.T.; Moechars, D.; Arancibia-Carcamo, I.L.; Fiers, M.; et al. Microglia-astrocyte crosstalk in the amyloid plaque niche of an Alzheimer’s disease mouse model, as revealed by spatial transcriptomics. Cell Rep. 2024, 43, 114216. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Nisa Awan, M.U.; Bai, J. The Mechanism and Function of Glia in Parkinson’s Disease. Front. Cell. Neurosci. 2022, 16, 903469. [Google Scholar] [CrossRef]
- Trainor, A.R.; MacDonald, D.S.; Penney, J. Microglia: Roles and genetic risk in Parkinson’s disease. Front. Neurosci. 2024, 18, 1506358. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Dettmer, U. Astrocytes in Parkinson’s Disease: From Role to Possible Intervention. Cells 2023, 12, 2336. [Google Scholar] [CrossRef]
- Barker, R.A.; Saarma, M.; Svendsen, C.N.; Morgan, C.; Whone, A.; Fiandaca, M.S.; Luz, M.; Bankiewicz, K.S.; Fiske, B.; Isaacs, L.; et al. Neurotrophic factors for Parkinson’s disease: Current status, progress, and remaining questions. Conclusions from a 2023 workshop. J. Park. Dis. 2024, 14, 1659–1676. [Google Scholar] [CrossRef]
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Mantovani, S.; Robertson, A.A.B.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10, eaah4066. [Google Scholar] [CrossRef]
- Lee, E.; Hwang, I.; Park, S.; Hong, S.; Hwang, B.; Cho, Y.; Son, J.; Yu, J.W. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 2019, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Dang, T.; Zhou, Y.; Zhou, Y.Z.; Zhao, R.; Wang, M. Targeting microglial NLRP3 in the SNc region as a promising disease-modifying therapy for Parkinson’s disease. Brain Behav. 2022, 12, e2784. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Zheng, R.; Liu, Y.; Ruan, Y.; Lin, Z.H.; Xue, N.J.; Chen, Y.; Zhang, B.R.; Pu, J.L. Parkin regulates microglial NLRP3 and represses neurodegeneration in Parkinson’s disease. Aging Cell 2023, 22, e13834. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. White Matter in Crisis: Oligodendrocytes and the Pathophysiology of Multiple Sclerosis. Cells 2025, 14, 1408. [Google Scholar] [CrossRef]
- Rawji, K.S.; Neumann, B.; Franklin, R.J.M. Glial aging and its impact on central nervous system myelin regeneration. Ann. N. Y. Acad. Sci. 2023, 1519, 34–45. [Google Scholar] [CrossRef]
- Rivellini, C.; Porrello, E.; Dina, G.; Mrakic-Sposta, S.; Vezzoli, A.; Bacigaluppi, M.; Gullotta, G.S.; Chaabane, L.; Leocani, L.; Marenna, S.; et al. JAB1 deletion in oligodendrocytes causes senescence-induced inflammation and neurodegeneration in mice. J. Clin. Investig. 2022, 132, e145071. [Google Scholar] [CrossRef] [PubMed]
- Kamermans, A.; Planting, K.E.; Jalink, K.; van Horssen, J.; de Vries, H.E. Reactive astrocytes in multiple sclerosis impair neuronal outgrowth through TRPM7-mediated chondroitin sulfate proteoglycan production. Glia 2019, 67, 68–77. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, X.; Liu, J. Neuroinflammation in Vascular Cognitive Impairment and Dementia: Current Evidence, Advances, and Prospects. Int. J. Mol. Sci. 2022, 23, 6224. [Google Scholar] [CrossRef]
- Valles, S.L.; Iradi, A.; Aldasoro, M.; Vila, J.M.; Aldasoro, C.; de la Torre, J.; Campos-Campos, J.; Jorda, A. Function of Glia in Aging and the Brain Diseases. Int. J. Med. Sci. 2019, 16, 1473–1479. [Google Scholar] [CrossRef]
- Paradise, M.B.; Naismith, S.L.; Norrie, L.M.; Graeber, M.B.; Hickie, I.B. The role of glia in late-life depression. Int. Psychogeriatr. 2012, 24, 1878–1890. [Google Scholar] [CrossRef]
- Stockmeier, C.A.; Rajkowska, G. Cellular abnormalities in depression: Evidence from postmortem brain tissue. Dialogues Clin. Neurosci. 2004, 6, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Khundakar, A.A.; Thomas, A.J. Morphometric changes in early- and late-life major depressive disorder: Evidence from postmortem studies. Int. Psychogeriatr. 2009, 21, 844–854. [Google Scholar] [CrossRef]
- Khundakar, A.A.; Thomas, A.J. Cellular morphometry in late-life depression: A review of postmortem studies. Am. J. Geriatr. Psychiatry 2014, 22, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Molecular mechanism of caloric restriction mimetics-mediated neuroprotection of age-related neurodegenerative diseases: An emerging therapeutic approach. Biogerontology 2023, 24, 679–708. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, J.; Montgomery, A.; Grossberg, G. Intermittent fasting and neurocognitive disorders: What the evidence shows. J. Nutr. Health Aging 2025, 29, 100480. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, H.; Ding, N.; Li, X.; Chen, X.; Chen, Z. Beneficial Effects on Brain Micro-Environment by Caloric Restriction in Alleviating Neurodegenerative Diseases and Brain Aging. Front. Physiol. 2021, 12, 715443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, M.; Chen, W.; Liu, Z. The neuroprotective effects of intermittent fasting on brain aging and neurodegenerative diseases via regulating mitochondrial function. Free Radic. Biol. Med. 2022, 182, 206–218. [Google Scholar] [CrossRef]
- Olmedillas Del Moral, M.; Fröhlich, N.; Figarella, K.; Mojtahedi, N.; Garaschuk, O. Effect of Caloric Restriction on the in vivo Functional Properties of Aging Microglia. Front. Immunol. 2020, 11, 750. [Google Scholar] [CrossRef]
- Hein, Z.M.; Arbain, M.F.F.; Kumar, S.; Mehat, M.Z.; Hamid, H.A.; Che Ramli, M.D.; Che Mohd Nassir, C.M.N. Intermittent Fasting as a Neuroprotective Strategy: Gut–Brain Axis Modulation and Metabolic Reprogramming in Neurodegenerative Disorders. Nutrients 2025, 17, 2266. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Y.; Zhao, X. The Effects and Mechanisms of n-3 and n-6 Polyunsaturated Fatty Acids in the Central Nervous System. Cell. Mol. Neurobiol. 2025, 45, 25. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Cole, R.M.; Deems, N.P.; Belury, M.A.; Barrientos, R.M. Fatty food, fatty acids, and microglial priming in the adult and aged hippocampus and amygdala. Brain Behav. Immun. 2020, 89, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, A.; Flowers, A.; Hudson, C.; Chaput, D.; Guergues, J.; Stevens, S.M., Jr.; Bickford, P.C. Polyphenol Supplementation Reverses Age-Related Changes in Microglial Signaling Cascades. Int. J. Mol. Sci. 2021, 22, 6373. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, M.; Tu, X.; Mo, X.; Zhang, L.; Yang, B.; Wang, F.; Kim, Y.-B.; Huang, C.; Chen, L.; et al. Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients 2024, 16, 3305. [Google Scholar] [CrossRef]
- Pereira, Q.C.; Dos Santos, T.W.; Fortunato, I.M.; Ribeiro, M.L. The Molecular Mechanism of Polyphenols in the Regulation of Ageing Hallmarks. Int. J. Mol. Sci. 2023, 24, 5508. [Google Scholar] [CrossRef]
- Jalouli, M.; Rahman, M.A.; Biswas, P.; Rahman, H.; Harrath, A.H.; Lee, I.S.; Kang, S.; Choi, J.; Park, M.N.; Kim, B. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: A comprehensive review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Ceccarelli, V.; Codini, M.; Fettucciari, K.; Calvitti, M.; Cataldi, S.; Albi, E.; Vecchini, A.; Beccari, T. The Polyunsaturated Fatty Acid EPA, but Not DHA, Enhances Neurotrophic Factor Expression through Epigenetic Mechanisms and Protects against Parkinsonian Neuronal Cell Death. Int. J. Mol. Sci. 2022, 23, 16176. [Google Scholar] [CrossRef]
- Maugeri, G.; Amato, A.; Evola, G.; D’Agata, V.; Musumeci, G. Addressing the Effect of Exercise on Glial Cells: Focus on Ependymal Cells. J. Integr. Neurosci. 2024, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Corrigan, F.; Baune, B.T. Effects of physical exercise on central nervous system functions: A review of brain region specific adaptations. J. Mol. Psychiatry 2015, 3, 3. [Google Scholar] [CrossRef]
- Ferrer-Uris, B.; Ramos, M.A.; Busquets, A.; Angulo-Barroso, R. Can exercise shape your brain? A review of aerobic exercise effects on cognitive function and neuro-physiological underpinning mechanisms. AIMS Neurosci. 2022, 9, 150–174. [Google Scholar] [CrossRef]
- Zare, N.; Bishop, D.J.; Levinger, I.; Febbraio, M.A.; Broatch, J.R. Exercise intensity matters: A review on evaluating the effects of aerobic exercise intensity on muscle-derived neuroprotective myokines. Alzheimers Dement. 2025, 11, e70056. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, M.; Saadat, M.; Ghorbanpour, A.M.; Moradikor, N. Experimental and clinical evidence of physical exercise on BDNF and cognitive function: A comprehensive review from molecular basis to therapy. Brain Behav. Immun. Integr. 2023, 3, 100017. [Google Scholar] [CrossRef]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef]
- Strohm, A.O.; Majewska, A.K. Physical exercise regulates microglia in health and disease. Front. Neurosci. 2024, 18, 1420322. [Google Scholar] [CrossRef]
- Matsui, T.; Omuro, H.; Liu, Y.F.; Soya, M.; Shima, T.; McEwen, B.S.; Soya, H. Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proc. Natl. Acad. Sci. USA 2017, 114, 6358–6363. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Agata, V.; Musumeci, G. Role of exercise in the brain: Focus on oligodendrocytes and remyelination. Neural Regen. Res. 2023, 18, 2645–2646. [Google Scholar] [CrossRef]
- Graciani, A.L.; Gutierre, M.U.; Coppi, A.A.; Arida, R.M.; Gutierre, R.C. Myelin, aging, and physical exercise. Neurobiol. Aging 2023, 127, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.H.; Tobiume, M.; Re, D.B.; Kariya, S. Physical Exercise Counteracts Aging-Associated White Matter Demyelination Causing Cognitive Decline. Aging Dis. 2024, 15, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chao, F.L.; Lu, W.; Zhang, L.; Huang, C.X.; Yang, S.; Qiu, X.; Yang, H.; Zhao, Y.Y.; Wang, S.R.; et al. Long-Term Running Exercise Delays Age-Related Changes in White Matter in Rats. Front. Aging Neurosci. 2020, 12, 590530. [Google Scholar] [CrossRef]
- Qiu, D.; Zhou, S.; Donnelly, J.; Xia, D.; Zhao, L. Aerobic exercise attenuates abnormal myelination and oligodendrocyte differentiation in 3xTg-AD mice. Exp. Gerontol. 2023, 182, 112293. [Google Scholar] [CrossRef]
- Norling, A.M.; Lipsitz, L.A. Exercise to Mitigate Cerebrovascular Aging: A Geroscience Perspective. J. Gerontol. A. Biol. Sci. Med. Sci. 2024, 79, glae083. [Google Scholar] [CrossRef]
- Kumar, A. Editorial: Neuroinflammation and Cognition. Front. Aging Neurosci. 2018, 10, 413. [Google Scholar] [CrossRef]
- Wixey, J.A.; Sukumar, K.R.; Pretorius, R.; Lee, K.M.; Colditz, P.B.; Bjorkman, S.T.; Chand, K.K. Ibuprofen Treatment Reduces the Neuroinflammatory Response and Associated Neuronal and White Matter Impairment in the Growth Restricted Newborn. Front. Physiol. 2019, 10, 541. [Google Scholar] [CrossRef]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Hanke, A.; Dewachter, I.; Kuiperi, C.; O’Banion, K.; Klockgether, T.; Van Leuven, F.; Landreth, G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain 2005, 128, 1442–1453. [Google Scholar] [CrossRef]
- Sekiyama, K.; Fujita, M.; Sekigawa, A.; Takamatsu, Y.; Waragai, M.; Takenouchi, T.; Sugama, S.; Hashimoto, M. Ibuprofen ameliorates protein aggregation and astrocytic gliosis, but not cognitive dysfunction, in a transgenic mouse expressing dementia with Lewy bodies-linked P123H β-synuclein. Neurosci. Lett. 2012, 515, 97–101. [Google Scholar] [CrossRef]
- Elsisi, N.S.; Darling-Reed, S.; Lee, E.Y.; Oriaku, E.T.; Soliman, K.F. Ibuprofen and apigenin induce apoptosis and cell cycle arrest in activated microglia. Neurosci. Lett. 2005, 375, 91–96. [Google Scholar] [CrossRef]
- Costa, T.; Fernandez-Villalba, E.; Izura, V.; Lucas-Ochoa, A.M.; Menezes-Filho, N.J.; Santana, R.C.; de Oliveira, M.D.; Araújo, F.M.; Estrada, C.; Silva, V.; et al. Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. J. Neuroimmune Pharmacol. 2021, 16, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Márquez Loza, A.; Elias, V.; Wong, C.P.; Ho, E.; Bermudez, M.; Magnusson, K.R. Effects of ibuprofen on cognition and NMDA receptor subunit expression across aging. Neuroscience 2017, 344, 276–292. [Google Scholar] [CrossRef]
- Rogers, J.T.; Liu, C.C.; Zhao, N.; Wang, J.; Putzke, T.; Yang, L.; Shinohara, M.; Fryer, J.D.; Kanekiyo, T.; Bu, G. Subacute ibuprofen treatment rescues the synaptic and cognitive deficits in advanced-aged mice. Neurobiol. Aging 2017, 53, 112–121. [Google Scholar] [CrossRef]
- Hou, B.; Yin, J.; Liu, S.; Guo, J.; Zhang, B.; Zhang, Z.; Yang, L.; Tan, X.; Long, Y.; Feng, S.; et al. Inhibiting the NLRP3 Inflammasome with MCC950 Alleviates Neurological Impairment in the Brain of EAE Mice. Mol. Neurobiol. 2024, 61, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Yu, X.; Li, D.; Liu, N.; Xue, X.; Fu, J. Periventricular Microglia Polarization and Morphological Changes Accompany NLRP3 Inflammasome-Mediated Neuroinflammation after Hypoxic-Ischemic White Matter Damage in Premature Rats. J. Immunol. Res. 2023, 2023, 5149306. [Google Scholar] [CrossRef] [PubMed]
- Talley, S.; Nguyen, T.; Van Ye, L.; Valiauga, R.; DeCarlo, J.; Mustafa, J.; Cook, B.; White, F.A.; Campbell, E.M. Characterization of age-associated inflammasome activation reveals tissue specific differences in transcriptional and post-translational inflammatory responses. Immun. Ageing 2024, 21, 60. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Qin, D.; Chen, H.; Wang, J.; Wang, J.; Song, S.; Wang, C.; Wang, Y.; Liu, S.; et al. The role of brain derived neurotrophic factor in central nervous system. Front. Aging Neurosci. 2022, 14, 986443. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, C.F.; Andressoo, J.O. Biology of GDNF and its receptors—Relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef]
- Hayes, C.A.; Wilson, D.; De Leon, M.A.; Mustapha, M.J.; Morales, S.; Odden, M.C.; Ashpole, N.M. Insulin-like growth factor-1 and cognitive health: Exploring cellular, preclinical, and clinical dimensions. Front. Neuroendocrinol. 2025, 76, 101161. [Google Scholar] [CrossRef]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflug. Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Zota, I.; Chanoumidou, K.; Gravanis, A.; Charalampopoulos, I. Stimulating myelin restoration with BDNF: A promising therapeutic approach for Alzheimer’s disease. Front. Cell. Neurosci. 2024, 18, 1422130. [Google Scholar] [CrossRef]
- Xiao, J.; Wong, A.W.; Willingham, M.M.; van den Buuse, M.; Kilpatrick, T.J.; Murray, S.S. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals 2010, 18, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.J.; Zhang, M.; Chen, S.; Chen, W.F. Anti-inflammatory effect of IGF-1 is mediated by IGF-1R cross talk with GPER in MPTP/MPP+-induced astrocyte activation. Mol. Cell. Endocrinol. 2021, 519, 111053. [Google Scholar] [CrossRef]
- Rusin, D.; Vahl Becirovic, L.; Lyszczarz, G.; Krueger, M.; Benmamar-Badel, A.; Vad Mathiesen, C.; Sigurðardóttir Schiöth, E.; Lykke Lambertsen, K.; Wlodarczyk, A. Microglia-Derived Insulin-like Growth Factor 1 Is Critical for Neurodevelopment. Cells 2024, 13, 184. [Google Scholar] [CrossRef]
- Sonntag, W.E.; Deak, F.; Ashpole, N.; Toth, P.; Csiszar, A.; Freeman, W.; Ungvari, Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front. Aging Neurosci. 2013, 5, 27. [Google Scholar] [CrossRef]
- Xie, L.; Fang, Q.; Wei, X.; Zhou, L.; Wang, S. Exogenous insulin-like growth factor 1 attenuates sevoflurane anesthesia-induced cognitive dysfunction in aged rats. J. Neurophysiol. 2021, 125, 2117–2124. [Google Scholar] [CrossRef]
- Pinto-Benito, D.; Paradela-Leal, C.; Ganchala, D.; de Castro-Molina, P.; Arevalo, M.A. IGF-1 regulates astrocytic phagocytosis and inflammation through the p110α isoform of PI3K in a sex-specific manner. Glia 2022, 70, 1153–1169. [Google Scholar] [CrossRef]
- Pan, L.; Cho, K.S.; Wei, X.; Xu, F.; Lennikov, A.; Hu, G.; Tang, J.; Guo, S.; Chen, J.; Kriukov, E.; et al. IGFBPL1 is a master driver of microglia homeostasis and resolution of neuroinflammation in glaucoma and brain tauopathy. Cell Rep. 2023, 42, 112889. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell. Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef]
- Kramer, E.R.; Liss, B. GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett. 2015, 589, 3760–3772. [Google Scholar] [CrossRef]
- Rickert, U.; Grampp, S.; Wilms, H.; Spreu, J.; Knerlich-Lukoschus, F.; Held-Feindt, J.; Lucius, R. Glial Cell Line-Derived Neurotrophic Factor Family Members Reduce Microglial Activation via Inhibiting p38MAPKs-Mediated Inflammatory Responses. J. Neurodegener. Dis. 2014, 2014, 369468. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, F.; Min, S.; Chen, J.; Yang, J.; Wang, X. Glial cell line-derived neurotrophic factor (GDNF) attenuates the peripheral neuromuscular dysfunction without inhibiting the activation of spinal microglia/monocyte. BMC Geriatr. 2018, 18, 110. [Google Scholar] [CrossRef]
- Flores, I.O.; Treviño, S.; Díaz, A. Neurotrophic fragments as therapeutic alternatives to ameliorate brain aging. Neural Regen. Res. 2023, 18, 51–56. [Google Scholar] [CrossRef]
- Zagrebelsky, M.; Korte, M. Are TrkB receptor agonists the right tool to fulfill the promises for a therapeutic value of the brain-derived neurotrophic factor? Neural Regen. Res. 2024, 19, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008, 10, 291–315. [Google Scholar] [CrossRef]
- Madsen, H.B.; Navarro, C.; Gasparini, E.; Park, J.H.; Li, Z.; Croteau, D.L.; Bohr, V.A. Urolithin A and nicotinamide riboside differentially regulate innate immune defenses and metabolism in human microglial cells. Front. Aging Neurosci. 2024, 16, 1503336. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Pan, X.; Chen, Q.; Wang, J.; Kong, X.; Li, Y.; Liu, H.; Hou, X.; Wang, Y. Nicotinamide mononucleotide mitigates neuroinflammation by enhancing GPX4-mediated ferroptosis defense in microglia. Brain Res. 2024, 1845, 149197. [Google Scholar] [CrossRef]
- Yusri, K.; Jose, S.; Vermeulen, K.S.; Tan, T.C.M.; Sorrentino, V. The role of NAD+ metabolism and its modulation of mitochondria in aging and disease. NPJ Metab. Health Dis. 2025, 3, 26. [Google Scholar] [CrossRef]
- Kodali, M.; Attaluri, S.; Madhu, L.N.; Shuai, B.; Upadhya, R.; Gonzalez, J.J.; Rao, X.; Shetty, A.K. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell 2021, 20, e13277. [Google Scholar] [CrossRef]
- Abou Zaki, R.; El-Osta, A. Metformin: Decelerates biomarkers of aging clocks. Signal Transduct. Target. Ther. 2024, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, L.; Makarczyk, M.J.; Feng, P.; Zhang, J. The Anti-Aging Mechanism of Metformin: From Molecular Insights to Clinical Applications. Molecules 2025, 30, 816. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.; Marcuzzi, A.; Gonelli, A.; Celeghini, C.; Maximova, N.; Rimondi, E. Mitochondria-Targeted Antioxidants, an Innovative Class of Antioxidant Compounds for Neurodegenerative Diseases: Perspectives and Limitations. Int. J. Mol. Sci. 2023, 24, 3739. [Google Scholar] [CrossRef]
- Tung, C.; Varzideh, F.; Farroni, E.; Mone, P.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Elamipretide: A Review of Its Structure, Mechanism of Action, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 944. [Google Scholar] [CrossRef]
- Chen, C.; Chan, A.; Wen, H.; Chung, S.H.; Deng, W.; Jiang, P. Stem and Progenitor Cell-Derived Astroglia Therapies for Neurological Diseases. Trends Mol. Med. 2015, 21, 715–729. [Google Scholar] [CrossRef]
- Zeldich, E.; Rajkumar, S. Identity and Maturity of iPSC-Derived Oligodendrocytes in 2D and Organoid Systems. Cells 2024, 13, 674. [Google Scholar] [CrossRef]
- Albert, K.; Niskanen, J.; Kälvälä, S.; Lehtonen, Š. Utilising Induced Pluripotent Stem Cells in Neurodegenerative Disease Research: Focus on Glia. Int. J. Mol. Sci. 2021, 22, 4334. [Google Scholar] [CrossRef]
- Mertens, J.; Reid, D.; Lau, S.; Kim, Y.; Gage, F.H. Aging in a Dish: iPSC-Derived and Directly Induced Neurons for Studying Brain Aging and Age-Related Neurodegenerative Diseases. Annu. Rev. Genet. 2018, 52, 271–293. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, X.J.; Fu, X.; Zhang, Y.; Zhan, Y.; Liu, J.; Zhao, L.; Xia, C.L. Engrafted primary type-2 astrocytes improve the recovery of the nigrostriatal pathway in a rat model of Parkinson’s disease. Mol. Cell. Biochem. 2021, 476, 619–631. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, M.; Jian, T.; Li, J.; Yang, C.; Ma, Q.; Deng, P.; Wang, Y.; Huang, M.; Wang, H.; et al. Engrafted glial progenitor cells yield long-term integration and sensory improvement in aged mice. Stem Cell Res. Ther. 2022, 13, 285. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Qi, Y.B.; Gao, Y.N.; Chen, W.G.; Zhou, T.; Zang, Y.; Li, J. Astrocyte metabolism and signaling pathways in the CNS. Front. Neurosci. 2023, 17, 1217451. [Google Scholar] [CrossRef] [PubMed]
- Roumes, H.; Pellerin, L.; Bouzier-Sore, A.K. Astrocytes as metabolic suppliers to support neuronal activity and brain functions. Essays Biochem. 2023, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Mozafari, S.; Glatza, M.; Starost, L.; Velychko, S.; Hallmann, A.L.; Cui, Q.L.; Schambach, A.; Kim, K.P.; Bachelin, C.; et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl. Acad. Sci. USA 2017, 114, E2243–E2252. [Google Scholar] [CrossRef] [PubMed]
- Stöberl, N.; Maguire, E.; Salis, E.; Shaw, B.; Hall-Roberts, H. Human iPSC-derived glia models for the study of neuroinflammation. J. Neuroinflamm. 2023, 20, 231. [Google Scholar] [CrossRef]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef]
- Hardison, J.L.; Nistor, G.; Gonzalez, R.; Keirstead, H.S.; Lane, T.E. Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response. Exp. Neurol. 2006, 197, 420–429. [Google Scholar] [CrossRef]
- Vieira, R.; Mariani, J.N.; Huynh, N.P.T.; Stephensen, H.J.T.; Solly, R.; Tate, A.; Schanz, S.; Cotrupi, N.; Mousaei, M.; Sporring, J.; et al. Young glial progenitor cells competitively replace aged and diseased human glia in the adult chimeric mouse brain. Nat. Biotechnol. 2024, 42, 719–730. [Google Scholar] [CrossRef]
- Quan, F.S.; Chen, J.; Zhong, Y.; Ren, W.Z. Comparative effect of immature neuronal or glial cell transplantation on motor functional recovery following experimental traumatic brain injury in rats. Exp. Ther. Med. 2016, 12, 1671–1680. [Google Scholar] [CrossRef][Green Version]
- Hébert, J.M.; Vijg, J. Cell Replacement to Reverse Brain Aging: Challenges, Pitfalls, and Opportunities. Trends Neurosci. 2018, 41, 267–279. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Cook, W.H.; Young, D. AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front. Mol. Neurosci. 2021, 13, 618020. [Google Scholar] [CrossRef] [PubMed]
- Martier, R.; Konstantinova, P. Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock. Front. Neurosci. 2020, 14, 580179. [Google Scholar] [CrossRef] [PubMed]
- Katsu-Jiménez, Y.; Loría, F.; Corona, J.C.; Díaz-Nido, J. Gene Transfer of Brain-derived Neurotrophic Factor (BDNF) Prevents Neurodegeneration Triggered by FXN Deficiency. Mol. Ther. 2016, 24, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Revilla, S.; Ursulet, S.; Álvarez-López, M.J.; Castro-Freire, M.; Perpiñá, U.; García-Mesa, Y.; Bortolozzi, A.; Giménez-Llort, L.; Kaliman, P.; Cristòfol, R.; et al. Lenti-GDNF gene therapy protects against Alzheimer’s disease-like neuropathology in 3xTg-AD mice and MC65 cells. CNS Neurosci. Ther. 2014, 20, 961–972. [Google Scholar] [CrossRef]
- Dolcetti, F.J.C.; Falomir-Lockhart, E.; Acuña, F.; Herrera, M.L.; Cervellini, S.; Barbeito, C.G.; Grassi, D.; Arevalo, M.A.; Bellini, M.J. IGF1 gene therapy in middle-aged female rats delays reproductive senescence through its effects on hypothalamic GnRH and kisspeptin neurons. Aging 2022, 14, 8615–8632. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, I.S.; Choi, S.G.; Lee, K.; Park, H.; Shin, J.; Kim, D.; Beom, J.; Yi, Y.Y.; Gupta, D.P.; et al. Rejuvenating aged microglia by p16ink4a-siRNA-loaded nanoparticles increases amyloid-β clearance in animal models of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 25. [Google Scholar] [CrossRef]
- Barragán-Álvarez, C.P.; Flores-Fernandez, J.M.; Hernández-Pérez, O.R.; Ávila-Gónzalez, D.; Díaz, N.F.; Padilla-Camberos, E.; Dublan-García, O.; Gómez-Oliván, L.M.; Diaz-Martinez, N.E. Recent advances in the use of CRISPR/Cas for understanding the early development of molecular gaps in glial cells. Front. Cell Dev. Biol. 2022, 10, 947769. [Google Scholar] [CrossRef]
- DeVos, S.L.; Miller, T.M. Antisense oligonucleotides: Treating neurodegeneration at the level of RNA. Neurotherapeutics 2013, 10, 486–497. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Cheong, C.S.Y.; Verma, K.; Tencomnao, T.; Brimson, J.M.; Prasansuklab, A. Role of Epigenetic Modulation in Neurodegenerative Diseases: Implications of Phytochemical Interventions. Antioxidants 2024, 13, 606. [Google Scholar] [CrossRef]
- Soreq, L.; UK Brain Expression Consortium; North American Brain Expression Consortium; Rose, J.; Soreq, E.; Hardy, J.; Trabzuni, D.; Cookson, M.R.; Smith, C.; Ryten, M.; et al. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep. 2017, 18, 557–570. [Google Scholar] [CrossRef]
- Baltan, S. Histone deacetylase inhibitors preserve function in aging axons. J. Neurochem. 2012, 123 (Suppl. S2), 108–115. [Google Scholar] [CrossRef] [PubMed]
- Umlauf, B.J.; Shusta, E.V. Exploiting BBB disruption for the delivery of nanocarriers to the diseased CNS. Curr. Opin. Biotechnol. 2019, 60, 146–152. [Google Scholar] [CrossRef]
- Senanayake, D.; Yapa, P.; Dabare, S.; Munaweera, I. Precision targeting of the CNS: Recent progress in brain-directed nanodrug delivery. RSC Adv. 2025, 15, 25910–25928. [Google Scholar] [CrossRef]
- Saksena, J.; Hamilton, A.E.; Gilbert, R.J.; Zuidema, J.M. Nanomaterial payload delivery to central nervous system glia for neural protection and repair. Front. Cell. Neurosci. 2023, 17, 1266019. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood-brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Saha, S.; Sachivkina, N.; Karamyan, A.; Novikova, E.; Chubenko, T. Advances in Nrf2 Signaling Pathway by Targeted Nanostructured-Based Drug Delivery Systems. Biomedicines 2024, 12, 403. [Google Scholar] [CrossRef]
- Costa, M.F.D.; Rösler, T.W.; Höglinger, G.U. Exploring the neuroprotective potential of Nrf2-pathway activators against annonacin toxicity. Sci. Rep. 2024, 14, 20123. [Google Scholar] [CrossRef]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies that Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; De, R.; Lee, K.T. Emerging strategies to fabricate polymeric nanocarriers for enhanced drug delivery across blood-brain barrier: An overview. Adv. Colloid Interface Sci. 2023, 320, 103008. [Google Scholar] [CrossRef]
- Romero-Ben, E.; Goswami, U.; Soto-Cruz, J.; Mansoori-Kermani, A.; Mishra, D.; Martin-Saldaña, S.; Muñoz-Ugartemendia, J.; Sosnik, A.; Calderón, M.; Beloqui, A.; et al. Polymer-based nanocarriers to transport therapeutic biomacromolecules across the blood-brain barrier. Acta Biomater. 2025, 196, 17–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).