Therapy for Dupuytren’s Disease (II): Collagenase Therapy vs. Limited Fasciectomy—A Long-Term Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Collagenase
2.2. Open Surgical Limited Fasciectomy
2.3. Follow-Up Examination
2.4. Questionnaires/Patient-Reported Outcome Measures (PROMs)
2.5. Statistical Analysis
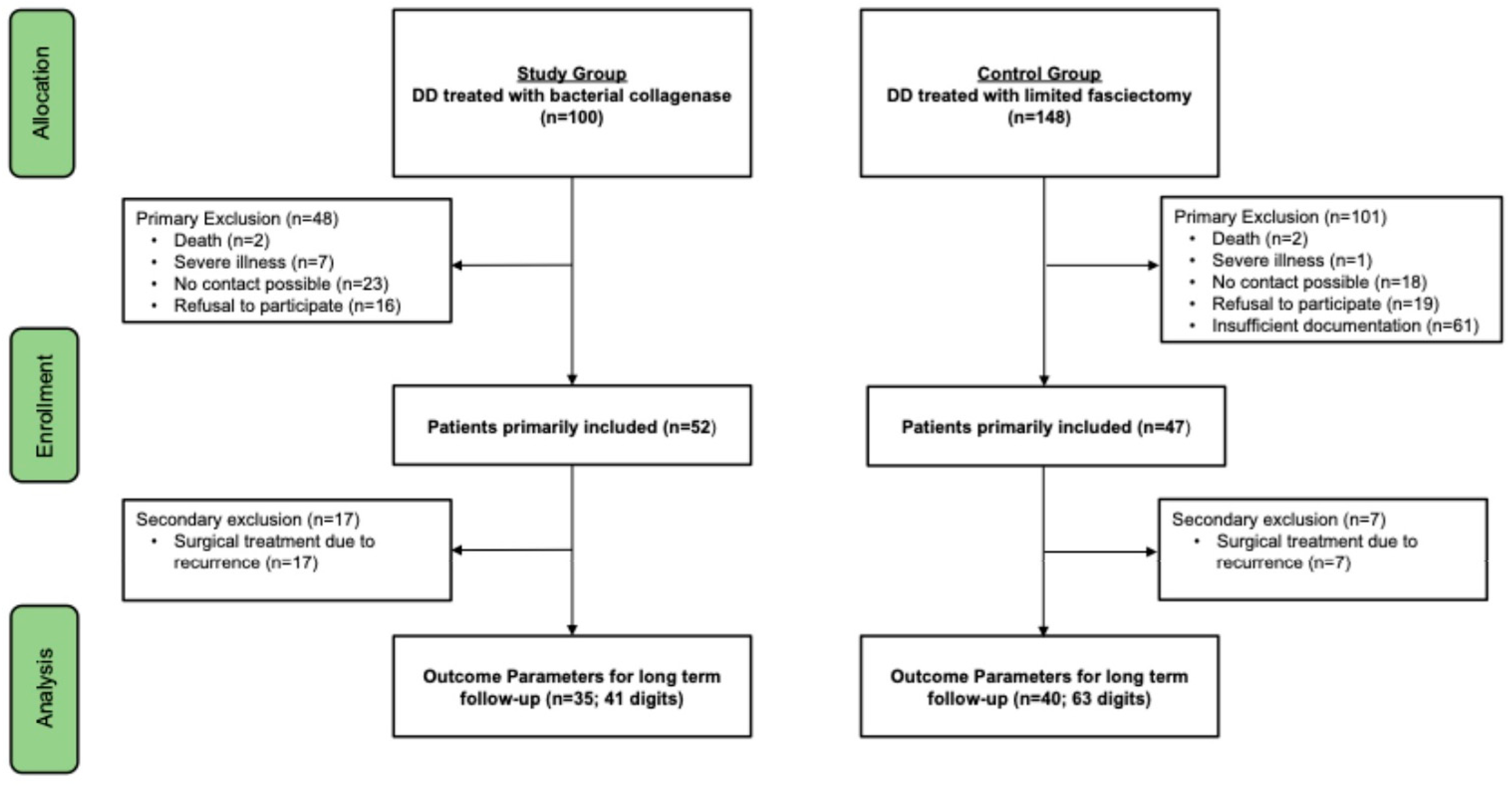
3. Results
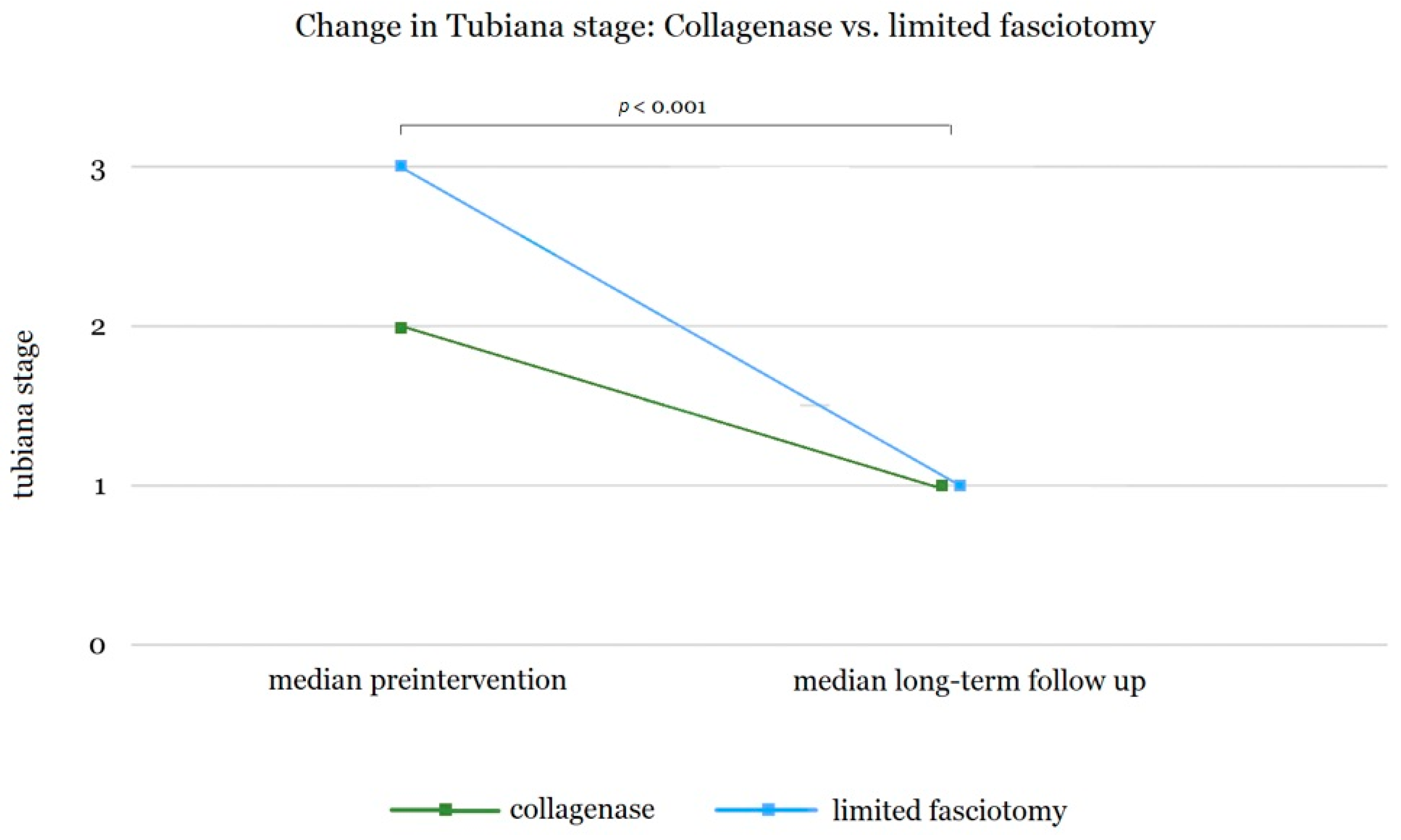
3.1. Comparison of Long-Term Outcomes of Collagenase Therapy and Limited Fasciectomy
3.2. Strength and Sensitivity of the Hand
3.3. Patient-Reported Outcome Measures (PROMs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belusa, L.; Selzer, A.M.; Partecke, B.D. Description of Dupuytren disease by the Basel physician and anatomist Felix Plater in 1614. Handchir. Mikrochir. Plast. Chir. 1995, 27, 272–275. [Google Scholar]
- McFarlane, R.M. On the origin and spread of Dupuytren’s disease. J. Hand Surg. Am. 2002, 27, 385–390. [Google Scholar] [CrossRef]
- Barsky, H.K. Guillaume Dupuytren: A Surgeon in His Place and Time; Vantage Press: New York, NY, USA, 1984. [Google Scholar]
- Wolfe, S.W.; Pederson, W.C.; Kozin, S.H.; Cohen, M.S. Green’s Operative Hand Surgery, 7th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2017; Volume 1. [Google Scholar]
- Lanting, R.; Broekstra, D.C.; Werker, P.M.N.; van den Heuvel, E.R. A systematic review and meta-analysis on the prevalence of Dupuytren disease in the general population of Western countries. Plast. Reconstr. Surg. 2014, 133, 593–603. [Google Scholar] [CrossRef]
- Wilbrand, S.; Ekbom, A.; Gerdin, B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J. Hand Surg. Br. 1999, 24, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Diep, G.K.; Agel, J.; Adams, J.E. Prevalence of palmar fibromatosis with and without contracture in asymptomatic patients. J. Plast. Surg. Hand Surg. 2015, 49, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.S.; Hentz, V.R. The treatment of Dupuytren disease. J. Hand Surg. Am. 2011, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Henry, M. Dupuytren’s disease: Current state of the art. Hand 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Oppermann, J.; Unglaub, F.; Muller, L.P.; Low, S.; Hahn, P.; Spies, C.K. Percutaneous needle aponeurotomy for Dupuytren’s contracture. Orthopade 2017, 46, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Vesper, U.S.; Mehling, I.M.; Arsalan-Werner, A.; Sauerbier, M. Primary intervention in Dupuytren’s disease. Orthopade 2017, 46, 336–341. [Google Scholar] [CrossRef]
- Chen, N.C.; Srinivasan, R.C.; Shauver, M.J.; Chung, K.C. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand 2011, 6, 250–255. [Google Scholar] [CrossRef]
- Ruettermann, M.; Hermann, R.M.; Khatib-Chahidi, K.; Werker, P.M.N. Dupuytren’s Disease-Etiology and Treatment. Dtsch. Arztebl. Int. 2021, 118, 781–788. [Google Scholar] [CrossRef]
- Spies, C.K.; Muller, L.P.; Skouras, E.; Bassemir, D.; Hahn, P.; Unglaub, F. Percutaneous needle aponeurotomy for Dupuytren’s disease. Oper. Orthop. Traumatol. 2016, 28, 12–19. [Google Scholar] [CrossRef]
- Jorgensen, R.W.; Jensen, C.H.; Jorring, S. Three-Year Recurrence of Dupuytren Contracture after Needle Fasciotomy or Collagenase Injection: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2023, 151, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Holzer, L.A.; Holzer, G. Collagenase Clostridum histolyticum in the management of Dupuytren’s contracture. Handchir. Mikrochir. Plast. Chir. 2011, 43, 269–274. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Xiapex EPAR (European Public Assessment Report) Product Information In European Medicines Agency. Available online: https://ec.europa.eu/health/documents/community-register/2015/20151123133365/anx_133365_en.pdf (accessed on 7 January 2025).
- Keller, M.; Arora, R.; Schmiedle, G.; Kastenberger, T. Treatment of Dupuytren’s disease with collagenase Clostridium histolyticum. Orthopade 2017, 46, 321–327. [Google Scholar] [CrossRef]
- Müller, A. Pfizer: Vorläufig kein Xiapex für deutsche Patienten. Pharmazeutische Zeitung. ABDA—Bundesvereinigung Deutscher Apothekerverbände e. V., Berlin. 2012. Available online: https://www.pharmazeutische-zeitung.de/2012-05/pfizer-vorlaeufig-kein-xiapex-fuer-deutsche-patienten/#:~:text=Pfizer%20wird%20sein%20neues%20Medikament,Qualit%C3%A4t%20im%20Gesundheitswesen%20(IQWiG) (accessed on 7 January 2025).
- Passiatore, M.; De Vitis, R.; Taccardo, G. Xiapex will no longer be distributed in Europe: Our concerns and our hopes relative to collagenase. Hand Surg. Rehabil. 2020, 39, 466. [Google Scholar] [CrossRef]
- Endo, Inc. XIAFLEX®—The Only FDA-Approved Nonsurgical Treatment for Dupuytren’s Contracture When a “Cord” Can Be Felt. Available online: https://dupuytrens-contracture.xiaflex.com/patient/ (accessed on 7 January 2025).
- Wachtel, N.; Dingler, F.R.; Nurnberger, T.; Vollbach, F.H.; Moellhoff, N.; Giunta, R.; Demmer, W. Therapy for Dupuytren’s Disease: Collagenase Therapy-A Long-Term Follow-Up Study. Life 2024, 14, 1275. [Google Scholar] [CrossRef] [PubMed]
- Passiatore, M.; Cilli, V.; Cannella, A.; Caruso, L.; Sassara, G.M.; Taccardo, G.; De Vitis, R. Long-term assessment of collagenase treatment for Dupuytren’s contracture: A 10-year follow-up study. World J. Orthop. 2024, 15, 355–362. [Google Scholar] [CrossRef]
- Steppe, C.; Cinclair, R.; Lies, S. A 10-Year Review of Collagenase Versus Fasciectomy in the Treatment of Dupuytren Contracture. Ann. Plast. Surg. 2024, 92, 642–646. [Google Scholar] [CrossRef]
- Pototschnig, A.; Volkmer, E.; Giunta, R.E. [Treatment of Dupuytren’s disease with collagenase injections in Germany: Efficacy and adverse effects in 110 treated joints]. Handchir. Mikrochir. Plast. Chir. 2017, 49, 154–161. [Google Scholar] [CrossRef]
- Gajdosik, R.L.; Bohannon, R.W. Clinical measurement of range of motion. Review of goniometry emphasizing reliability and validity. Phys. Ther. 1987, 67, 1867–1872. [Google Scholar] [CrossRef]
- Demmer, W.; Meyer, E.; Ehrl, D.; Volkmer, E.; Lukas, B.; Knie, N.F.; Giunta, R.E.; Wachtel, N. Postoperative Benefits of Soft Tissue Wrist Arthroscopy: Retro- and Prospective Analyses of Outcome Measures. J. Clin. Med. 2024, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, V.; Weber, K.; Volland, G.; Kashman, N. Reliability and validity of grip and pinch strength evaluations. J. Hand Surg. Am. 1984, 9, 222–226. [Google Scholar] [CrossRef]
- Dellon, A.L.; Mackinnon, S.E.; Crosby, P.M. Reliability of two-point discrimination measurements. J. Hand Surg. Am. 1987, 12, 693–696. [Google Scholar] [CrossRef]
- Towfigh, H. Handchirurgie; Springer: Heidelberg, Germany, 2011. [Google Scholar]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- Williams, N. Dash. Occup. Med. 2014, 64, 67–68. [Google Scholar] [CrossRef]
- Sanjuan-Cervero, R.; Gomez-Herrero, D.; Vazquez-Ferreiro, P.; Sanjuan-Arago, A.; Poquet-Jornet, J.E.; Carrer-Hueso, J. Sensitivity and Specificity of the Unite Rhumatologique Des Affections De La Main (URAM) Scale for Dupuytren Contracture: A Systematic Review and Meta-Analyses. Cureus 2022, 14, e21636. [Google Scholar] [CrossRef] [PubMed]
- Beaudreuil, J.; Allard, A.; Zerkak, D.; Gerber, R.A.; Cappelleri, J.C.; Quintero, N.; Lasbleiz, S.; Bernabe, B.; Orcel, P.; Bardin, T.; et al. Unite Rhumatologique des Affections de la Main (URAM) scale: Development and validation of a tool to assess Dupuytren’s disease-specific disability. Arthritis Care Res 2011, 63, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.C.; Badalamente, M.A.; Hentz, V.R.; Hotchkiss, R.N.; Kaplan, F.T.; Meals, R.A.; Smith, T.M.; Rodzvilla, J.; Group, C.I.S. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N. Engl. J. Med. 2009, 361, 968–979. [Google Scholar] [CrossRef]
- Arora, R.; Kaiser, P.; Kastenberger, T.J.; Schmiedle, G.; Erhart, S.; Gabl, M. Injectable collagenase Clostridium histolyticum as a nonsurgical treatment for Dupuytren’s disease. Oper. Orthop. Traumatol. 2016, 28, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lauritzson, A.; Atroshi, I. Collagenase injections for Dupuytren’s disease: Prospective cohort study assessing 2-year treatment effect durability. BMJ Open 2017, 7, e012943. [Google Scholar] [CrossRef]
- Naam, N.H. Functional outcome of collagenase injections compared with fasciectomy in treatment of Dupuytren’s contracture. Hand 2013, 8, 410–416. [Google Scholar] [CrossRef]
- Murphy, L.E.; Murphy, K.M.; Kilpatrick, S.M.; Thompson, N.W. The use of Collagenase Clostridium Histolyticum in the management of Dupuytren’s contracture-outcomes of a pilot study in a District General Hospital setting. Ulster Med. J. 2017, 86, 94–98. [Google Scholar] [PubMed]
- Watt, A.J.; Curtin, C.M.; Hentz, V.R. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J. Hand Surg. Am. 2010, 35, 534–539.e531. [Google Scholar] [CrossRef]
- Werlinrud, J.C.; Hansen, K.L.; Larsen, S.; Lauritsen, J. Five-year results after collagenase treatment of Dupuytren disease. J. Hand Surg. Eur. Vol. 2018, 43, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Peimer, C.A.; Blazar, P.; Coleman, S.; Kaplan, F.T.; Smith, T.; Lindau, T. Dupuytren Contracture Recurrence Following Treatment With Collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-Year Data. J. Hand Surg. Am. 2015, 40, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Leclere, F.M.; Kohl, S.; Varonier, C.; Unglaub, F.; Vogelin, E. Range of motion, postoperative rehabilitation and patient satisfaction in MCP and PIP joints affected by Dupuytren Tubiana stage 1–3: Collagenase enzymatic fasciotomy or limited fasciectomy? A clinical study in 52 patients. Arch. Orthop. Trauma. Surg. 2018, 138, 1623–1631. [Google Scholar] [CrossRef]
- Hupez, A.D.C.; Van Innis, F.; Troussel, S.; Libouton, X.; Lequint, T. Comparative study of collagenase injections versus fasciectomy in dupuytren’s contracture: A 1-year follow-up. Louvain Médical 2017, 231–237. [Google Scholar]
- Thoma, A.; Murphy, J.; Gallo, L.; Ayeni, B.; Thabane, L. Randomized Controlled Trial Comparing the Clinical Effectiveness of Collagenase Injection (Xiaflex((R))) and Palmar Fasciectomy in the Management of Dupuytren’s Contracture. Plast Surg 2024, 32, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Harryson, M.; Eklund, M.; Arner, M.; Wilbrand, S. Patient-Reported Outcome in Dupuytren’s Disease Treated With Fasciectomy, Collagenase or Needle Fasciotomy: A Swedish Registry Study. J. Hand Surg. Glob. Online 2023, 5, 733–739. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, R.; Passiatore, M.; Perna, A.; Careri, S.; Cilli, V.; Taccardo, G. Seven-year clinical outcomes after collagenase injection in patients with Dupuytren’s disease: A prospective study. J. Orthop. 2020, 21, 218–222. [Google Scholar] [CrossRef]
- Clesham, K.; Sheridan, G.A.; Murphy, E.P.; SP, O.C.; ME, O.S. Collagenase and the Treatment of Dupuytren Contracture: Efficacy of Treatment and Patient Satisfaction. J. Hand Surg. Asian Pac. Vol. 2022, 27, 141–147. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yasunaga, H.; Kakinoki, R.; Tsubokawa, N.; Morita, A.; Tanaka, K.; Sakai, A.; Kurahashi, T.; Hirata, H.; Ce, C.J.s.G. The CeCORD-J study on collagenase injection versus aponeurectomy for Dupuytren’s contracture compared by hand function and cost effectiveness. Sci. Rep. 2022, 12, 9094. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Tharmanathan, P.; Arundel, C.; Welch, C.; Wu, Q.; Leighton, P.; Armaou, M.; Johnson, N.; James, S.; Cooke, J.; et al. Collagenase Injection versus Limited Fasciectomy for Dupuytren’s Contracture. N. Engl. J. Med. 2024, 391, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Alhebshi, Z.A.; Bamuqabel, A.O.; Alqurain, Z.; Dahlan, D.; Wasaya, H.I.; Al Saedi, Z.S.; Alqarni, G.S.; Alqarni, D.; Ghalimah, B. Comparing Complications and Patient Satisfaction Following Injectable Collagenase Versus Limited Fasciectomy for Dupuytren’s Disease: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e53147. [Google Scholar] [CrossRef] [PubMed]
- Beecher, S.M.; Wilkinson, J.E.; Cuggy, C.; O’Shaughnessy, M. Patient’s perspective of treatment in Dupuytren’s Disease: Collagenase versus limited fasciectomy. J. Hand Surg. Eur. Vol. 2022, 47, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Tharmanathan, P.; Arundel, C.; Welch, C.; Wu, Q.; Leighton, P.; Armaou, M.; Corbacho, B.; Johnson, N.; James, S.; et al. Collagenase injection versus limited fasciectomy surgery to treat Dupuytren’s contracture in adult patients in the UK: DISC, a non-inferiority RCT and economic evaluation. Health Technol. Assess. 2024, 28, 1–262. [Google Scholar] [CrossRef] [PubMed]
- Altziebler, J.; Hubmer, M.; Parvizi, D.; Spendel, S.; Rab, M.; Kamolz, L.-P. Dupuytren’s contracture: The status and impact of collagenase Clostridium histolyticum treatment in Austria. Saf. Health 2017, 3, 12. [Google Scholar] [CrossRef]
- Denkler, K.A.; Vaughn, C.J.; Dolan, E.L.; Hansen, S.L. Evidence-Based Medicine: Options for Dupuytren’s Contracture: Incise, Excise, and Dissolve. Plast. Reconstr. Surg. 2017, 139, 240e–255e. [Google Scholar] [CrossRef]
- Herrera, F.A.; Mitchell, S.; Elzik, M.; Roostaeian, J.; Benhaim, P. Modified percutaneous needle aponeurotomy for the treatment of dupuytren’s contracture: Early results and complications. Hand 2015, 10, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Obed, D.; Salim, M.; Schlottmann, F.; Bingoel, A.S.; Panayi, A.C.; Dastagir, K.; Vogt, P.M.; Koenneker, S. Short-term efficacy and adverse effects of collagenase clostridium histolyticum injections, percutaneous needle fasciotomy and limited fasciectomy in the treatment of Dupuytren’s contracture: A network meta-analysis of randomized controlled trials. BMC Musculoskelet. Disord. 2022, 23, 939. [Google Scholar] [CrossRef] [PubMed]
- Denkler, K. Surgical complications associated with fasciectomy for dupuytren’s disease: A 20-year review of the English literature. Eplasty 2010, 10, e15. [Google Scholar]
- van Rijssen, A.L.; Ter Linden, H.; Werker, P.M.N. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: Percutaneous needle fasciotomy versus limited fasciectomy. Plast. Reconstr. Surg. 2012, 129, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Bundesausschuss, G. Anlage XII—Beschlüsse über die Nutzenbewertung von Arzneimitteln mit neuen Wirkstoffen nach § 35a SGB V—Mikrobielle Collagenase aus Clostridium histolyticum. Bundesanzeiger. 2012. Available online: https://www.g-ba.de/downloads/39-261-1475/2012-04-19_AM-RL-XII_Collagenase_BAnz.pdf (accessed on 7 January 2025).
- Neuwirth, M.; Binter, A.; Pipam, W.; Rab, M. Comparative Cost Effectiveness of Clostridium Histolyticum Collagenase (Xiapex(R)) and Partial Fasciectomy for the Treatment of Dupuytren’s Contracture in Austria. Handchir. Mikrochir. Plast. Chir. 2016, 48, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Belcher, H.J. A single-centre cost comparison analysis of collagenase injection versus surgical fasciectomy for Dupuytren’s contracture of the hand. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 368–372. [Google Scholar] [CrossRef]

| Parameter | Possible Response |
|---|---|
| Pain and evaluation of therapy choice | |
| Subjective pain in the treated hand Subjective positive and negative aspects of the treatment In retrospect: same choice of therapy | yes/no open question yes/no |
| Collagenase * | Limited Fasciectomy | |
|---|---|---|
| Total | 35 | 40 |
| Male | 28 | 37 |
| Female | 7 | 3 |
| Age at the time of treatment (Mean ± SD) | 68 (8.4) | 67 (8.7) |
| Affected digits | ||
| Total | 41 | 69 |
| Middle finger | 5 | 7 |
| Ring finger | 21 | 27 |
| Little finger | 15 | 32 |
| Collagenase | Limited Fasciectomy | ||
|---|---|---|---|
| Grip Strength | Treated (kg ± SD) | 36.6 (11.4) | 35.3 (8.8) |
| Untreated (kg ± SD) | 34.8 (11.1) | 34.9 (8.8) | |
| Significance of difference between sides (p) | 0.079 | 0.571 | |
| Pinch Test | Treated (kg ± SD) | 3.2 (1.8) | 3.1 (1.8) |
| Untreated (kg ± SD) | 3.0 (1.5) | 3.1 (1.6) | |
| Significance of difference between sides (p) | 0.339 | 0.917 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachtel, N.; Dingler, F.R.; Kuhlmann, C.; Mert, S.; Haas-Lützenberger, E.M.; Alt, V.; Moellhoff, N.; Giunta, R.; Demmer, W. Therapy for Dupuytren’s Disease (II): Collagenase Therapy vs. Limited Fasciectomy—A Long-Term Comparative Study. Life 2025, 15, 76. https://doi.org/10.3390/life15010076
Wachtel N, Dingler FR, Kuhlmann C, Mert S, Haas-Lützenberger EM, Alt V, Moellhoff N, Giunta R, Demmer W. Therapy for Dupuytren’s Disease (II): Collagenase Therapy vs. Limited Fasciectomy—A Long-Term Comparative Study. Life. 2025; 15(1):76. https://doi.org/10.3390/life15010076
Chicago/Turabian StyleWachtel, Nikolaus, Francesca Romana Dingler, Constanze Kuhlmann, Sinan Mert, Elisabeth Maria Haas-Lützenberger, Verena Alt, Nicholas Moellhoff, Riccardo Giunta, and Wolfram Demmer. 2025. "Therapy for Dupuytren’s Disease (II): Collagenase Therapy vs. Limited Fasciectomy—A Long-Term Comparative Study" Life 15, no. 1: 76. https://doi.org/10.3390/life15010076
APA StyleWachtel, N., Dingler, F. R., Kuhlmann, C., Mert, S., Haas-Lützenberger, E. M., Alt, V., Moellhoff, N., Giunta, R., & Demmer, W. (2025). Therapy for Dupuytren’s Disease (II): Collagenase Therapy vs. Limited Fasciectomy—A Long-Term Comparative Study. Life, 15(1), 76. https://doi.org/10.3390/life15010076







