Regulation of LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells via TLR4-Mediated NF-κB and MAPK Signaling Pathways by Lactoferrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Chemicals, and Antibodies
2.2. Source of Bovine in Mammary Epithelial Cells
2.3. Experimental Infection
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Cell Viability Assay
2.6. Western Blot Analysis
2.7. Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
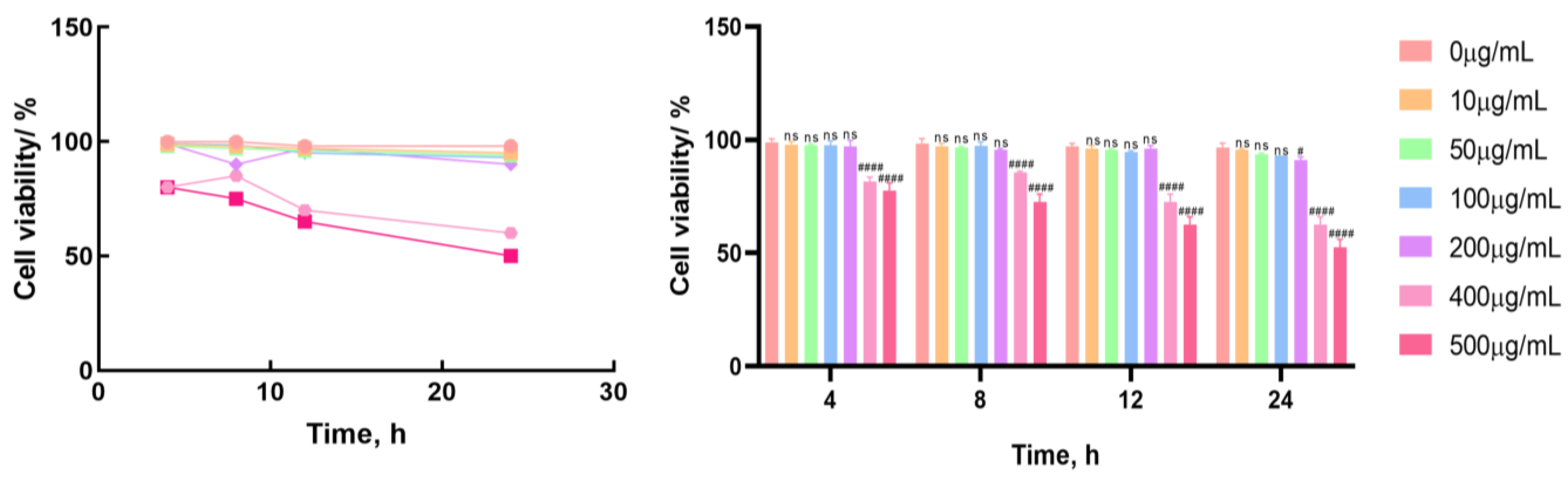
3.1. Effect of LF on the Activity of Bovine Mammary Epithelial Cells
3.2. Pretreatment with LF Reduces LPS-Induced Inflammatory Responses in BMECs
3.3. Post-Treatment with LF Promotes LPS-Induced Inflammatory Responses in BMECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangoni, M.L.; Epand, R.F.; Rosenfeld, Y.; Peleg, A.; Barra, D.; Epand, R.M.; Shai, Y. Lipopolysaccharide, a Key Molecule Involved in the Synergism between Temporins in Inhibiting Bacterial Growth and in Endotoxin Neutralization. J. Biol. Chem. 2008, 283, 22907–22917. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K. Innate Immune Sensing of Pathogens and Danger Signals by Cell Surface Toll-like Receptors. Semin. Immunol. 2007, 19, 3–10. [Google Scholar] [CrossRef]
- Leslie, K.E.; Petersson-Wolfe, C.S. Assessment and Management of Pain in Dairy Cows with Clinical Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cesnik, C.; Schwartz, K.; Foxman, B. Lactation Mastitis. JAMA 2003, 289, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, P.; Sachivkina, N.; Vatnikov, Y.; Shabunin, S.; Engashev, S.; Kontsevaya, S.; Karamyan, A.; Bokov, D.; Kuznetsova, O.; Vasilieva, E. Role of Microorganisms Isolated from Cows with Mastitis in Moscow Region in Biofilm Formation. Vet. World 2021, 14, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Porcherie, A.; Gilbert, F.B.; Germon, P.; Cunha, P.; Trotereau, A.; Rossignol, C.; Winter, N.; Berthon, P.; Rainard, P. IL-17A Is an Important Effector of the Immune Response of the Mammary Gland to Escherichia coli Infection. J. Immunol. 2016, 196, 803–812. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, Z.; Zhang, S.; Cao, J.; Mao, W.; Fu, Y.; Su, N.; Ding, Y.; Zhao, J.; Gu, B.; et al. Braun Lipoprotein Protects against Escherichia coli-Induced Inflammatory Responses and Lethality in Mice. Microbiol. Spectr. 2023, 11, e0354122. [Google Scholar] [CrossRef]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef]
- An, J.; Xu, Y.; Kong, Z.; Xie, Y.; Tabys, D.; Ma, M.; Cao, X.; Ren, H.; Liu, N. Effect of Lactoferrin and Its Digests on Differentiation Activities of Bone Mesenchymal Stem Cells. J. Funct. Foods 2019, 57, 202–210. [Google Scholar] [CrossRef]
- Vogel, P.; Donoviel, M.S.; Read, R.; Hansen, G.M.; Hazlewood, J.; Anderson, S.J.; Sun, W.; Swaffield, J.; Oravecz, T. Incomplete Inhibition of Sphingosine 1-Phosphate Lyase Modulates Immune System Function yet Prevents Early Lethality and Non-Lymphoid Lesions. PLoS ONE 2009, 4, e4112. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. A Structural Perspective on Lactoferrin Function. Biochem. Cell Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.-Y.; Yang, X.-P.; Li, Q.; Wu, L.-H.; Shen, Q.-R.; Zhao, F.-J. Changes in Antibiotic Concentrations and Antibiotic Resistome during Commercial Composting of Animal Manures. Environ. Pollut. 2016, 219, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Ryman, V.E.; Packiriswamy, N.; Sordillo, L.M. Role of Endothelial Cells in Bovine Mammary Gland Health and Disease. Anim. Health Res. Rev. 2015, 16, 135–149. [Google Scholar] [CrossRef]
- Zhao, X.; Lacasse, P. Mammary Tissue Damage during Bovine Mastitis: Causes and Control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef]
- Cheng, W.N.; Jeong, C.H.; Seo, H.G.; Han, S.G. Moringa Extract Attenuates Inflammatory Responses and Increases Gene Expression of Casein in Bovine Mammary Epithelial Cells. Animals 2019, 9, 391. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, R.; Lönnerdal, B. Biochemical and Molecular Impacts of Lactoferrin on Small Intestinal Growth and Development during Early Life. Biochem. Cell Biol. 2012, 90, 476–484. [Google Scholar] [CrossRef]
- Roussel, P.; Cunha, P.; Porcherie, A.; Petzl, W.; Gilbert, F.B.; Riollet, C.; Zerbe, H.; Rainard, P.; Germon, P. Investigating the Contribution of IL-17A and IL-17F to the Host Response during Escherichia coli Mastitis. Vet. Res. 2015, 46, 56. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, M.; Su, H.; Zhao, F.; Wang, D.; Zhang, Y.; Cao, G.; Zhang, Y. Regulation of Inflammatory Responses of Cow Mammary Epithelial Cells through MAPK Signaling Pathways of IL-17A Cytokines. Animals 2024, 14, 1572. [Google Scholar] [CrossRef]
- Zhang, J.L.; Han, X.; Shan, Y.J.; Zhang, L.W.; Du, M.; Liu, M.; Yi, H.X.; Ma, Y. Effect of Bovine Lactoferrin and Human Lactoferrin on the Proliferative Activity of the Osteoblast Cell Line MC3T3-E1 in Vitro. J. Dairy. Sci. 2018, 101, 1827–1833. [Google Scholar] [CrossRef]
- Håversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.A.; Mattsby-Baltzer, I. Lactoferrin Down-Regulates the LPS-Induced Cytokine Production in Monocytic Cells via NF-κB. Cell Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef]
- Diarra, M.S.; Petitclerc, D.; Deschênes, E.; Lessard, N.; Grondin, G.; Talbot, B.G.; Lacasse, P. Lactoferrin against Staphylococcus aureus Mastitis. Lactoferrin Alone or in Combination with Penicillin G on Bovine Polymorphonuclear Function and Mammary Epithelial Cells Colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. 2003, 95, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.; Zhang, P.; Zhong, Q.; Sun, Z. Application of Traditional Chinese Herbal Medicine By-Products as Dietary Feed Supplements and Antibiotic Replacements in Animal Production. Curr. Drug Metab. 2019, 20, 54–64. [Google Scholar] [CrossRef] [PubMed]
- El-Halfawy, O.M.; Klett, J.; Ingram, R.J.; Loutet, S.A.; Murphy, M.E.P.; Martín-Santamaría, S.; Valvano, M.A. Antibiotic Capture by Bacterial Lipocalins Uncovers an Extracellular Mechanism of Intrinsic Antibiotic Resistance. mBio 2017, 8, e00225-17. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-Lipopolysaccharide (LPS) Binding as Key to Antibacterial and Antiendotoxic Effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dafwang, I.I.; Cook, M.E.; Sunde, M.L.; Bird, H.R. Bursal, Intestinal, and Spleen Weights and Antibody Response of Chicks Fed Subtherapeutic Levels of Dietary Antibiotics. Poult. Sci. 1985, 64, 634–639. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, S.; Gu, B.; Cao, J.; Mao, W.; Yao, Y.; Zhao, J.; Ren, P.; Zhang, K.; Liu, B. Codonopsis pilosula Polysaccharides Attenuate Escherichia coli-Induced Acute Lung Injury in Mice. Food Funct. 2022, 13, 7999–8011. [Google Scholar] [CrossRef]
- Galvão, K.N.; Santos, N.R.; Galvão, J.S.; Gilbert, R.O. Association between Endometritis and Endometrial Cytokine Expression in Postpartum Holstein Cows. Theriogenology 2011, 76, 290–299. [Google Scholar] [CrossRef]
- Losfeld, M.-E.; Khoury, D.E.; Mariot, P.; Carpentier, M.; Krust, B.; Briand, J.-P.; Mazurier, J.; Hovanessian, A.G.; Legrand, D. The Cell Surface Expressed Nucleolin Is a Glycoprotein That Triggers Calcium Entry into Mammalian Cells. Exp. Cell Res. 2009, 315, 357–369. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, Y.; Qian, Y.; Jiang, X.; Zhang, S.; Liu, B.; Cao, J.; Song, Y.; Mao, W. Staphylococcus aureus Increases Prostaglandin E2 Secretion in Cow Neutrophils by Activating TLR2, TLR4, and NLRP3 Inflammasome Signaling Pathways. Front. Microbiol. 2023, 14, 1163261. [Google Scholar] [CrossRef]
- Roura, E.; Homedes, J.; Klasing, K.C. Prevention of Immunologic Stress Contributes to the Growth-Permitting Ability of Dietary Antibiotics in Chicks. J. Nutr. 1992, 122, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Li, Y.; Sangild, P.T.; Bering, S.B.; Chatterton, D.E.W. Effects of Bovine Lactoferrin on the Immature Porcine Intestine. Br. J. Nutr. 2014, 111, 321–331. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Sequences (5′-3′) | Accession Number |
|---|---|---|
| β-actin | F: 5′-CCAAGGCCAACCGTGAGAAGAT-3′ R: 5′-CCACGTTCCGTGAGGATCTTCA-3′ | NM_173979.3 |
| TNF-α | F: 5′-CAACGGTGTGAAGCTGGAAGAC-3′ R: 5′-TGAAGAGGACCTGTGAGTAGATGAG-3′ | NM_173966.3 |
| Il-1β | F: 5′-ATGAAGAGCTGCATCCAACACCTG-3′ R: 5′-ACCGACACCACCTGCCTGAAG-3′ | NM_174093.1 |
| IL-8 | F: 5′-GCTGGCTGTTGCTCTCTTGG-3′ R: 5′-GGGTGGAAAGGTGTGGAATGTG-3′ | NM_173925.2 |
| IL-6 | F: 5′-ATGATGAGTGTGAAAGCAGCAAGG-3′ R: 5′-TGATACTCCAGAAGACCAGCAGTG-3 | NM_173923.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhang, R.; Zhang, Y.; Zhang, M.; Su, H.; Zhao, F.; Wang, D.; Cao, G.; Zhang, Y. Regulation of LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells via TLR4-Mediated NF-κB and MAPK Signaling Pathways by Lactoferrin. Life 2025, 15, 69. https://doi.org/10.3390/life15010069
Zhang K, Zhang R, Zhang Y, Zhang M, Su H, Zhao F, Wang D, Cao G, Zhang Y. Regulation of LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells via TLR4-Mediated NF-κB and MAPK Signaling Pathways by Lactoferrin. Life. 2025; 15(1):69. https://doi.org/10.3390/life15010069
Chicago/Turabian StyleZhang, Kai, Ruizhen Zhang, Yuanyuan Zhang, Min Zhang, Hong Su, Feifei Zhao, Daqing Wang, Guifang Cao, and Yong Zhang. 2025. "Regulation of LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells via TLR4-Mediated NF-κB and MAPK Signaling Pathways by Lactoferrin" Life 15, no. 1: 69. https://doi.org/10.3390/life15010069
APA StyleZhang, K., Zhang, R., Zhang, Y., Zhang, M., Su, H., Zhao, F., Wang, D., Cao, G., & Zhang, Y. (2025). Regulation of LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells via TLR4-Mediated NF-κB and MAPK Signaling Pathways by Lactoferrin. Life, 15(1), 69. https://doi.org/10.3390/life15010069






