The Potential Effect of Bualuang (White Nelumbo nucifera Gaertn.) Extract on Sperm Quality and Metabolomic Profiles in Mancozeb-Induced Oxidative Stress in Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Preparation
2.2. Plant Preparation and Extraction
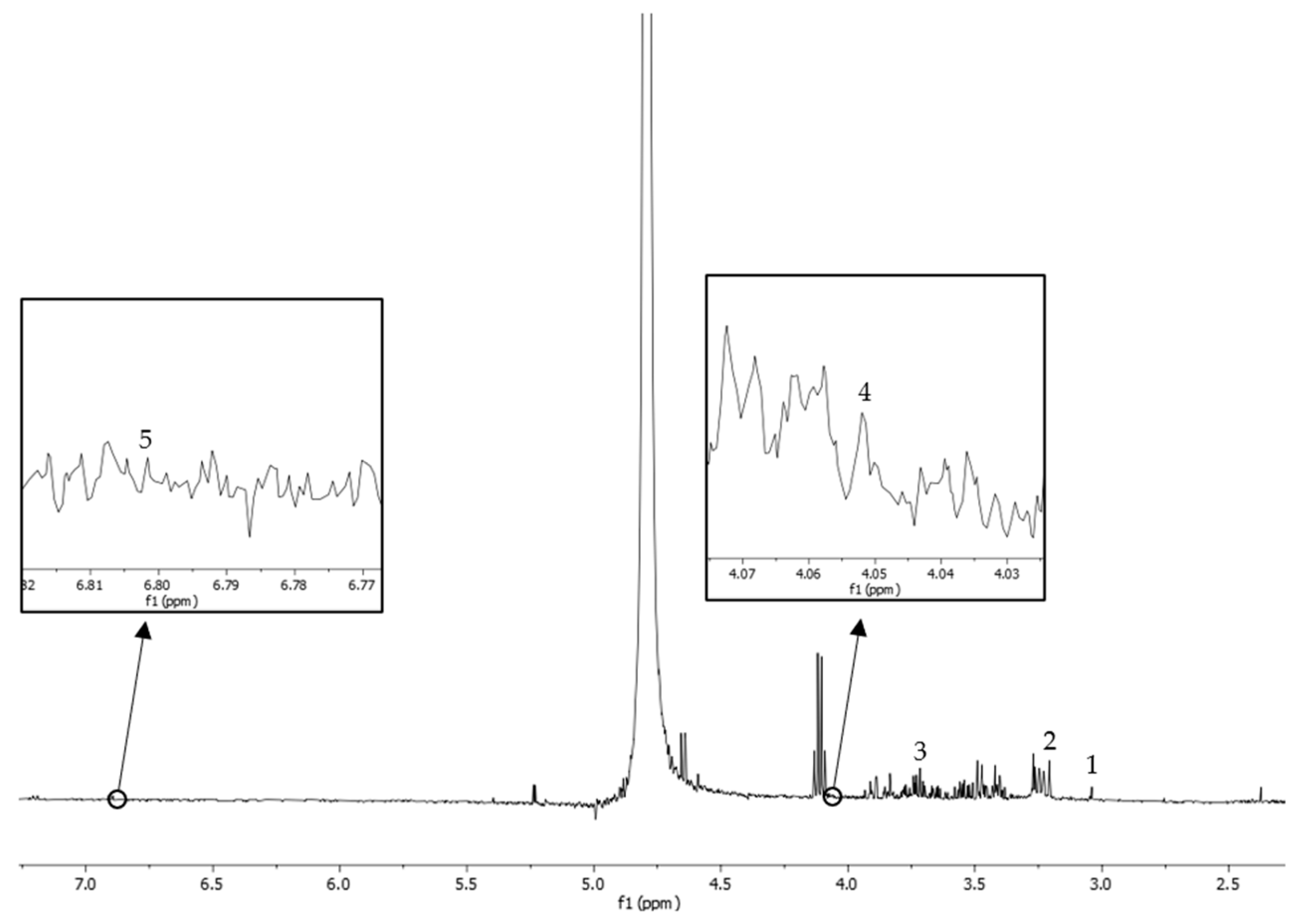
2.3. Phytochemical Screening of WNPE Using Proton Nuclear Magnetic Resonance (1H-NMR)
2.4. Mineral Assay with Atomic Absorption Spectroscopy (AAS)
2.5. Animals
2.6. Experimental Design
- –
- Group I (control group): male rats received 1 mL of distilled water.
- –
- Group II–IV: male rats were orally gavaged with WNPE that was dissolved in distilled water at doses of 0.55, 1.10, and 2.20 mg/kg, respectively.
- –
- Group V (vehicle control): male rats were orally gavaged with olive oil.
- –
- Group VI (toxic group): male rats were orally gavaged with MZ 500 mg/kg, which is the toxicity dose that was recommended in a previous study [39].
- –
- Group VII–IX (treatment group): male rats were orally co-gavaged with WNPE at doses of 0.55, 1.10, and 2.20 mg/kg, respectively, and with MZ.
2.7. Metabolomic Activity Analysis by 1H-NMR
2.8. Sperm Quality Investigation
2.8.1. Sperm Concentration and Sperm Motility Assay
2.8.2. Sperm Viability and Acrosome Integrity Assay
2.8.3. Sperm Morphology Assay
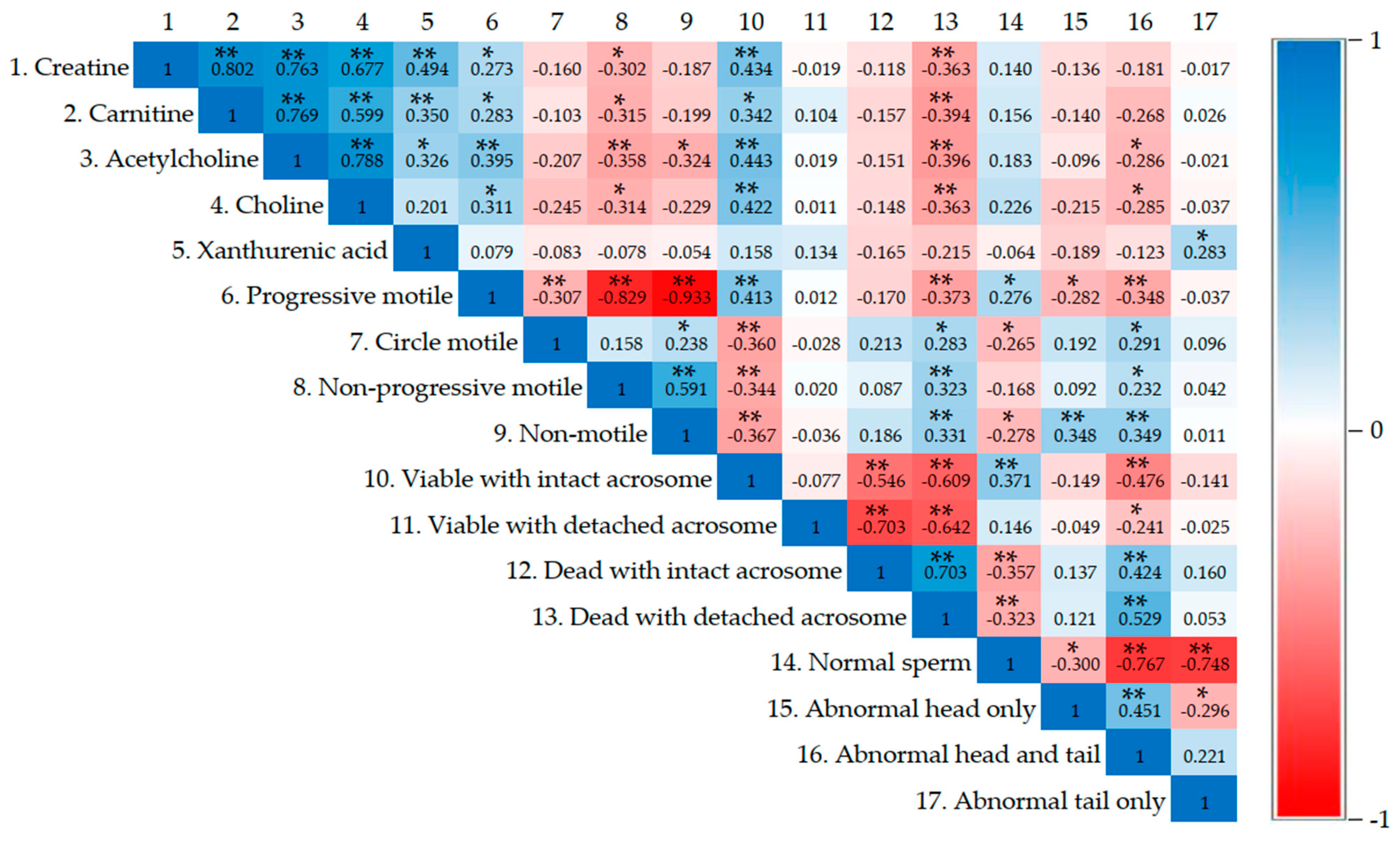
2.9. Correlation Analysis of Metabolomic Profiles and Sperm Quality
2.10. Statistical Analysis
3. Results
3.1. Phytochemical Screening of WNPE Using Proton Nuclear Magnetic Resonance (1H-NMR)
3.2. Analysis of Minerals in WNPE with AAS
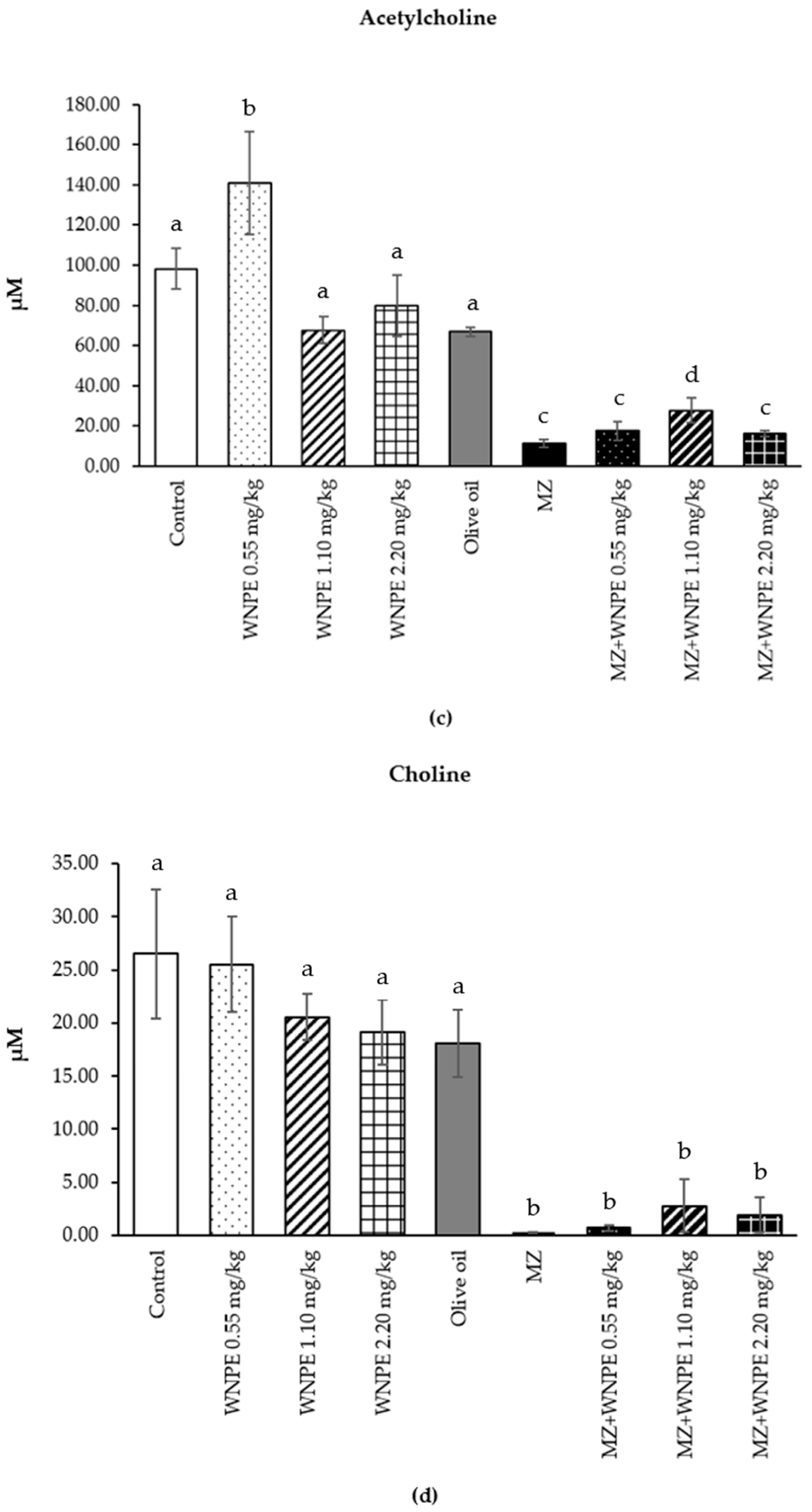
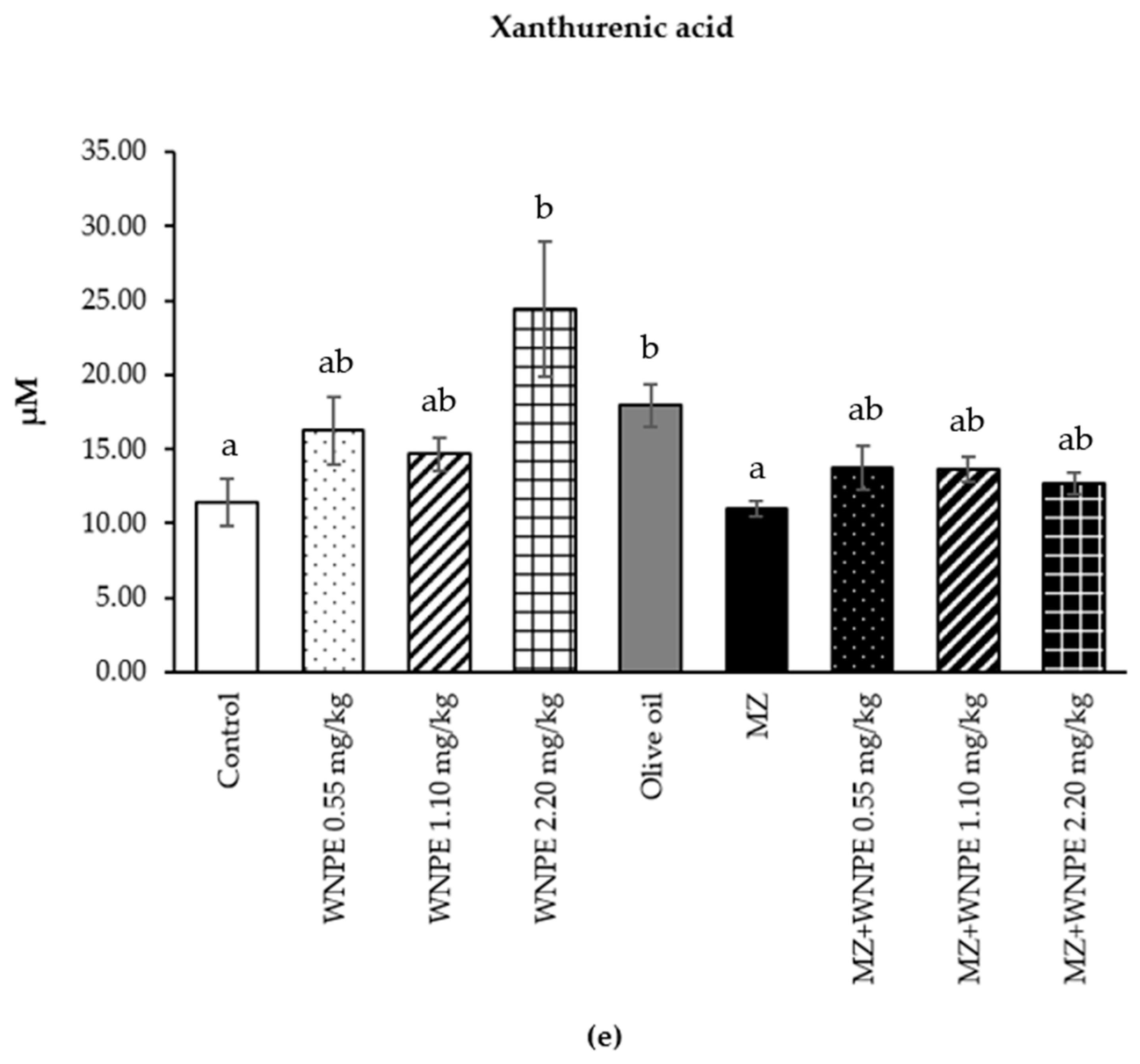
3.3. Effect of White N. nucifera Petal Extract on Metabolomic Profile Determined by 1H-NMR
3.4. Sperm Quality
3.4.1. Sperm Concentration and Sperm Motility
3.4.2. Sperm Viability and Acrosome Integrity
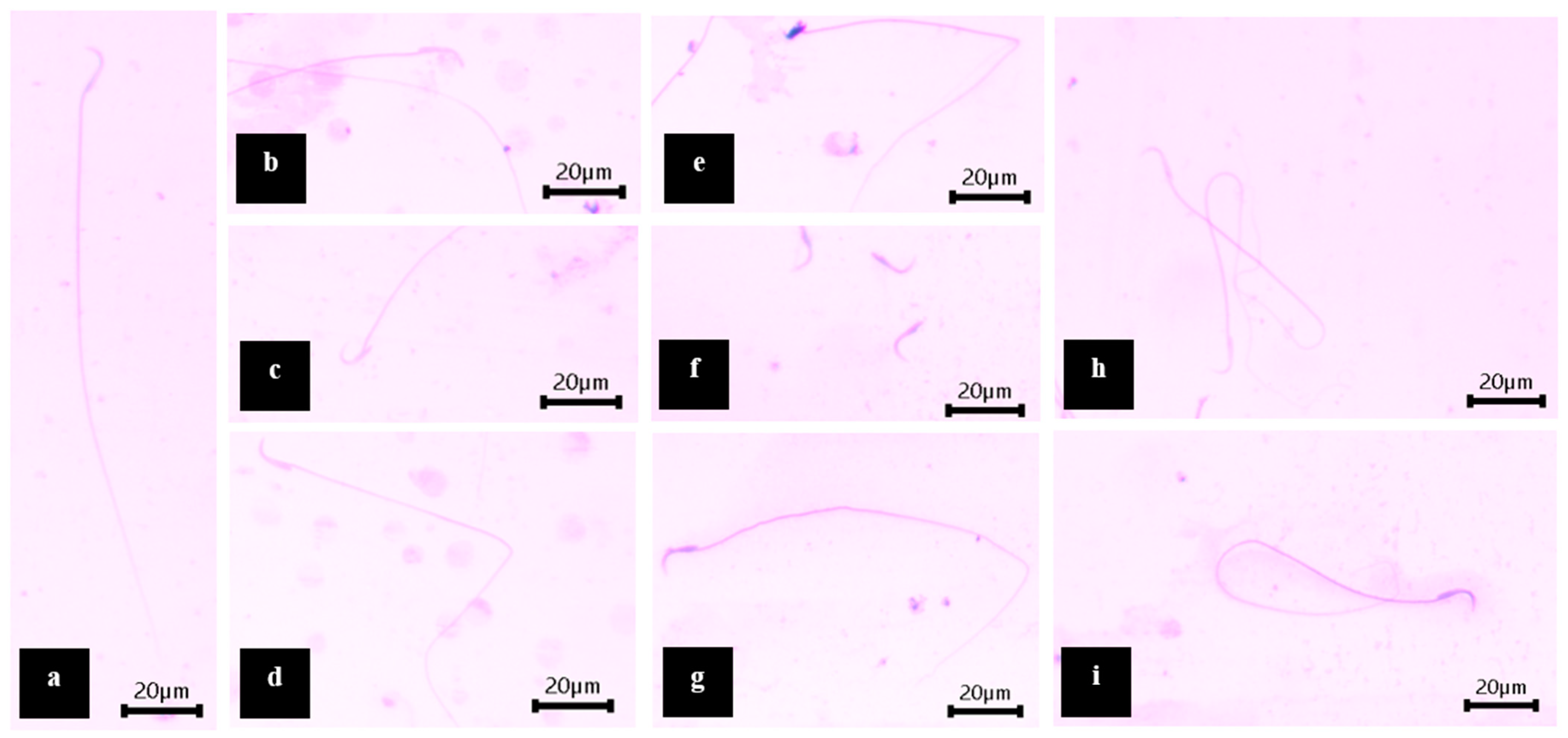
3.4.3. Sperm Morphology
3.5. Correlation Analysis of Metabolomic Profiles and Sperm Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina-Pastor, P.; Triacchini, G. The 2018 European Union report on pesticide residues in food. EFSA J. 2020, 18, e06057. [Google Scholar] [PubMed]
- Kumar, R.; Nehra, M.; Kumar, D.; Saharan, B.S.; Chawla, P.; Sadh, P.K.; Manuja, A.; Duhan, J.S. Evaluation of cytotoxicity, release behavior and phytopathogens control by Mancozeb-Loaded Guar Gum nanoemulsions for sustainable agriculture. J. Xenobiot. 2023, 13, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Runkle, J.; Flocks, J.; Economos, J.; Dunlop, A.L. A systematic review of Mancozeb as a reproductive and developmental hazard. Environ. Int. 2017, 99, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Dhaneshwar, A.; Hardej, D. Disruption of mitochondrial complexes, cytotoxicity, and apoptosis results from Mancozeb exposure in transformed human colon cells. Environ. Toxicol. Pharmacol. 2021, 84, 103614. [Google Scholar] [CrossRef]
- Skalny, A.; Aschner, M.; Paoliello, M.; Santamaria, A.; Nikitina, N.; Rejniuk, V.; Jiang, Y.; Rocha, J.; Tinkov, A. Endocrine-disrupting activity of mancozeb. Arh. Za Farm. 2021, 71, 491. [Google Scholar] [CrossRef] [PubMed]
- Nuchniyom, P.; Intui, K.; Laoung-on, J.; Jaikang, C.; Quiggins, R.; Photichai, K.; Sudwan, P. Effects of Nelumbo nucifera Gaertn. petal tea extract on hepatotoxicity and oxidative stress induced by mancozeb in rat model. Toxics 2023, 11, 480. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, R.; Bao, J.; Gong, Y.; Wang, X. Mancozeb induces nephrotoxicity by impairing the oxidative phosphorylation pathway: A transcriptome study. Ecotoxicol. Environ. Saf. 2023, 249, 114471. [Google Scholar] [CrossRef] [PubMed]
- Intui, K.; Nuchniyom, P.; Laoung-on, J.; Jaikang, C.; Quiggins, R.; Sudwan, P. Neuroprotective effect of white Nelumbo nucifera Gaertn. petal tea in rats poisoned with mancozeb. Foods 2023, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Ksheerasagar, R.L.; Kaliwal, B.B. Temporal effects of mancozeb on testes, accessory reproductive organs and biochemical constituents in albino mice. Environ. Toxicol. Pharmacol. 2003, 15, 9–17. [Google Scholar] [CrossRef]
- Kackar, R.; Srivastava, M.; Raizada, R. Assessment of toxicological effects of mancozeb in male rats after chronic exposure. Int. J. Environ. Biol. 1999, 37, 553–559. [Google Scholar]
- Laoung-on, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Effect of Nelumbo nucifera petals extract on antioxidant activity and sperm quality in Charolais cattle sperm induced by mancozeb. Plants 2022, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Panuwet, P.; Siriwong, W.; Prapamontol, T.; Ryan, P.B.; Fiedler, N.; Robson, M.G.; Barr, D.B. Agricultural pesticide management in Thailand: Status and population health risk. Environ. Sci. Policy 2012, 17, 72–81. [Google Scholar] [CrossRef]
- Ashok, A.; Gurpriya, V.; Chloe, O.; Stefan, S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar] [PubMed]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrin. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Sedha, S.; Kumar, S.; Shukla, S. Role of oxidative stress in male reproductive dysfunctions with reference to phthalate compounds. Urol. J. 2015, 12, 2304–2316. [Google Scholar] [CrossRef]
- Bansal, A.; Bilaspuri, G. Effect of ferrous sulphate and ascorbic acid on motility, viability and lipid peroxidation of crossbred cattle bull spermatozoa. Animal 2008, 2, 100–104. [Google Scholar] [CrossRef]
- Zargari, F.; Rahaman, M.S.; KazemPour, R.; Hajirostamlou, M. Arsenic, oxidative stress and reproductive system. J. Xenobiot. 2022, 12, 214–222. [Google Scholar] [CrossRef]
- Mallidis, C.; Green, B.; Rogers, D.; Agbaje, I.; Hollis, J.; Migaud, M.; Amigues, E.; McClure, N.; Browne, R. Metabolic profile changes in the testes of mice with streptozotocin--induced type 1 diabetes mellitus. Int. J. Androl. 2009, 32, 156–165. [Google Scholar] [CrossRef]
- Mendes, R.; Cardoso, C.; Pestana, C. Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem. 2009, 112, 1038–1045. [Google Scholar] [CrossRef]
- Hage, D.S. Immunoassays. Anal. Chem. 1999, 71, 294–304. [Google Scholar] [CrossRef]
- Donnan, J.R.; Ungar, W.J.; Mathews, M.; Rahman, P. Systematic review of thiopurine methyltransferase genotype and enzymatic testing strategies. Ther. Drug Monit. 2011, 33, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Brindle, J.T.; Antti, H.; Holmes, E.; Tranter, G.; Nicholson, J.K.; Bethell, H.W.; Clarke, S.; Schofield, P.M.; McKilligin, E.; Mosedale, D.E. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat. Med. 2002, 8, 1439–1445. [Google Scholar] [CrossRef]
- Ghafarizadeh, A.A.; Malmir, M.; Naderi Noreini, S.; Faraji, T.; Ebrahimi, Z. The effect of vitamin E on sperm motility and viability in asthenoteratozoospermic men: In vitro study. Andrologia 2021, 53, e13891. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.; Orabueze, I.; Okorie, N.H. Antioxidants properties of natural and synthetic chemical compounds: Therapeutic effects on biological system. Acta Sci. Pharm. Sci. 2019, 3, 28–42. [Google Scholar] [CrossRef]
- Lee, B.M.; Park, K.-K. Beneficial and adverse effects of chemopreventive agents. Mutat. Res. 2003, 523, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, T.; Har-Vardi, I.; Harlev, A.; Friger, M.; Hamou, B.; Barac, T.; Levitas, E.; Saphier, O. Antioxidants and polyphenols: Concentrations and relation to male infertility and treatment success. Oxid. Med. Cell Longev. 2016, 2016, 9140925. [Google Scholar] [CrossRef]
- Sudwan, P.; Saenphet, K.; Aritajat, S.; Sitasuwan, N. Effects of Boesenbergia rotunda (L.) Mansf. on sexual behaviour of male rats. Asian J. Androl. 2007, 9, 849–855. [Google Scholar] [CrossRef]
- Sudwan, P.; Saenphet, K.; Saenphet, S.; Wongsawad, C. Sperm density and ultrastructure of Sertoli cells in male rats treated with Kaempferia parviflora Wall. Ex Baker extract. Southeast Asian J. Trop. Med. Public Health 2007, 38, 249. [Google Scholar]
- Laoung-on, J.; Saenphet, K.; Jaikang, C.; Sudwan, P. Effect of Moringa oleifera Lam. leaf tea on sexual behavior and reproductive function in male rats. Plants 2021, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.; Okdah, Y.; El-Adly, E. Effect of ginger (Zingiber officinale) on mancozeb fungicide induced testicular damage in albino rats. Aust. J. Basic Appl. Sci. 2009, 3, 1328–1333. [Google Scholar]
- Laoung-on, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical screening, antioxidant and sperm viability of Nelumbo nucifera petal extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Saha, K.; Pal, M.; Saha, B. Effect of Nelumbo nucifera rhizome extract on blood sugar level in rats. J. Ethnopharmacol. 1997, 58, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Panth, N. Phytochemical profile and biological activity of Nelumbo nucifera. Evid. Based Complement. Alternat. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Pinthong, D.; Hano, C. Flavonoids from Nelumbo nucifera Gaertn., a medicinal plant: Uses in traditional medicine, phytochemistry and pharmacological activities. Medicines 2018, 5, 127. [Google Scholar] [CrossRef]
- Guo, H. Cultivation of lotus (Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China. Genet. Resour. Crop Evol. 2009, 56, 323–330. [Google Scholar] [CrossRef]
- Ministry of Public Health. Thai Hercal Pharmacopoeia; Ministry of Public Health: Bangkok, Thailand, 2019; Volume 1.
- Wong, W.Y.; Flik, G.; Groenen, P.M.; Swinkels, D.W.; Thomas, C.M.; Copius-Peereboom, J.H.; Merkus, H.M.; Steegers-Theunissen, R.P. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod. Toxicol. 2001, 15, 131–136. [Google Scholar] [CrossRef]
- Mohammadi-Sardoo, M.; Mandegary, A.; Nabiuni, M.; Nematollahi-Mahani, S.-N.; Amirheidari, B. Mancozeb induces testicular dysfunction through oxidative stress and apoptosis: Protective role of N-acetylcysteine antioxidant. Toxicol. Ind. Health 2018, 34, 798–811. [Google Scholar] [CrossRef]
- Sudwan, P.; Saenphet, K.; Saenphet, S.; Suwansirikul, S. Effect of Kaempferia parviflora Wall. ex. Baker on sexual activity of male rats and its toxicity. Southeast Asian J. Trop. Med. Public Health 2006, 37, 210. [Google Scholar] [PubMed]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Ryan, D.; Brennan, L.; Tenori, L.; Luchinat, C.; Gao, X.; Zeri, A.C.; Gowda, G.N. Recommendations and standardization of biomarker quantification using NMR-based metabolomics with particular focus on urinary analysis. J. Proteome Res. 2016, 15, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Somtua, P.; Jaikang, C.; Konguthaithip, G.; Intui, K.; Watcharakhom, S.; O’Brien, T.E.; Amornlertwatana, Y. Postmortem alteration of purine metabolism in coronary artery disease. Metabolites 2023, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Yotarlai, S.; Chaisuksunt, V.; Saenphet, K.; Sudwan, P. Effects of Boesenbergia rotunda juice on sperm qualities in male rats. J. Med. Plant Res. 2011, 5, 3861–3867. [Google Scholar]
- Najafi, A.; Mohammadi, H.; Sharifi, S.D.; Rahimi, A. Apigenin supplementation substantially improves rooster sperm freezability and post-thaw function. Sci. Rep. 2024, 14, 4527. [Google Scholar] [CrossRef]
- Lai, Y.; Xi, Y.; Shao, M.; Cui, X.; Wei, X.; Li, L.; Wang, Y.; Fan, H. Myricetin reduces the reproductive toxicity of cyclophosphamide in male mice. Wei Sheng Yan Jiu 2020, 49, 790–794. [Google Scholar]
- Pei, Y.; Yang, L.; Wu, L.; He, H.; Geng, G.; Xu, D.; Chen, H.; Li, Q. Combined effect of apigenin and ferulic acid on frozen--thawed boar sperm quality. Anim. Sci. 2018, 89, 956–965. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Corsaro, R.; Giamalidi, M.; Collodel, G. Human sperm as an in vitro model to assess the efficacy of antioxidant supplements during sperm handling: A narrative review. Antioxidants 2023, 12, 1098. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Lu, G.; Gu, J. Pharmacological activity of flavonoid quercetin and its therapeutic potential in testicular injury. Nutrients 2023, 15, 2231. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, J.; Zhu, Z.; Zhao, A.; Zhou, Y.; Ying, H.; Zhang, Q. Luteolin ameliorates testis injury and blood–testis barrier disruption through the Nrf2 signaling pathway and by upregulating Cx43. Mol. Nutr. Food Res. 2019, 63, 1800843. [Google Scholar] [CrossRef] [PubMed]
- Zahra, M.; Marefat, G.N.; Ghasem Golmohammadi, M.; Mohsen, S.; Seyed, A.Z.; Mohammad, A.A.; Nazarian, H. Protective effect of gallic acid on testicular tissue, sperm parameters, and DNA fragmentation against toxicity induced by cyclophosphamide in adult NMRI mice. Urol. J. 2020, 17, 78–85. [Google Scholar] [CrossRef]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bruemmert, J.; Coy, R.; Squires, E.; Graham, J. Effect of pyruvate on the function of stallion spermatozoa stored for up to 48 hours. J. Anim. Sci. 2002, 80, 12–18. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.-H.; Chae, Y.-J.; Ogawa, H.; Lee, M.-H.; Gerton, G.L. Creatine synthesis and transport systems in the male rat reproductive tract. Biol. Reprod. 1998, 58, 1437–1444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Draper, R.P.; Timbrell, J.A. Urinary creatine as a potential marker of testicular damage: Effect of vasectomy. Reprod. Toxicol. 1996, 10, 79–85. [Google Scholar] [CrossRef]
- Dajas-Bailador, F.; Wonnacott, S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004, 25, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Silva, R.; Carrageta, D.F.; Alves, M.G.; Seco-Rovira, V.; Oliveira, P.F.; de Lourdes Pereira, M. Carbamate pesticides: Shedding light on their impact on the male reproductive system. Int. J. Mol. Sci. 2022, 23, 8206. [Google Scholar] [CrossRef]
- Abdullah, F.; Nor-Ashikin, M.N.K.; Agarwal, R.; Kamsani, Y.S.; Abd Malek, M.; Bakar, N.S.; Kamal, A.-A.M.; Sarbandi, M.-S.; Rahman, N.-S.A.; Musa, N.H. Glutathione (GSH) improves sperm quality and testicular morphology in streptozotocin-induced diabetic mice. Asian J. Androl. 2021, 23, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.; Agarwal, A.; Majzoub, A.; Panner Selvam, M.K.; Baskaran, S.; Henkel, R.; Elbardisi, H. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants 2020, 9, 219. [Google Scholar] [CrossRef]
- Komori, K.; Tsujimura, A.; Ishijima, S.; Tanjapatkul, P.; Fujita, K.; Matsuoka, Y.; Takao, T.; Miyagawa, Y.; Takada, S.; Okuyama, A. Comparative study of sperm motility analysis system and conventional microscopic semen analysis. Reprod. Med. Biol. 2006, 5, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Saddein, E.; Haghpanah, T.; Nematollahi-Mahani, S.N.; Seyedi, F.; Ezzatabadipour, M. Preventative effects of vitamin E on testicular damage and sperm parameters in the first-generation mice pups due to pre-and postnatal mancozeb exposure. J. Toxicol. 2019, 2019, 4763684. [Google Scholar] [CrossRef] [PubMed]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, C.; Ma, X.; Yan, X.; Xiong, B.; Shen, W.; Yin, S.; Zhang, H.; Sun, Q.; Zhao, Y. Muscarinic acetylcholine receptor M5 is involved in spermatogenesis through the modification of cell–cell junctions. Reproduction 2021, 162, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Laoung-on, J.; Ounjaijean, S.; Sudwan, P.; Boonyapranai, K. Phytochemical screening, antioxidant effect and sperm quality of the Bomba ceiba stamen extracts on Charolais cattle sperm induced by ferrous sulfate. Plants 2024, 13, 960. [Google Scholar] [CrossRef]
- Lehti, M.S.; Sironen, A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, A.; Satrio, F.A.; Indriastuti, R.; Rosyada, Z.N.A.; Pardede, B.P.; Agil, M.; Purwantara, B. Sperm head morphology alterations associated with chromatin instability and lack of protamine abundance in frozen-thawed sperm of Indonesian local bulls. Animals 2023, 13, 2433. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Haenen, G.R.; Vervoort, L.; Moalin, M. Flavonoids seen through the energy perspective. Int. J. Mol. Sci. 2021, 23, 187. [Google Scholar] [CrossRef]
- Martin, L.J.; Touaibia, M. Improvement of testicular steroidogenesis using flavonoids and isoflavonoids for prevention of late-onset male hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Ros-Santaella, J.L.; Pintus, E. Plant extracts as alternative additives for sperm preservation. Antioxidants 2021, 10, 772. [Google Scholar] [CrossRef]

| Group | Mineral Composition (mg/g of Plant Extract) | ||
|---|---|---|---|
| Mg | Zn | Ca | |
| WNPE | 14.83 ± 0.04 | 0.11 ± 0.01 | 5.20 ± 0.05 |
| Group | Percentages of Motile Sperm | Percentages of Non-Motile Sperm | ||
|---|---|---|---|---|
| Progressive | Circle | Non-Progressive | ||
| Control (n = 8) | 61.31 ± 1.82 a | 2.06 ± 0.27 | 11.13 ± 1.50 ab | 25.50 ± 1.78 acd |
| 0.55 mg/kg (n = 8) | 79.50 ± 3.91 b | 0.94 ± 0.11 | 8.63 ± 1.95 a | 10.94 ± 2.28 b |
| 1.10 mg/kg (n = 8) | 68.00 ± 1.39 c | 1.56 ± 0.53 | 9.38 ± 0.99 a | 21.06 ± 0.85 d |
| 2.20 mg/kg (n = 8) | 64.81 ± 3.92 ac | 1.31 ± 0.33 | 12.56 ± 2.03 ab | 21.31 ± 2.61 ad |
| Olive oil (n = 8) | 60.38 ± 2.58 a | 2.75 ± 0.69 | 11.81 ± 1.24 ab | 25.06 ± 1.33 ad |
| MZ (n = 8) | 39.50 ± 8.07 d | 2.44 ± 0.64 | 19.06 ± 3.40 b | 39.69 ± 5.00 e |
| MZ + 0.55 mg/kg (n = 8) | 74.94 ± 4.46 bc | 1.19 ± 0.23 | 12.31 ± 3.33 ab | 11.56 ± 1.48 bc |
| MZ + 1.10 mg/kg (n = 8) | 57.88 ± 2.79 a | 2.06 ± 0.41 | 15.31 ± 1.85 b | 25.38 ± 1.01 a |
| MZ + 2.20 mg/kg (n = 8) | 58.50 ± 3.89 a | 2.06 ± 0.48 | 15.13 ± 2.29 b | 24.31 ± 2.06 ad |
| Group | Number of Viable Sperm | Number of Dead Sperm | ||
|---|---|---|---|---|
| Intact | Detached | Intact | Detached | |
| Control (n = 8) | 33.88 ± 4.74 a | 45.75 ± 2.80 ab | 12.88 ± 3.58 a | 7.50 ± 2.08 a |
| 0.55 mg/kg (n = 8) | 41.00 ± 6.15 a | 41.75 ± 6.15 ab | 10.88 ± 3.49 a | 6.38 ± 2.24 a |
| 1.10 mg/kg (n = 8) | 38.25 ± 6.24 a | 33.13 ± 5.60 bc | 17.50 ± 4.89 a | 11.13 ± 4.20 ab |
| 2.20 mg/kg (n = 8) | 35.75 ± 7.07 a | 36.75 ± 6.95 bc | 17.13 ± 6.52 a | 10.50 ± 2.96 ab |
| Olive oil (n = 8) | 28.63 ± 5.10 ab | 47.38 ± 5.41 ab | 14.88 ± 4.55 a | 9.13 ± 2.14 a |
| MZ (n = 8) | 14.88 ± 4.78 b | 20.88 ± 6.52 c | 32.63 ± 4.95 b | 31.63 ± 7.26 c |
| MZ + 0.55 mg/kg (n = 8) | 33.75 ± 6.77 a | 43.38 ± 8.07 ab | 11.38 ± 5.54 a | 11.50 ± 6.00 ab |
| MZ + 1.10 mg/kg (n = 8) | 25.75 ± 3.96 ab | 59.75 ± 5.85 a | 6.00 ± 2.07 a | 8.50 ± 1.90 a |
| MZ + 2.20 mg/kg (n = 8) | 15.38 ± 3.41 b | 50.25 ± 6.96 ab | 12.25 ± 2.21 a | 22.13 ± 3.68 bc |
| Group | Number of Normal Sperm | Number of Abnormal Sperm | ||
|---|---|---|---|---|
| Head Only | Head and Tail | Tail Only | ||
| Control (n = 8) | 57.50 ± 4.72 a | 5.88 ± 0.74 a | 7.50 ± 1.70 a | 29.13 ± 3.51 |
| 0.55 mg/kg (n = 8) | 58.38 ± 4.72 a | 6.88 ± 1.60 a | 7.63 ± 2.14 a | 27.13 ± 4.62 |
| 1.10 mg/kg (n = 8) | 55.00 ± 5.89 a | 7.00 ± 1.02 a | 7.75 ± 2.06 a | 30.25 ± 5.34 |
| 2.20 mg/kg (n = 8) | 49.75 ± 5.06 a | 6.38 ± 1.49 a | 8.13 ± 2.36 a | 35.75 ± 4.62 |
| Olive oil (n = 8) | 52.25 ± 5.39 a | 8.63 ± 1.19 a | 9.00 ± 1.71 a | 30.13 ± 4.17 |
| MZ (n = 8) | 34.63 ± 3.90 b | 13.50 ± 1.99 b | 21.25 ± 3.00 b | 30.63 ± 2.58 |
| MZ + 0.55 mg/kg (n = 8) | 57.25 ± 3.66 a | 5.63 ± 1.28 a | 7.50 ± 1.38 a | 29.63 ± 3.05 |
| MZ + 1.10 mg/kg (n = 8) | 62.13 ± 3.21 a | 5.38 ± 0.98 a | 7.00 ± 1.09 a | 25.50 ± 2.68 |
| MZ + 2.20 mg/kg (n = 8) | 55.25 ± 4.38 a | 5.50 ± 1.72 a | 8.88 ± 1.47 a | 30.38 ± 3.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laoung-on, J.; Nuchniyom, P.; Intui, K.; Jaikang, C.; Saenphet, K.; Boonyapranai, K.; Konguthaithip, G.; Outaitaveep, N.; Phankhieo, S.; Sudwan, P. The Potential Effect of Bualuang (White Nelumbo nucifera Gaertn.) Extract on Sperm Quality and Metabolomic Profiles in Mancozeb-Induced Oxidative Stress in Male Rats. Life 2025, 15, 6. https://doi.org/10.3390/life15010006
Laoung-on J, Nuchniyom P, Intui K, Jaikang C, Saenphet K, Boonyapranai K, Konguthaithip G, Outaitaveep N, Phankhieo S, Sudwan P. The Potential Effect of Bualuang (White Nelumbo nucifera Gaertn.) Extract on Sperm Quality and Metabolomic Profiles in Mancozeb-Induced Oxidative Stress in Male Rats. Life. 2025; 15(1):6. https://doi.org/10.3390/life15010006
Chicago/Turabian StyleLaoung-on, Jiraporn, Pimchanok Nuchniyom, Ketsarin Intui, Churdsak Jaikang, Kanokporn Saenphet, Kongsak Boonyapranai, Giatgong Konguthaithip, Nopparuj Outaitaveep, Sasitorn Phankhieo, and Paiwan Sudwan. 2025. "The Potential Effect of Bualuang (White Nelumbo nucifera Gaertn.) Extract on Sperm Quality and Metabolomic Profiles in Mancozeb-Induced Oxidative Stress in Male Rats" Life 15, no. 1: 6. https://doi.org/10.3390/life15010006
APA StyleLaoung-on, J., Nuchniyom, P., Intui, K., Jaikang, C., Saenphet, K., Boonyapranai, K., Konguthaithip, G., Outaitaveep, N., Phankhieo, S., & Sudwan, P. (2025). The Potential Effect of Bualuang (White Nelumbo nucifera Gaertn.) Extract on Sperm Quality and Metabolomic Profiles in Mancozeb-Induced Oxidative Stress in Male Rats. Life, 15(1), 6. https://doi.org/10.3390/life15010006







