Two Small Peptides from Buthus martensii Hydrolysates Exhibit Antitumor Activity Through Inhibition of TNF-α-Mediated Signal Transduction Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of B. martensii Hydrolysates
2.3. LC-MS-Based Identification of Small Peptides
2.4. In Silico Prediction of Therapeutic Targets
2.5. Assessment of the Inflammatory Microenvironment In Vitro and In Vivo
2.6. Assessment of Protein Expression Levels
2.7. Statistical Analysis
3. Results and Discussion
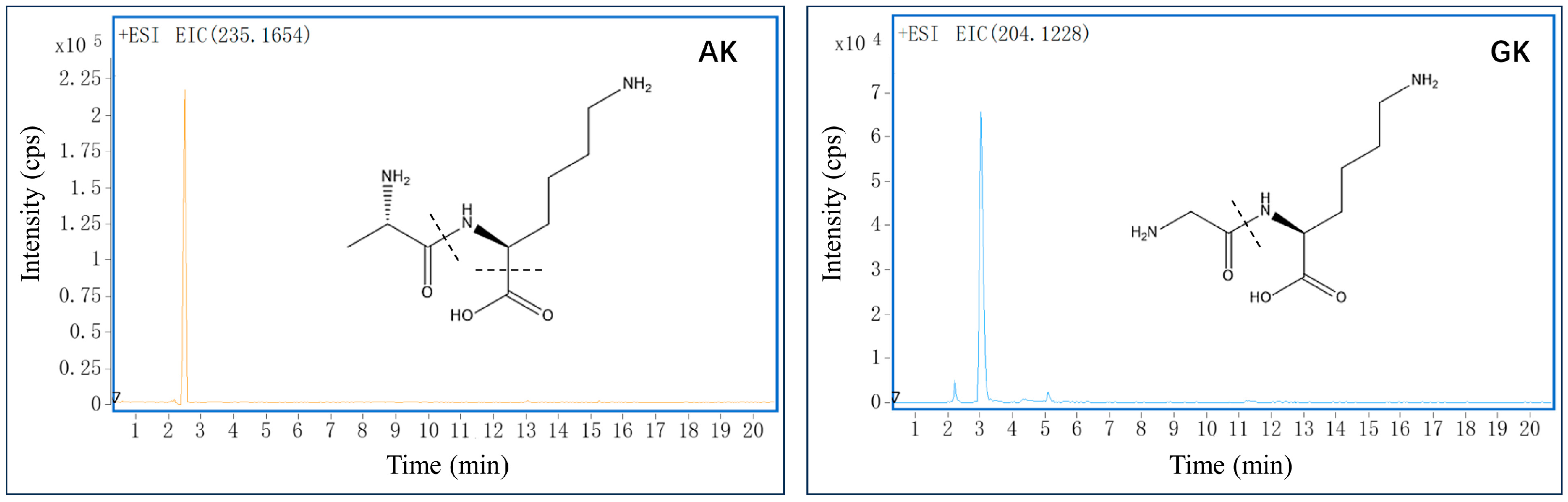
3.1. Identification of Small Peptides in B. martensii Hydrolysates
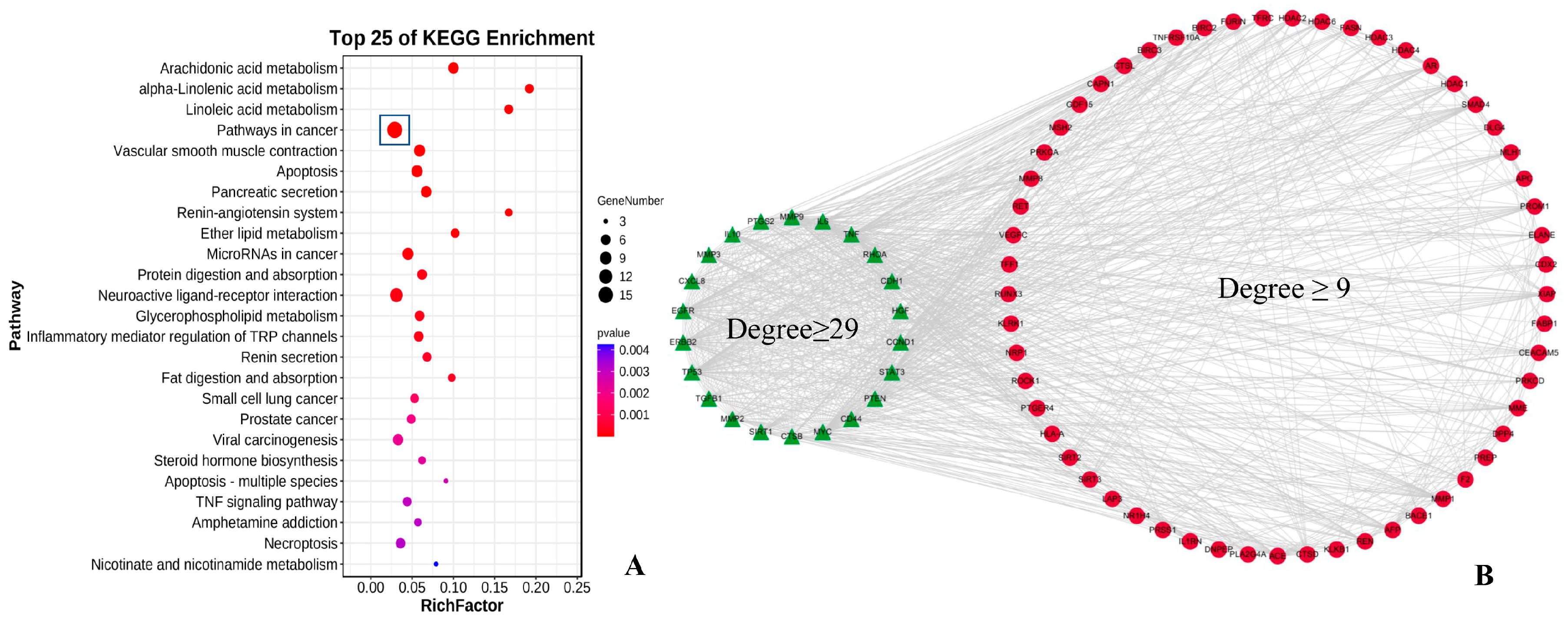
3.2. Bioinformatics Target Analysis
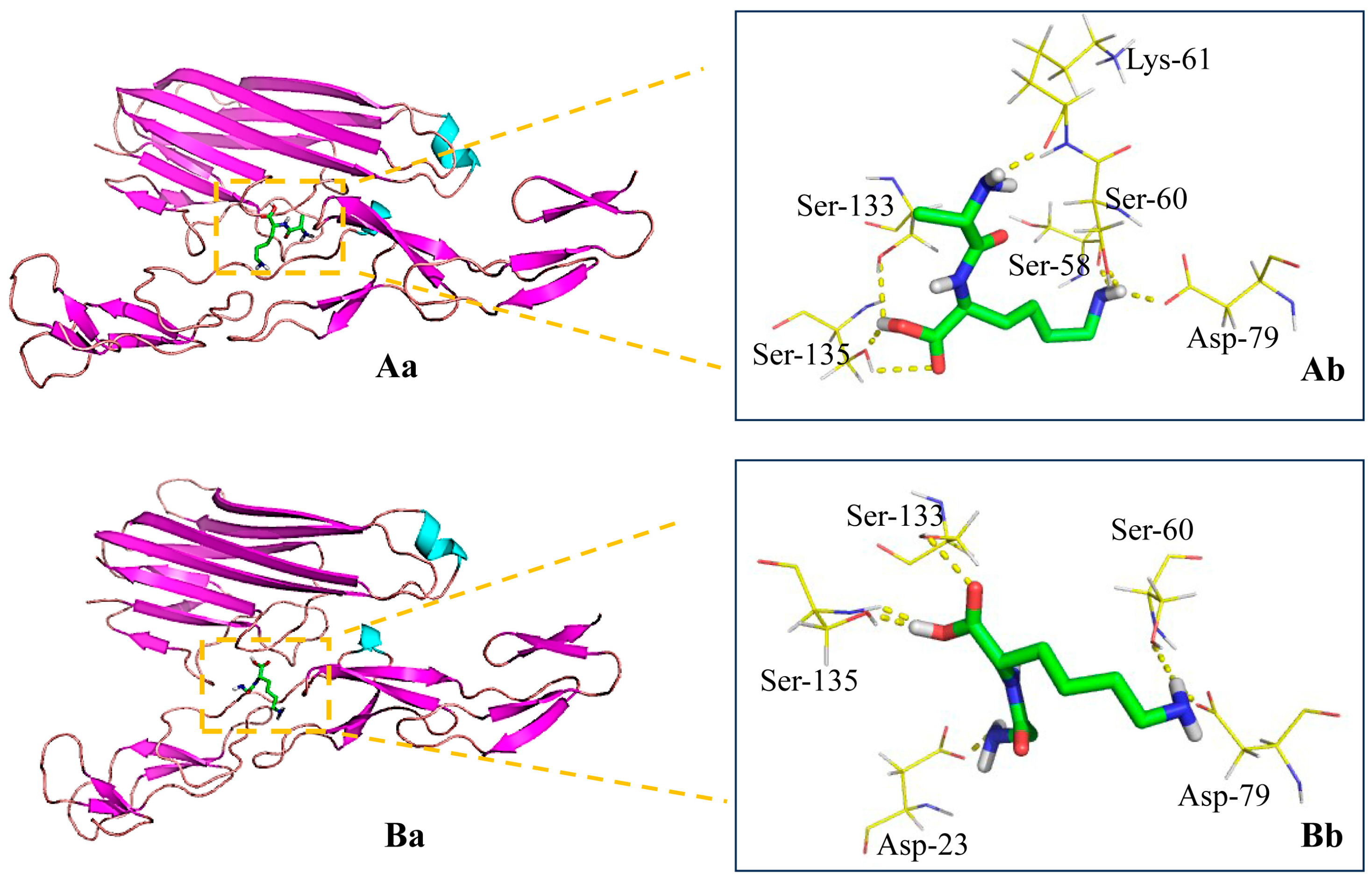
3.3. Binding of the Peptides to TNFR
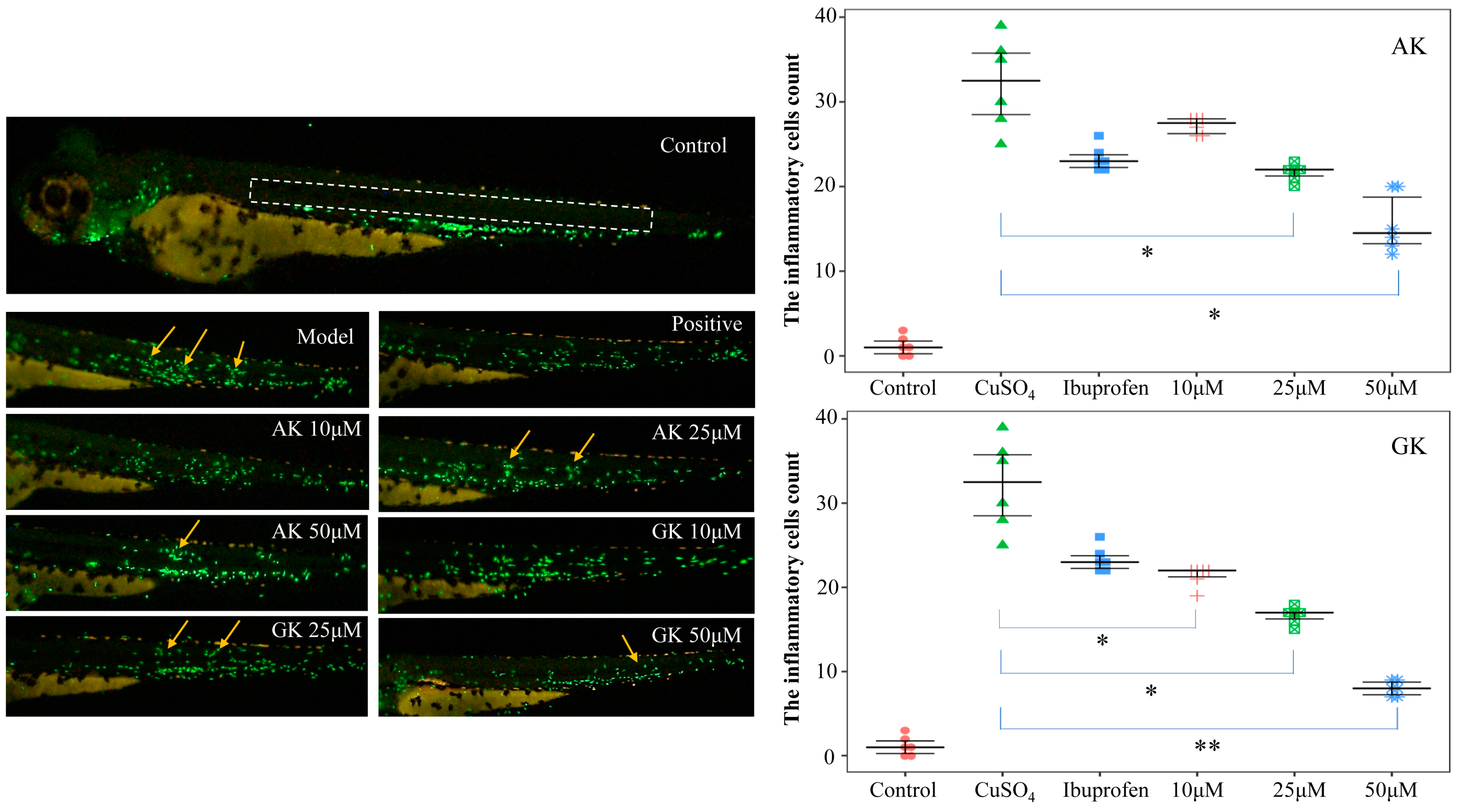
3.4. Effects of AK and GK on the Inflammatory Microenvironment
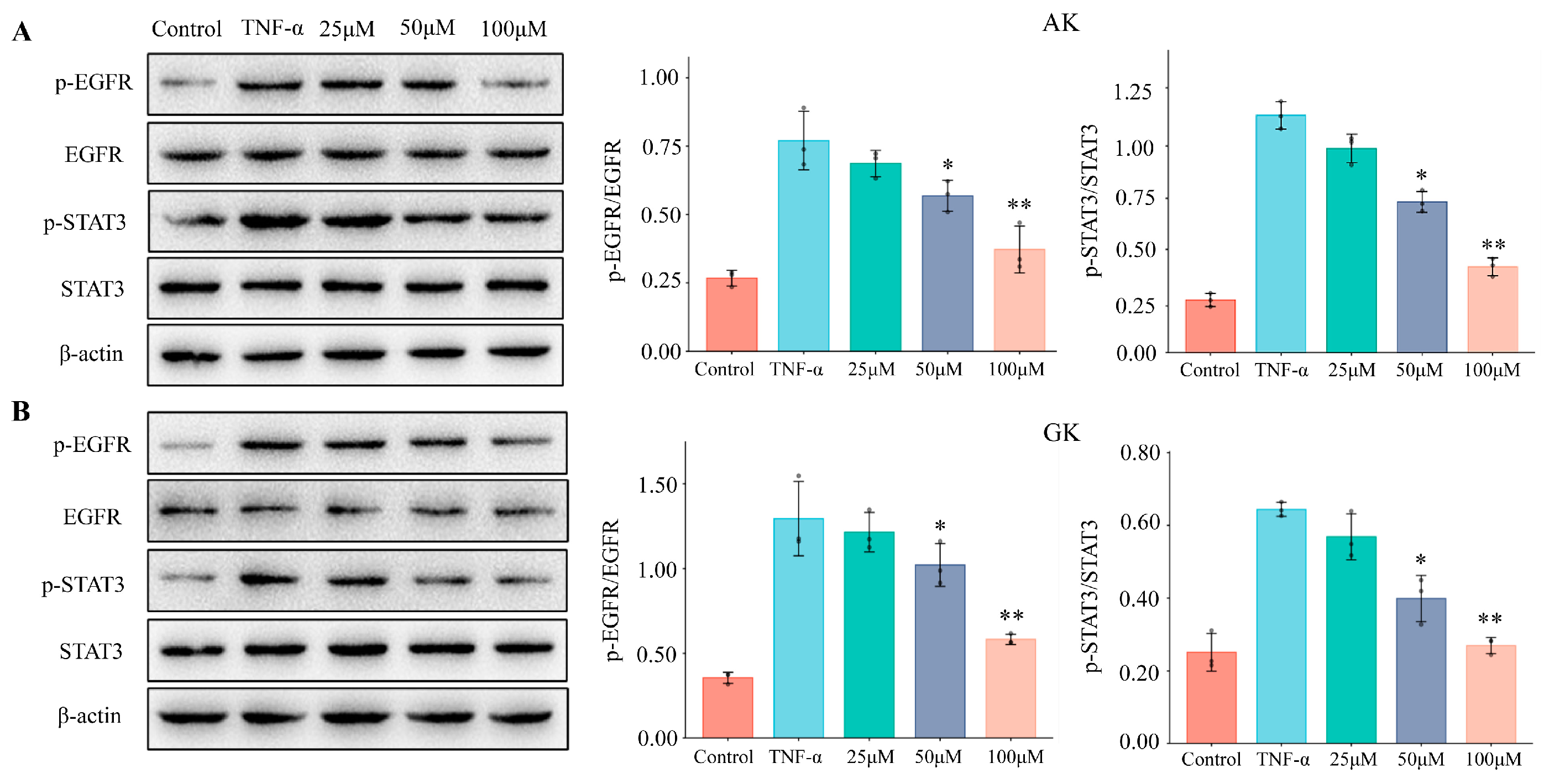
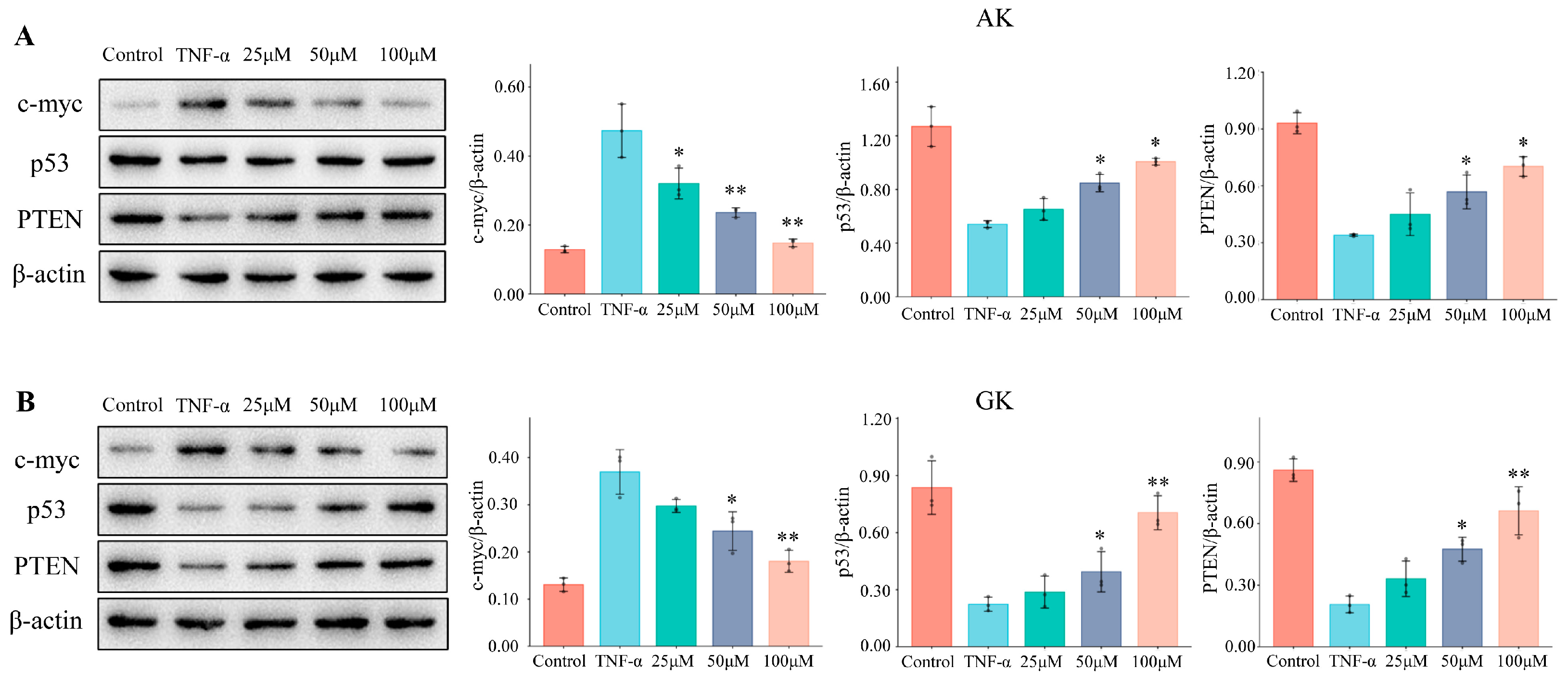
3.5. The Effects of AK and GK on Protein Expression Levels in MGC-803 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.M.; Huang, K.; Shi, M.X.; Gong, H.; Han, M.Y.; Tian, W.J.; Wang, X.Y.; Zhang, D.K. MicroRNAs in Helicobacter pylori-infected gastric cancer: Function and clinical application. Pharmacol. Res. 2024, 2024, 107216. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Bhatia, K.; Coban, S. Inflammation and Gastric Cancer. Diseases 2022, 10, 35. [Google Scholar] [CrossRef]
- Scaria, B.; Sood, S.; Raad, C.; Khanafer, J.; Jayachandiran, R.; Pupulin, A.; Grewal, S.; Okoko, M.; Arora, M.; Miles, L.; et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. Int. J. Mol. Sci. 2020, 21, 8480. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Song, X.; Guo, Y.N.; Song, P.; Duan, D.Z.; Chen, Z.S. Natural products: A promising therapeutics for targeting tumor angiogenesis. Front. Oncol. 2021, 11, 772915. [Google Scholar] [CrossRef]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour necrosis factor-α as a tumour promoter. Eur. J. Cancer 2006, 42, 745–750. [Google Scholar] [CrossRef]
- Bornschein, J.; Kandulski, A.; Selgrad, M.; Malfertheiner, P. From Gastric Inflammation to Gastric Cancer. Dig. Dis. 2010, 28, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, H.; Gao, S.J.; Wang, J.X. Review on Pharmacological Activities of the Peptides from Scorpion Buthus martensii Karsch. J. Pharm. Drug Dev. 2015, 3, 202. [Google Scholar]
- Dardevet, L.; Rani, D.; Aziz, T.A.E.; Bazin, I.; Sabatier, J.-M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A Helpful Natural Scorpion Peptide to Diagnose Glioma and Fight Tumor Invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef]
- Santos, F.F.; Silva, M.; de Matos, I.M. Scorpion Venom Peptides as Therapeutic Agents in Cardiovascular Diseases: A Systematic Review. Int. J. Pept. Res. Ther. 2025, 31, 12. [Google Scholar] [CrossRef]
- Mendes, L.C.; Viana, G.M.M.; Nencioni, A.L.A.; Pimenta, D.C.; Beraldo-Neto, E. Scorpion Peptides and Ion Channels: An Insightful Review of Mechanisms and Drug Development. Toxins 2023, 15, 238. [Google Scholar] [CrossRef]
- Almaaytah, A.; Albalas, Q. Scorpion venom peptides with no disulfide bridges: A review. Peptides 2014, 51, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, H.; Lai, F.R.; Yang, M.Y.; Li, X.F.; Tang, Y.Q. Isolation and identification of a novel anticoagulant peptide from enzymatic hydrolysates of scorpion (Buthus martensii Karsch) protein. Food Res. Int. 2014, 64, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Kong, C.; Wang, X.X.; Hou, H.R.; Yu, H.X.; Wang, L.Z.; Li, P.H.; Li, X.B.; Zhang, Y.; Han, L.W.; et al. LC-MS Analysis of ginsenosides in different parts of panax quinquefolius and their potential for coronary disease improvement. Planta Medica 2023, 89, 764–772. [Google Scholar] [CrossRef]
- Qaiser, H.; Saeed, M.; Nerukh, D.; Ul-Haq, Z. Structural insight into TNF-α inhibitors through combining pharmacophore-based virtual screening and molecular dynamic simulation. J. Biomol. Struct. Dyn. 2020, 39, 5920–5939. [Google Scholar] [CrossRef]
- Zang, S.L.; Tang, Q.L.; Dong, F.R.; Liu, H.; Li, L.L.; Guo, F.; Pan, X.D.; Lin, H.R.; Zeng, W.B.; Cai, Z.X.; et al. Curcumol inhibits the proliferation of gastric adenocarcinoma MGC-803 cells via downregulation of IDH1. Oncol. Rep. 2017, 38, 3583–3591. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, H.N.; Wang, L.Z.; Zhang, S.S.; Wang, F.X.; Lin, H.W.; Gao, S.; Li, X.B.; Liu, K.C. Anti-inflammatory peptides and metabolomics-driven biomarkers discovery from sea cucumber protein hydrolysates. J. Food Sci. 2021, 86, 3540–3549. [Google Scholar] [CrossRef]
- Sun, Y.; Du, C.L.; Wang, B.; Zhang, Y.L.; Liu, X.Y.; Ren, G.P. Up-regulation of eEF1A2 promotes proliferation and inhibits apoptosis in prostate cancer. Biochem. Biophys. Res. Commun. 2014, 450, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.F.; Qiao, C.X.; Cheng, X.F.; Wang, H.Z.; Liu, Q. Treatment of advanced gastric cancer using Guben Gongdu therapy: Analysis of 90 cases. World Chin. J. Dig. 2016, 24, 1087–1091. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Sharif, P.M.; Jabbari, P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine 2020, 130, 155066. [Google Scholar] [CrossRef]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can Cancer Get the Message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.B.; Lü, M.H.; Fan, Y.H.; Cao, Y.L.; Zhang, Z.R.; Yang, S.M. Macrophages in tumor microenvironments and the progression of tumors. J. Immunol. Res. 2012, 2012, 948098. [Google Scholar] [CrossRef]
- Yoo, J.; Perez, C.E.R.; Nie, W.X.; Edwards, R.A.; Sinnett-Smith, J.; Rozengurt, E. TNF-α induces upregulation of EGFR expression and signaling in human colonic myofibroblasts. AJP Gastrointest. Liver Physiol. 2012, 302, G805–G814. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.S.; Chan, L.K.Y.; Wong, E.C.H.; Hui, C.W.C.; Sneddon, K.; Cheung, T.H.; Yim, S.F.; Lee, J.H.S.; Yeung, C.S.Y.; Chung, T.K.H.; et al. A loop of cancer-stroma-cancer interaction promotes peritoneal metastasis of ovarian cancer via TNFα-TGFα-EGFR. Oncogene 2017, 36, 3576–3587. [Google Scholar] [CrossRef]
- Giraud, A.S.; Menheniott, T.R.; Judd, L.M. Targeting STAT3 in gastric cancer. Expert Opin. Ther. Targets 2012, 16, 889–901. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Orouei, S.; Zarrin, V.; Rahmani Moghadam, E.; Zabolian, A.; Mohammadi, S.; Hushmandi, K.; Gharehaghajlou, Y.; Makvandi, P.; et al. STAT3 Pathway in Gastric Cancer: Signaling, Therapeutic Targeting and Future Prospects. Biology 2020, 9, 126. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Zhou, Z.X.; Ji, Y.L. Suppression of EGFR-STAT3 signaling inhibits tumorigenesis in a lung cancer cell line. Int. J. Clin. Exp. Med. 2014, 7, 2096. [Google Scholar]
- Wu, J.; Patmore, D.M.; Jousma, E.; Eaves, D.W.; Breving, K.; Patel, A.V.; Schwartz, E.B.; Fuchs, J.R.; Cripe, T.P.; Stemmer-Rachamimov, A.O.; et al. EGFR-STAT3 signaling promotes formation of malignant peripheral nerve sheath tumors. Oncogene 2014, 33, 173–180. [Google Scholar] [CrossRef]
- Gao, F.Y.; Li, X.T.; Xu, K.; Wang, R.T.; Guan, X.X. c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Commun. Signal. 2023, 21, 28. [Google Scholar] [CrossRef]
- Zilfou, J.T.; Lowe, S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009, 1, a001883. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, J.Y.; He, L.N.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Domon-Dell, C.; Kang, J.; Chung, D.H.; Freund, J.N.; Evers, B.M. Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-α/nuclear factor-κB (NF-κB)-inducing kinase/NF-κB pathway is linked to a default IκB-α autoregulatory loop. J. Biol. Chem. 2004, 279, 4285–4291. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharjee, A.; Ali, A.; Mandal, N.C.; Mandal, S.C.; Pal, M. Chronic inflammation and cancer: Potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J. Inflamm. 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Zhang, S.; Tang, J.; Hou, H.; Wang, L.; Lin, H.; Zhang, X.; Jin, M. Two Small Peptides from Buthus martensii Hydrolysates Exhibit Antitumor Activity Through Inhibition of TNF-α-Mediated Signal Transduction Pathways. Life 2025, 15, 105. https://doi.org/10.3390/life15010105
Zhu M, Zhang S, Tang J, Hou H, Wang L, Lin H, Zhang X, Jin M. Two Small Peptides from Buthus martensii Hydrolysates Exhibit Antitumor Activity Through Inhibition of TNF-α-Mediated Signal Transduction Pathways. Life. 2025; 15(1):105. https://doi.org/10.3390/life15010105
Chicago/Turabian StyleZhu, Mengshuang, Shanshan Zhang, Jiyang Tang, Hairong Hou, Lizhen Wang, Houwen Lin, Xuanming Zhang, and Meng Jin. 2025. "Two Small Peptides from Buthus martensii Hydrolysates Exhibit Antitumor Activity Through Inhibition of TNF-α-Mediated Signal Transduction Pathways" Life 15, no. 1: 105. https://doi.org/10.3390/life15010105
APA StyleZhu, M., Zhang, S., Tang, J., Hou, H., Wang, L., Lin, H., Zhang, X., & Jin, M. (2025). Two Small Peptides from Buthus martensii Hydrolysates Exhibit Antitumor Activity Through Inhibition of TNF-α-Mediated Signal Transduction Pathways. Life, 15(1), 105. https://doi.org/10.3390/life15010105






