The Etiological and Antimicrobial Susceptibility Profiles of the Bacteria Obtained from Ovine Caseous Lymphadenitis Cases in the Çankırı Region, Türkiye
Abstract
1. Introduction
2. Material and Method
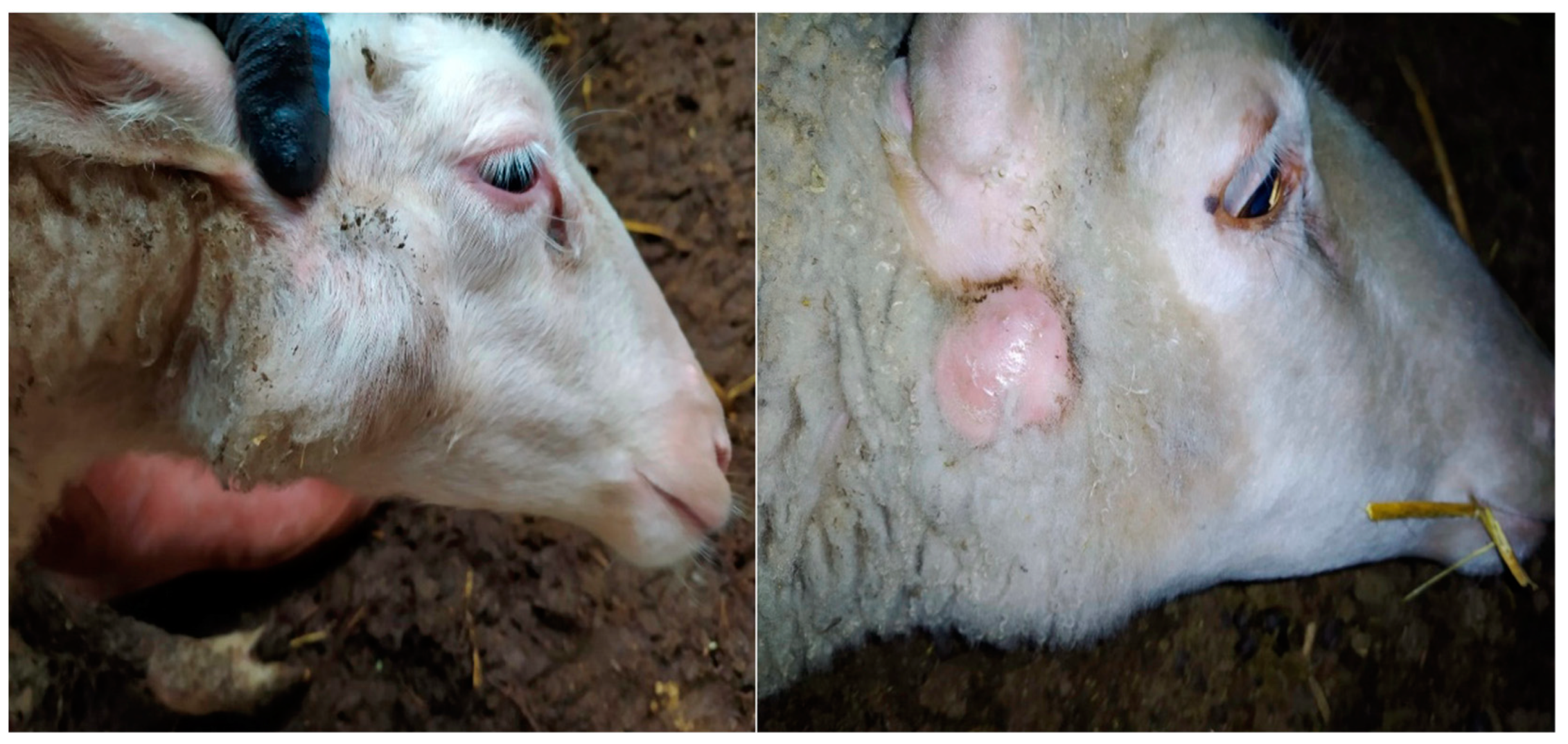
2.1. Acquisition of Samples
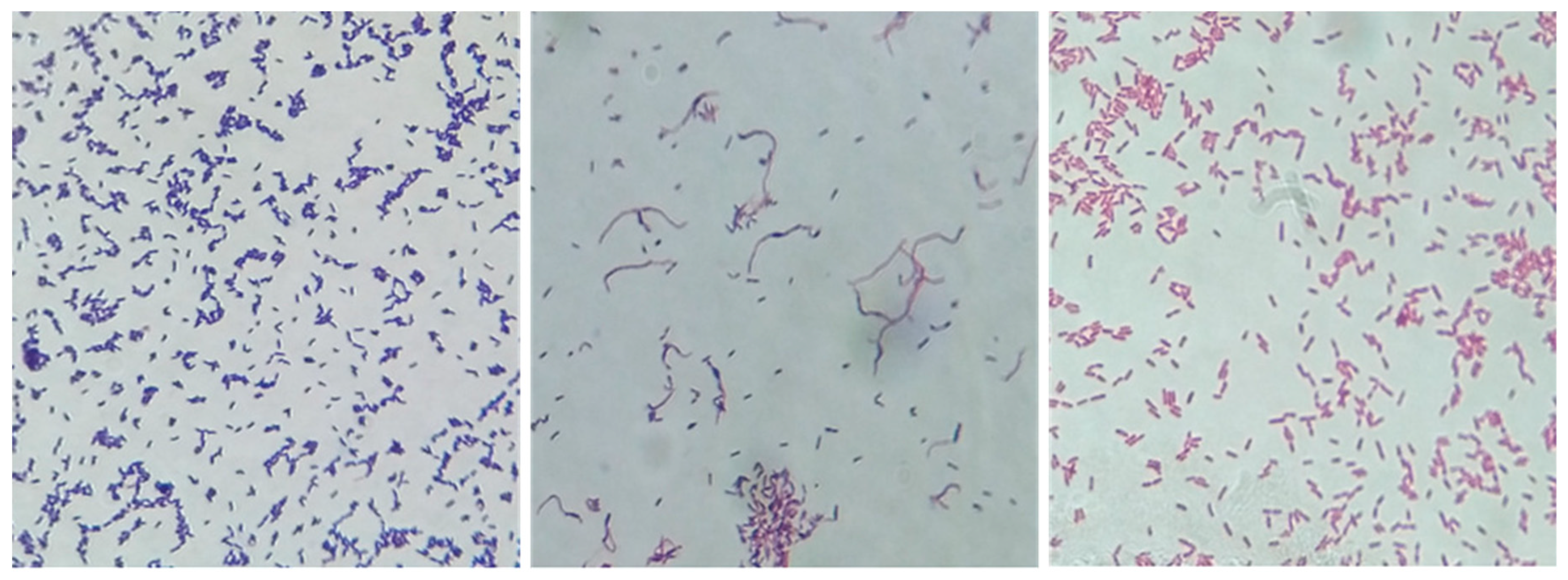
2.2. Bacterial Culture
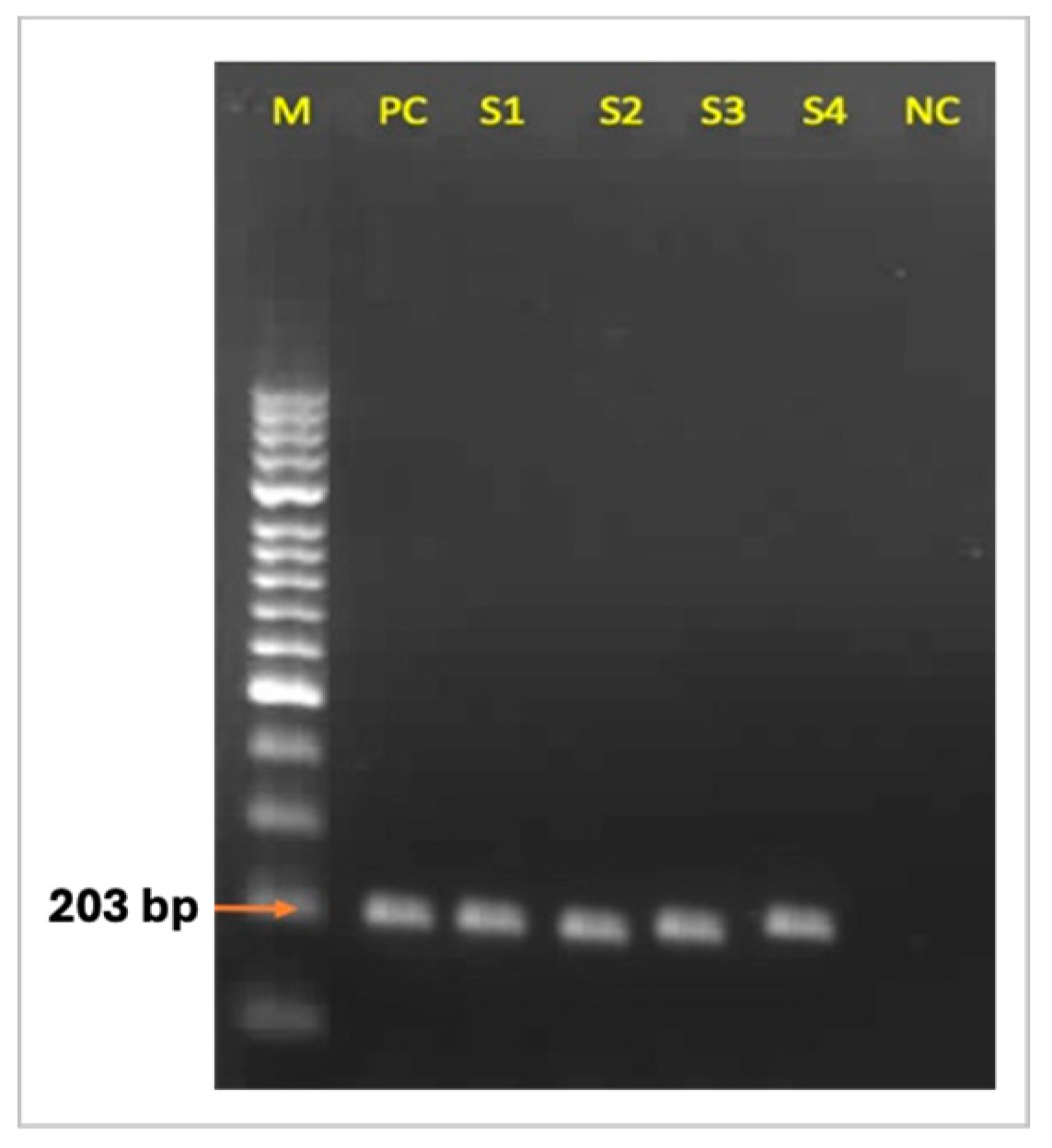
2.3. C. Pseudotuberculosis-Specific PCR
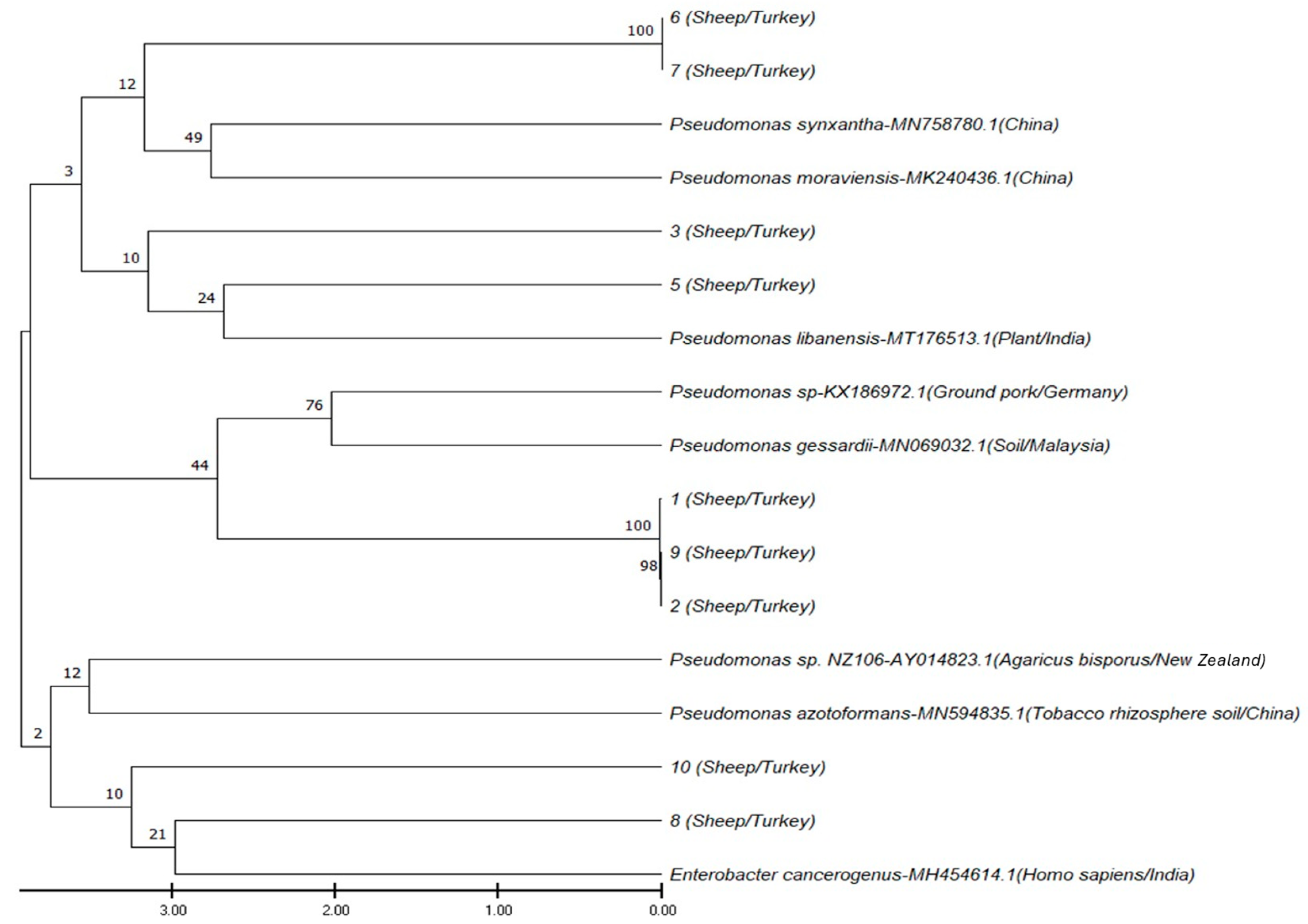
2.4. 16S rRNA Gene Sequencing and Phylogenetic Analysis
2.5. Antibiotic Sensitivity Test
3. Results
3.1. Bacterial Culture Findings
3.2. C. Pseudotuberculosis-Specific PCR Findings
3.3. 16S rRNA Gene Sequencing and Phylogenetic Analysis Findings
3.4. Antibiotic Sensitivity Test Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Günaydın, G. Koyun yetiştiriciliğinin ekonomi politiği. Uludag Üniv. Ziraat Fak. Derg. 2009, 23, 15–32. Available online: https://dergipark.org.tr/tr/download/article-file/154093 (accessed on 7 July 2024). (In Turkish).
- De Pinho, R.B.; De Oliveira Silva, M.T.; Bezerra, F.S.B.; Borsuk, S. Vaccines for caseous lymphadenitis: Up-to-date and forward-looking strategies. Appl. Microbiol. Biotechnol. 2021, 105, 2287–2296. [Google Scholar] [CrossRef]
- Khanamir, R.A.; Issa, N.A.; Abdulrahman, R.F. First study on molecular epidemiology of caseous lymphadenitis in slaughtered sheep and goats in Duhok Province, Iraq. Open Vet. J. 2023, 13, 588–598. [Google Scholar] [CrossRef]
- Zidan, K.H.; Mazloum, K.; Saran, M.A.; Hatem, M.E. Abscesses in dromedary camels, sheep and goats etiology and pathology. In Proceedings of the 1st International Scientific of Pathology Conference, Faculty of Veterinary Medicine-Cairo University, Sheraton Dreamland Hotel Conference Center, Giza, Egypt, 25–27 April 2013; Available online: https://www.researchgate.net/publication/272090416_ABSCESSES_IN_DROMEDARY_CAMELS_SHEEP_AND_GOATS_ETIOLOGY_AND_PATHOLOGY (accessed on 7 July 2024).
- Al-Harbi, K.B. Prevalence and etiology of abscess disease of sheep and goats at Qassim region, Saudi Arabia. Vet. World 2011, 4, 495–499. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef]
- De La Fuente, R.; De Las Heras, M.; Torrijos, C.; De Tejada, P.D.; Pérez-Sancho, M.; Carrión, F.J.; Dominguez-Bernal, G. Isolation frequency of bacteria causing lymphadenitis and abscesses in small ruminants in central Spain. Small Rumin. Res. 2017, 154, 5–8. [Google Scholar] [CrossRef]
- Batey, R.G. Pathogenesis of caseous lymphadenitis in sheep and goats. Aust. Vet. J. 1986, 63, 269–272. [Google Scholar] [CrossRef]
- Osman, A.Y.; Nordin, M.L.; Kadir, A.A.; Saharee, A.A. The epidemiology and pathophysiology of caseous lymphadenitis: A review. J. Vet. Med. Res. 2018, 5, 1129. [Google Scholar] [CrossRef]
- Pepin, M.; Cannella, D.; Fontaine, J.J.; Pittet, J.C.; Le Pape, A. Ovine mononuclear phagocytes in situ: Identification by monoclonal antibodies and involvement in experimental pyogranulomas. J. Leukoc. Biol. 1992, 51, 188–198. [Google Scholar] [CrossRef]
- Baird, G.J.; Fontaine, M.C. Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J. Comp. Pathol. 2007, 137, 179–210. [Google Scholar] [CrossRef]
- Torky, H.A.; Saad, H.M.; Khaliel, S.A.; Kassih, A.T.; Sabatier, J.M.; Batiha, G.E.S.; Hetta, H.F.; Elghazaly, E.M.; De Waard, M. Isolation and molecular characterization of Corynebacterium pseudotuberculosis: Association with proinflammatory cytokines in caseous lymphadenitis pyogranulomas. Animals 2023, 13, 296. [Google Scholar] [CrossRef]
- Ilhan, Z. Detection of Corynebacterium pseudotuberculosis from sheep lymph nodes by PCR. Rev. Med. Vet. 2013, 164, 60–66. [Google Scholar]
- Sakmanoğlu, A.; Hadimli, H.H.; Erganiş, O.; Pınarkara, Y.; Sayın, Z.; Kav, K. Identification and antimicrobial susceptibility of Corynebacterium pseudotuberculosis isolated from sheep. Eurasian J. Vet. Sci. 2015, 31, 116–121. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Akça, D.; Büyük, F.; Karakaya, E.; Coşkun, M.R.; Çelik, E.; Şahin, M.; Çelebi, Ö.; Otlu, S.; Gülmez Sağlam, A.; Eray, B.; et al. Molecular characterization of Bacillus anthracis isolates recovered from nomic andnonnomic hosts. Turk. J. Vet. Anim. Sci. 2022, 46, 44–51. [Google Scholar] [CrossRef]
- Wayne, P.A. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing. Inform. Suppl. 2011, 31, 100–121. Available online: https://www.sid.ir/paper/655659/en (accessed on 19 July 2024).
- Connor, K.M.; Quirie, M.M.; Baird, G.; Donachie, W. Characterization of United Kingdom isolates of Corynebacterium pseudotuberculosis using pulsed-field gel electrophoresis. J. Clin. Microbiol. 2000, 38, 2633–2637. [Google Scholar] [CrossRef]
- Abdulrahman, R.F. Virulence potential, antimicrobial susceptibility and phylogenetic analysis of corynebacterium pseudotuberculosis isolated from caseous lymphadenitis in sheep and goats in duhok city, Iraq. Adv. Anim. Vet. Sci. 2021, 9, 919–925. [Google Scholar] [CrossRef]
- Ali, A.D.; Mahmoud, A.A.; Khadr, A.M.; Elshemey, T.M.; Abdelrahman, A.H. Corynebacterium pseudotuberculosis: Disease prevalence, lesion distribution, and diagnostic comparison through microbiological culture and molecular diagnosis. Alex. J. Vet. Sci. 2016, 51, 189–198. [Google Scholar] [CrossRef]
- Mazreku, N.; Sylejmani, D.; Hamidi, A.; Robaj, A. The effect of vitamin h in aseptic laminitis prevention and its impact on blood indicators in dairy cows. Bulg. J. Agric. Sci. 2017, 23, 315–318. [Google Scholar]
- Saleh, W.M.M.; Lafta, M.H.; Abdulrazaq, A.W.; Habib, H.N.; Naeem, L.A. Bacteriological and histopathological evaluation of infectious lymphadenitis caused by Pseudomonas aeruginosa in awasi sheep. Adv. Anim. Vet. Sci. 2019, 7, 378–382. [Google Scholar] [CrossRef]
- Ruchi Tiwari, R.; Yadav, S.K.; Singh, S.; Gangwar, N.K. Bacterial etiology of skin and wound infections along with antibiogram profiling in reference to emerging antibiotic resistance in animals. Adv. Anim. Vet. Sci. 2015, 3, 259–268. [Google Scholar] [CrossRef]
- Latif, N.A.A.; Abdullah, F.F.J.; Othman, A.M.; Rina, A.; Chung, E.L.T.; Zamri-Saad, M.; Saharee, A.A.; Haron, A.W.; Lila, M.A.M. Isolation and detection of Corynebacterium pseudotuberculosis in the reproductive organs and associated lymph nodes of non-pregnant does experimentally inoculated through intradermal route in chronic form. Vet. World 2015, 8, 924–927. [Google Scholar] [CrossRef]
- Umer, M.; Abba, Y.; Abdullah, F.F.J.; Saleh, W.M.M.; Haron, A.W.; Saharee, A.A.; Ariff, A.B.; Baiee, F.H.A.; Hambali, I.U.; Sharif, A. Caseous lymphadenitis in small ruminants: An overview on reproductive implications. Int. J. Vet. Sci. Anim. Husb. 2017, 2, 23–31. [Google Scholar]
- Benito-Peña, A.; Peris, B.; Aduriz, G.; Martinez, J.; Corpa, J.M. Purulent nasomaxillary and mandibular osteomyelitis in sheep caused by Pseudomonas aeruginosa. Vet. Rec. 2010, 166, 115–116. [Google Scholar] [CrossRef]
- D’Agata, E.; Araos, R. Pseudomonas aeruginosa and other Pseudomonas species. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elseiver: Saunders, WB, USA, 2019; Volume 2, pp. 2686–2699. [Google Scholar]
- Abo-Amer, A.E.; Shobrak, M.Y. Antibiotic resistance and molecular characterization of Enterobacter cancerogenus isolated from wild birds in Taif province, Saudi Arabia. Thai J. Vet. Med. 2015, 45, 101–111. [Google Scholar] [CrossRef]
- Salimiyan Rizi, K.; Ghazvini, K.; Farsiani, H. Clinical and pathogenesis overview of Enterobacter infections. Rev. Clin. Med. 2020, 6, 146–154. [Google Scholar] [CrossRef]
- Washburn, K.E.; Bissett, W.T.; Fajt, V.R.; Libal, M.C.; Fosgate, G.T.; Miga, J.A.; Rockey, K.M. Comparison of three treatment regimens for sheep and goats with caseous lymphadenitis. J. Am. Vet. Med. Assoc. 2009, 234, 1162–1166. [Google Scholar] [CrossRef]
- Algammal, A. Molecular characterization and antibiotic susceptibility of Corynebacterium pseudotuberculosis isolated from sheep and goats suffering from caseous lymphadenitis. Zagazig Vet. J. 2016, 44, 1–8. [Google Scholar] [CrossRef]
- Abebe, D.; Sisay Tessema, T. Determination of Corynebacterium pseudotuberculosis prevalence and antimicrobial susceptibility pattern of isolates from lymph nodes of sheep and goats at an organic export abattoir, Modjo, Ethiopia. Lett. Appl. Microbiol. 2015, 61, 469–476. [Google Scholar] [CrossRef]
- El Damaty, H.M.; El-Demerdash, A.S.; Abd El-Aziz, N.K.; Yousef, S.G.; Hefny, A.A.; Abo Remela, E.M.; Shaker, A.; Elsohaby, I. Molecular characterization and antimicrobial susceptibilities of Corynebacterium pseudotuberculosis isolated from caseous lymphadenitis of smallholder sheep and goats. Animals 2023, 13, 2337. [Google Scholar] [CrossRef] [PubMed]
- İlhan, Z. In vitro antimicrobial susceptibility of Corynebacterium pseudotuberculosis isolated from sheep with caseous lymphadenitis. Kocatepe Vet. J. 2020, 13, 267–271. [Google Scholar] [CrossRef]
- Hussein, Z.T.; Mukhlif, S.S.; Taha, M.; Ali, S.A. Identification and characterization of Pseudomonas aeruginosa 16S rRNA gene isolated from contaminated soil with oil residues. In IOP Conference Series: Earth and Environmental Science, Proceedings of the Ninth National Conference on the Environment and Natural Resources (NCENR-2023), Basrah, Iraq, 8–9 Agust 2023; IOP Publishing: Bristol, UK, 2023; Volume 1215, p. 012001. [Google Scholar] [CrossRef]
- Lupo, A.; Haenni, M.; Madec, J.Y. Antimicrobial resistance in Acinetobacter spp. and Pseudomonas spp. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, H.L.; Ran, L.Y.; Li, C.Y.; Zhang, X.Y.; Su, H.N.; Shi, M.; Zhou, B.C.; Chen, X.L.; Zhang, Y.Z. Mechanistic insights into elastin degradation by pseudolysin, the major virulence factor of the opportunistic pathogen Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 9936. [Google Scholar] [CrossRef] [PubMed]
- Cabassi, C.S.; Sala, A.; Santospirito, D.; Alborali, G.L.; Carretto, E.; Ghibaudo, G.; Taddei, S. Activity of AMP2041 against human and animal multidrug resistant Pseudomonas aeruginosa clinical isolates. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 17. [Google Scholar] [CrossRef]
- Dapgh, A.N.; Hakim, A.S.; Abouelhag, H.A.; Abdou, A.M.; Elgabry, E.A. Detection of virulence and multidrug resistance operons in Pseudomonas aeruginosa isolated from Egyptian Baladi sheep and goat. Vet. World 2019, 12, 1524–1528. [Google Scholar] [CrossRef]
- Mahmood, A.N.; Aljobori, A.H. Isolation and identification of Pseudomonas aeruginosa from infected sheep and detection of phosolipase C (lecithinase). Iraqi J. Vet. Medicine 2015, 39, 28–32. [Google Scholar] [CrossRef]
- Saha, T.K.; Begum, F.; Kabir, S.L.; Islam, M.S.; Khan, M.S.R. Characterization of bacterial isolates from skin lesions of sheep, goat and cattle in different rearing condition. Asian J. Med. Biol. Res. 2019, 5, 117–125. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Diversity and antibiotic resistance in Pseudomonas spp. from drinking water. Sci. Total Environ. 2012, 426, 366–374. [Google Scholar] [CrossRef]
| Species | Primer Sequence (5′—3′) | Target Gene | PCR Component (25 μL) | Thermal Cycle | Amplicon (bp) | Reference |
|---|---|---|---|---|---|---|
| C. pseudotuberculosis | PLDF- ATAAGCGTAAGCAGGGAGCA PLDR1- ATCAGCGGTGATTGTCTTCC PLDR2- ATCAGCGGTGATTGTCTTCCAGG | pld |
| One cycle:
| 203 | [13] |
| Unknown species specific | 27F- AGAGTTTGATC(AC)TGGCTCAG 1492R- ACGG(CT)TTACCTTGTTACGACTT | 16S rRNA | One cycle:
| 1465 | [16] |
| Number of Isolate | Cultural and Phenotypic Characteristics | Species Name | NCBI Accession Number |
|---|---|---|---|
| 1 | Gram-negative, bacilli | P. helleri | OR512107 |
| 2 | Gram-negative, bacilli | P. synxantha | OR512109 |
| 3 | Gram-negative, bacilli | P. moraviensis | OR512111 |
| 5 | Gram-negative, bacilli | P. libanensis | OR512104 |
| 6 | Gram-negative, bacilli | P. fluorescens | OR512105 |
| 7 | Gram-negative, bacilli | P. fluorescens | OR512106 |
| 8 | Gram-negative, bacilli | P. azotoformans | OR512112 |
| 9 | Gram-negative, bacilli | P. gessardii | OR512108 |
| 10 | Gram-negative, cocobacilli | E. cancerogenus | OR512110 |
| Antibiotic (Concentration) | Susceptible | Intermediate | Resistant |
|---|---|---|---|
| KF (30 µg) | 0 (0%) | 0 (0%) | 30 (100%) |
| EFT (30 µg) | 0 (0%) | 0 (0%) | 30 (100%) |
| OBS (5 µg) | 0 (0%) | 0 (0%) | 30 (100%) |
| CFP (75 µg) | 27 (90%) | 0 (0%) | 3 (10%) |
| AML (25 µg) | 0 (0%) | 0 (0%) | 30 (100%) |
| LS (109 µg) | 27 (90%) | 0 (0%) | 3 (10%) |
| PNV (40 µg) | 18 (60%) | 0 (0%) | 12 (40%) |
| S (10 U) | 0 (0%) | 0 (0%) | 30 (100%) |
| BCDD (10 U) | 0 (0%) | 0 (0%) | 30 (100%) |
| Antibiotic (Concentration) | Susceptible | Intermediate | Resistant |
|---|---|---|---|
| KF (30 µg) | 0 (0%) | 0 (0%) | 8 (100%) |
| EFT (30 µg) | 0 (100%) | 0 (0%) | 8 (100%) |
| OBS (5 µg) | 0 (0%) | 0 (0%) | 8 (100%) |
| CFP (75 µg) | 8 (100%) | 0 (0%) | 0 (0%) |
| AML (25 µg) | 0 (100%) | 1 (11%) | 8 (89%) |
| LS (109 µg) | 7 (87.5%) | 0 (0%) | 1 (12.5%) |
| PNV (40 µg) | 0 (0%) | 0 (0%) | 8 (0%) |
| S (10 U) | 1 (12.5%) | 4 (50%) | 3 (37.5%) |
| BCDD (10 U) | 0 (0%) | 0 (0%) | 8 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarhane, S.; Büyük, F. The Etiological and Antimicrobial Susceptibility Profiles of the Bacteria Obtained from Ovine Caseous Lymphadenitis Cases in the Çankırı Region, Türkiye. Life 2024, 14, 1078. https://doi.org/10.3390/life14091078
Tarhane S, Büyük F. The Etiological and Antimicrobial Susceptibility Profiles of the Bacteria Obtained from Ovine Caseous Lymphadenitis Cases in the Çankırı Region, Türkiye. Life. 2024; 14(9):1078. https://doi.org/10.3390/life14091078
Chicago/Turabian StyleTarhane, Serdal, and Fatih Büyük. 2024. "The Etiological and Antimicrobial Susceptibility Profiles of the Bacteria Obtained from Ovine Caseous Lymphadenitis Cases in the Çankırı Region, Türkiye" Life 14, no. 9: 1078. https://doi.org/10.3390/life14091078
APA StyleTarhane, S., & Büyük, F. (2024). The Etiological and Antimicrobial Susceptibility Profiles of the Bacteria Obtained from Ovine Caseous Lymphadenitis Cases in the Çankırı Region, Türkiye. Life, 14(9), 1078. https://doi.org/10.3390/life14091078






