Role of Immune Cells and Immunotherapy in Multiple Myeloma
Abstract
1. Introduction
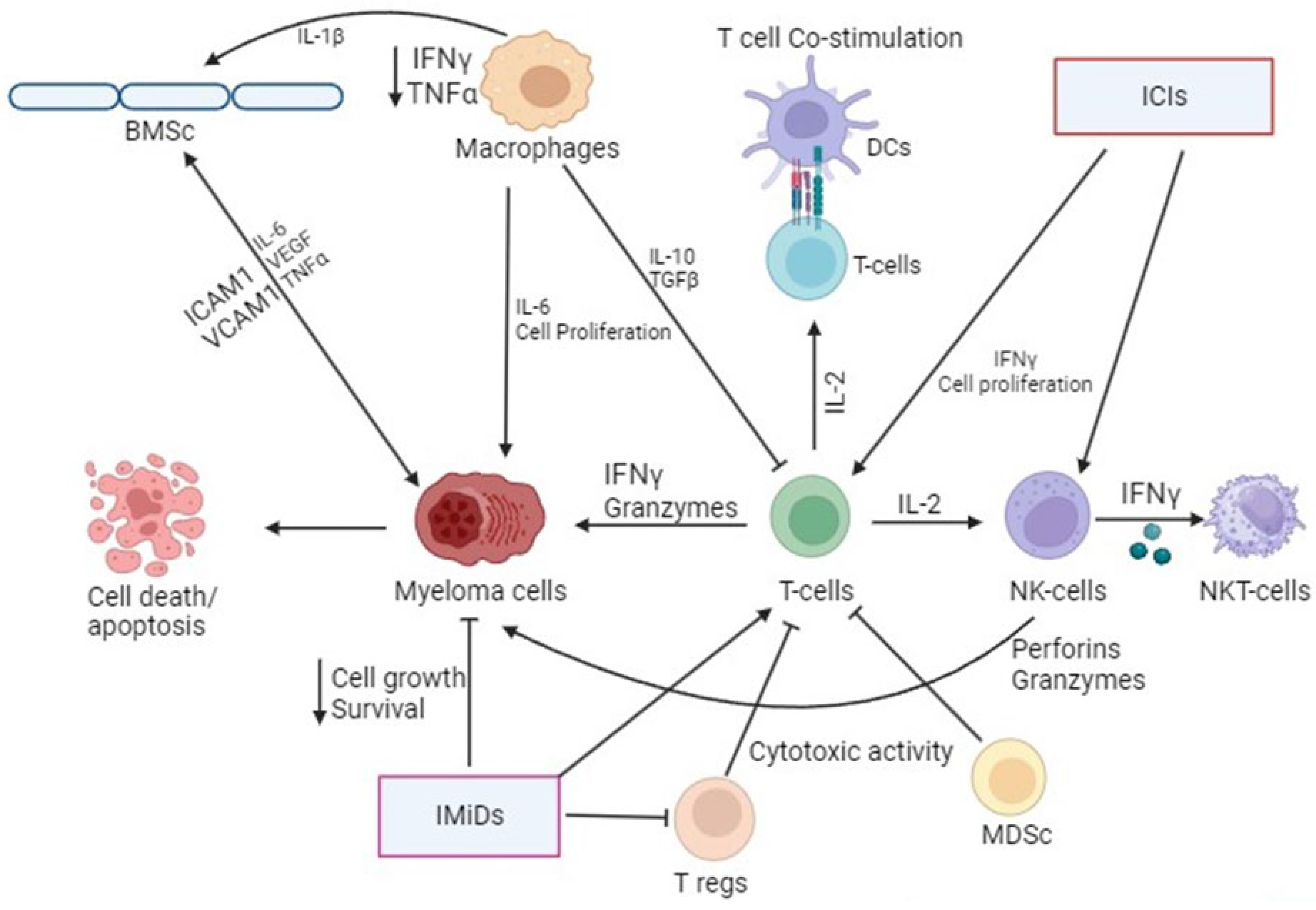
2. Role of Immune Cells in Multiple Myeloma (MM)
2.1. T Cells
2.2. Helper T Cells
2.3. Regulatory T Cells (Tregs)
2.4. B Cells
2.5. Macrophages
2.6. Myeloid-Derived Suppressor Cells (MDSCs)
2.7. Natural Killer (NK) and Natural Killer T Cells (NKT Cells)
2.8. Dendritic Cells (DCs)
3. Regulation of Immune Cells by Immunotherapy in Multiple Myeloma
3.1. Immunomodulatory Drugs
3.2. Immune Checkpoint Inhibitors (ICIs)
3.3. Chimeric Antigen Receptor (CAR)-T Cell Therapy
3.3.1. B-Cell Maturation Antigen (BCMA) CAR-T
3.3.2. Non-BCMA CAR-T
3.3.3. CAR-T Therapy Limitations and Toxicities
4. Limitations of Immunotherapy
5. Future Perspectives in the Field of Immunotherapy
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of Minimal Residual Disease with Superior Survival Outcomes in Patients with Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Zaknoen, S.L.; Kay, N.E. Immunoregulatory cell dysfunction in chronic B-cell leukemias. Blood Rev. 1990, 4, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Barlogie, B.; Epstein, J.; Selvanayagam, P.; Alexanian, R. Plasma cell myeloma—New biological insights and advances in therapy. Blood 1989, 73, 865–879. [Google Scholar] [CrossRef] [PubMed]
- ACS. Key Statistics about Multiple Myeloma. Available online: https://www.cancer.org/cancer/types/multiple-myeloma.html (accessed on 25 February 2024).
- Kopp, H.G.; Avecilla, S.T.; Hooper, A.T.; Rafii, S. The bone marrow vascular niche: Home of HSC differentiation and mobilization. Physiology 2005, 20, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Xu, H.; Wang, L.; Kryczek, I.; Wu, K.; Hu, Y.; Wang, G.; Zou, W. Bone marrow and the control of immunity. Cell. Mol. Immunol. 2012, 9, 11–19. [Google Scholar] [CrossRef]
- Harousseau, J.L. Multiple myeloma in the elderly: When to treat, when to go to transplant. Oncology 2010, 24, 992–998. [Google Scholar] [PubMed]
- Kumar, A.; Galeb, S.; Djulbegovic, B. Treatment of patients with multiple myeloma: An overview of systematic reviews. Acta Haematol. 2011, 125, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Lonial, S. Evidence-based mini-review: Treatment options for patients with relapsed/refractory myeloma previously treated with novel agents and high-dose chemotherapy and autologous stem-cell transplantation. Hematol. Am. Soc. Hematol. Educ. Program 2010, 2010, 310–313. [Google Scholar] [CrossRef]
- Serafini, P.; Meckel, K.; Kelso, M.; Noonan, K.; Califano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006, 203, 2691–2702. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Lokhorst, H.M.; Dimopoulos, M.; Cavo, M.; Morgan, G.; Einsele, H.; Kropff, M.; Schey, S.; Avet-Loiseau, H.; Ludwig, H.; et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat. Rev. 2011, 37, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Moschetta, M.; Manier, S.; Glavey, S.; Gorgun, G.T.; Roccaro, A.M.; Anderson, K.C.; Ghobrial, I.M. Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 2015, 263, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Solimando, A.G.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune Cells in the Myeloma Niche. Front. Oncol. 2020, 10, 599098. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Chauhan, D.; Anderson, K.C. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 2009, 23, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- George, L.L.; Deshpande, S.R.; Cortese, M.J.; Kendall, E.K.; Chattaraj, A.; Shah, Z.; Zhao, J.; Anwer, F. Emerging Targets and Cellular Therapy for Relapsed Refractory Multiple Myeloma: A Systematic Review. Clin. Lymphoma Myeloma Leuk. 2021, 21, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Sacco, A.; Leleu, X.; Ghobrial, I.M.; Roccaro, A.M. Bone marrow microenvironment in multiple myeloma progression. J. Biomed. Biotechnol. 2012, 2012, 157496. [Google Scholar] [CrossRef]
- Qureshi-Baig, K.; Kuhn, D.; Viry, E.; Pozdeev, V.I.; Schmitz, M.; Rodriguez, F.; Ullmann, P.; Koncina, E.; Nurmik, M.; Frasquilho, S.; et al. Hypoxia-induced autophagy drives colorectal cancer initiation and progression by activating the PRKC/PKC-EZR (ezrin) pathway. Autophagy 2020, 16, 1436–1452. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, R.; Hu, H.; Yu, L.; Tang, Q.; Tao, Y.; Liu, Z.; Li, J.; Wang, G. Integrative Analysis of Hypoxia-Associated Signature in Pan-Cancer. iScience 2020, 23, 101460. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.; Cawley, J.C. Abnormal monoclonal antibody-defined helper/suppressor T-cell subpopulations in multiple myeloma: Relationship to treatment and clinical stage. Br. J. Haematol. 1983, 53, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Raitakari, M.; Brown, R.D.; Gibson, J.; Joshua, D.E. T cells in myeloma. Hematol. Oncol. 2003, 21, 33–42. [Google Scholar] [CrossRef]
- Hayashi, T.; Hideshima, T.; Akiyama, M.; Raje, N.; Richardson, P.; Chauhan, D.; Anderson, K.C. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood 2003, 102, 1435–1442. [Google Scholar] [CrossRef]
- Davies, F.E.; Raje, N.; Hideshima, T.; Lentzsch, S.; Young, G.; Tai, Y.T.; Lin, B.; Podar, K.; Gupta, D.; Chauhan, D.; et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001, 98, 210–216. [Google Scholar] [CrossRef]
- Perez-Andres, M.; Almeida, J.; Martin-Ayuso, M.; Moro, M.J.; Martin-Nunez, G.; Galende, J.; Hernandez, J.; Mateo, G.; San Miguel, J.F.; Orfao, A.; et al. Characterization of bone marrow T cells in monoclonal gammopathy of undetermined significance, multiple myeloma, and plasma cell leukemia demonstrates increased infiltration by cytotoxic/Th1 T cells demonstrating a squed TCR-Vbeta repertoire. Cancer 2006, 106, 1296–1305. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, R.; Joshi, S.; Kumar, L.; Sharma, M. Dysregulation in T helper 1/T helper 2 cytokine ratios in patients with multiple myeloma. Leuk. Lymphoma 2010, 51, 920–927. [Google Scholar] [CrossRef]
- Guery, L.; Hugues, S. Th17 Cell Plasticity and Functions in Cancer Immunity. BioMed Res. Int. 2015, 2015, 314620. [Google Scholar] [CrossRef]
- Asadzadeh, Z.; Mohammadi, H.; Safarzadeh, E.; Hemmatzadeh, M.; Mahdian-Shakib, A.; Jadidi-Niaragh, F.; Azizi, G.; Baradaran, B. The paradox of Th17 cell functions in tumor immunity. Cell. Immunol. 2017, 322, 15–25. [Google Scholar] [CrossRef]
- Prabhala, R.H.; Pelluru, D.; Fulciniti, M.; Prabhala, H.K.; Nanjappa, P.; Song, W.; Pai, C.; Amin, S.; Tai, Y.T.; Richardson, P.G.; et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood 2010, 115, 5385–5392. [Google Scholar] [CrossRef]
- Bryant, C.; Suen, H.; Brown, R.; Yang, S.; Favaloro, J.; Aklilu, E.; Gibson, J.; Ho, P.J.; Iland, H.; Fromm, P.; et al. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013, 3, e148. [Google Scholar] [CrossRef]
- Kara, E.E.; McKenzie, D.R.; Bastow, C.R.; Gregor, C.E.; Fenix, K.A.; Ogunniyi, A.D.; Paton, J.C.; Mack, M.; Pombal, D.R.; Seillet, C.; et al. CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nat. Commun. 2015, 6, 8644. [Google Scholar] [CrossRef]
- Noonan, K.; Marchionni, L.; Anderson, J.; Pardoll, D.; Roodman, G.D.; Borrello, I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood 2010, 116, 3554–3563. [Google Scholar] [CrossRef]
- Hadjiaggelidou, C.; Katodritou, E. Regulatory T-Cells and Multiple Myeloma: Implications in Tumor Immune Biology and Treatment. J. Clin. Med. 2021, 10, 4588. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Muthu Raja, K.R.; Rihova, L.; Zahradova, L.; Klincova, M.; Penka, M.; Hajek, R. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS ONE 2012, 7, e47077. [Google Scholar] [CrossRef]
- Feyler, S.; von Lilienfeld-Toal, M.; Jarmin, S.; Marles, L.; Rawstron, A.; Ashcroft, A.J.; Owen, R.G.; Selby, P.J.; Cook, G. CD4+CD25+FoxP3+ regulatory T cells are increased whilst CD3+CD4−CD8−alphabetaTCR+ Double Negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br. J. Haematol. 2009, 144, 686–695. [Google Scholar] [CrossRef]
- Beyer, M.; Kochanek, M.; Giese, T.; Endl, E.; Weihrauch, M.R.; Knolle, P.A.; Classen, S.; Schultze, J.L. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood 2006, 107, 3940–3949. [Google Scholar] [CrossRef]
- Sakaguchi, S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004, 22, 531–562. [Google Scholar] [CrossRef]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742. [Google Scholar] [CrossRef]
- Vieira, P.L.; Christensen, J.R.; Minaee, S.; O’Neill, E.J.; Barrat, F.J.; Boonstra, A.; Barthlott, T.; Stockinger, B.; Wraith, D.C.; O’Garra, A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 2004, 172, 5986–5993. [Google Scholar] [CrossRef] [PubMed]
- Fehervari, Z.; Sakaguchi, S. Development and function of CD25+CD4+ regulatory T cells. Curr. Opin. Immunol. 2004, 16, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001, 182, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Mackinnon, S.; Lowdell, M.W. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 2012, 14, 1110–1118. [Google Scholar] [CrossRef]
- Bagg, A.; Becker, P.; Bezwoda, W.; van Rensburg, L.; Mendelow, B. Circulating monotypic B-cells in multiple myeloma: Association with lambda paraproteins. Br. J. Haematol. 1989, 72, 167–172. [Google Scholar] [CrossRef]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Khan, A.R.; Hams, E.; Floudas, A.; Sparwasser, T.; Weaver, C.T.; Fallon, P.G. PD-L1hi B cells are critical regulators of humoral immunity. Nat. Commun. 2015, 6, 5997. [Google Scholar] [CrossRef]
- Zhang, L.; Tai, Y.T.; Ho, M.; Xing, L.; Chauhan, D.; Gang, A.; Qiu, L.; Anderson, K.C. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J. 2017, 7, e547. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.F.; Flores-Langarica, A.; Bobat, S.; Dominguez Medina, C.C.; Cook, C.N.; Ross, E.A.; Lopez-Macias, C.; Henderson, I.R. B1b cells recognize protective antigens after natural infection and vaccination. Front. Immunol. 2014, 5, 535. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Lau, S.; Bai, C.; Degauque, N.; Holodick, N.E.; Steven, S.J.; Tumang, J.; Gao, W.; Rothstein, T.L. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009, 60, 3734–3743. [Google Scholar] [CrossRef] [PubMed]
- Enghard, P.; Humrich, J.Y.; Chu, V.T.; Grussie, E.; Hiepe, F.; Burmester, G.R.; Radbruch, A.; Berek, C.; Riemekasten, G. Class switching and consecutive loss of dsDNA-reactive B1a B cells from the peritoneal cavity during murine lupus development. Eur. J. Immunol. 2010, 40, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Gao, W.; Degauque, N.; Bai, C.; Lu, Y.; Kenny, J.; Oukka, M.; Strom, T.B.; Rothstein, T.L. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur. J. Immunol. 2007, 37, 2400–2404. [Google Scholar] [CrossRef] [PubMed]
- Maseda, D.; Candando, K.M.; Smith, S.H.; Kalampokis, I.; Weaver, C.T.; Plevy, S.E.; Poe, J.C.; Tedder, T.F. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. J. Immunol. 2013, 191, 2780–2795. [Google Scholar] [CrossRef] [PubMed]
- Prabhala, R.H.; Talluri, S.; Stekla, M.; Negrroiu, A.; Buonopane, M.; LaBlanc, K.; Heubeck, A.; Fulciniti, M.; Anderson, K.; Munshi, N. Defining Fundamental B Cell-Subset Dysfunction in Myeloma. Blood 2016, 128, 2085. [Google Scholar] [CrossRef]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; de Kroon, C.; Driel, W.V.; Tidy, J.; Marth, C.; et al. An international randomized phase III trial comparing radical hysterectomy and pelvic node dissection (RH) vs simple hysterectomy and pelvic node dissection (SH) in patients with low-risk early-stage cervical cancer (LRESCC): A Gynecologic Cancer Intergroup study led by the Canadian Cancer Trials Group (CCTG CX.5-SHAPE). J. Clin. Oncol. 2023, 41, LBA5511. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, Z.; Wang, S.; Zhang, X.; Qian, J.; Hong, S.; Li, H.; Wang, M.; Yang, J.; Yi, Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 2009, 114, 3625–3628. [Google Scholar] [CrossRef]
- Petty, A.J.; Yang, Y. Tumor-Associated Macrophages in Hematologic Malignancies: New Insights and Targeted Therapies. Cells 2019, 8, 1526. [Google Scholar] [CrossRef]
- Ribatti, D.; Moschetta, M.; Vacca, A. Macrophages in multiple myeloma. Immunol. Lett. 2014, 161, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.; Li, Y.; Wang, H.; Qi, N.; Wu, Y. Macrophages as a Double-Edged Weapon: The Use of Macrophages in Cancer Immunotherapy and Understanding the Cross-Talk Between Macrophages and Cancer. DNA Cell Biol. 2021, 40, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Denu, R.A.; Dollar, B.A.; Escalante, L.E.; Kuether, J.P.; Callander, N.S.; Asimakopoulos, F.; Hematti, P. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br. J. Haematol. 2012, 158, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, I.R.; Martner, A.; Pisklakova, A.; Condamine, T.; Chase, T.; Vogl, T.; Roth, J.; Gabrilovich, D.; Nefedova, Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J. Immunol. 2013, 190, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, J.; Liyadipitiya, T.; Brown, R.; Yang, S.; Suen, H.; Woodland, N.; Nassif, N.; Hart, D.; Fromm, P.; Weatherburn, C.; et al. Myeloid derived suppressor cells are numerically, functionally and phenotypically different in patients with multiple myeloma. Leuk. Lymphoma 2014, 55, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Gulla, A.; Correale, P.; Tagliaferri, P.; Tassone, P. Myeloid-derived suppressor cells in multiple myeloma: Pre-clinical research and translational opportunities. Front. Oncol. 2014, 4, 348. [Google Scholar] [CrossRef]
- Schmielau, J.; Finn, O.J. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001, 61, 4756–4760. [Google Scholar] [PubMed]
- Mantovani, G.; Maccio, A.; Madeddu, C.; Mura, L.; Gramignano, G.; Lusso, M.R.; Massa, E.; Mocci, M.; Serpe, R. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: Assessment of the most important laboratory indexes of cachexia and oxidative stress. J. Mol. Med. 2003, 81, 664–673. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Nefedova, Y.; Yoder, D.; Gabrilovich, D.I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 2004, 172, 989–999. [Google Scholar] [CrossRef]
- Serafini, P.; Mgebroff, S.; Noonan, K.; Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008, 68, 5439–5449. [Google Scholar] [CrossRef]
- Pan, P.Y.; Ma, G.; Weber, K.J.; Ozao-Choy, J.; Wang, G.; Yin, B.; Divino, C.M.; Chen, S.H. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010, 70, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Abramson, H.N. B-Cell Maturation Antigen (BCMA) as a Target for New Drug Development in Relapsed and/or Refractory Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 5192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Wang, H.; Xiong, S.; Li, Y.; Tao, Q.; Xiao, W.; Qin, H.; Wang, Y.; Zhai, Z. Tumor-induced CD14+HLA-DR−/low myeloid-derived suppressor cells correlate with tumor progression and outcome of therapy in multiple myeloma patients. Cancer Immunol. Immunother. 2015, 64, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, G.T.; Whitehill, G.; Anderson, J.L.; Hideshima, T.; Maguire, C.; Laubach, J.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013, 121, 2975–2987. [Google Scholar] [CrossRef] [PubMed]
- Terabe, M.; Khanna, C.; Bose, S.; Melchionda, F.; Mendoza, A.; Mackall, C.L.; Helman, L.J.; Berzofsky, J.A. CD1d-restricted natural killer T cells can down-regulate tumor immunosurveillance independent of interleukin-4 receptor-signal transducer and activator of transcription 6 or transforming growth factor-beta. Cancer Res. 2006, 66, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.J.; Cook, G. Immunotherapy in multiple myeloma—Possibility or probability? Br. J. Haematol. 2005, 130, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu. Rev. Immunol. 2005, 23, 877–900. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, H.R. NK1.1+ T cell receptor-alpha/beta+ cells: New clues to their origin, specificity, and function. J. Exp. Med. 1995, 182, 633–638. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Richter, J. Harnessing natural killer T (NKT) cells in human myeloma: Progress and challenges. Clin. Immunol. 2011, 140, 160–166. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Geller, M.D.; Chang, D.H.; Shimizu, K.; Fujii, S.; Dhodapkar, K.M.; Krasovsky, J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 2003, 197, 1667–1676. [Google Scholar] [CrossRef]
- Spanoudakis, E.; Hu, M.; Naresh, K.; Terpos, E.; Melo, V.; Reid, A.; Kotsianidis, I.; Abdalla, S.; Rahemtulla, A.; Karadimitris, A. Regulation of multiple myeloma survival and progression by CD1d. Blood 2009, 113, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Jurczyszyn, A.; Gdula-Argasinska, J.; Kosmaczewska, A.; Skotnicki, A.B. The role of the bone marrow microenvironment in the pathogenesis of multiple myeloma. Postepy Hig. Med. Dosw. 2015, 69, 521–533. [Google Scholar] [CrossRef]
- Avella Patino, D.M.; Radhakrishnan, V.; Suvilesh, K.N.; Manjunath, Y.; Li, G.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Warren, W.C.; Kaifi, J.T.; Mitchem, J.B. Epigenetic Regulation of Cancer Immune Cells. Semin. Cancer Biol. 2022, 83, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bodey, B.; Siegel, S.E.; Kaiser, H.E. Antigen presentation by dendritic cells and their significance in antineoplastic immunotherapy. In Vivo 2004, 18, 81–100. [Google Scholar] [PubMed]
- Garcia De Vinuesa, C.; Gulbranson-Judge, A.; Khan, M.; O’Leary, P.; Cascalho, M.; Wabl, M.; Klaus, G.G.; Owen, M.J.; MacLennan, I.C. Dendritic cells associated with plasmablast survival. Eur. J. Immunol. 1999, 29, 3712–3721. [Google Scholar] [CrossRef]
- Jego, G.; Palucka, A.K.; Blanck, J.P.; Chalouni, C.; Pascual, V.; Banchereau, J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Brimnes, M.K.; Svane, I.M.; Johnsen, H.E. Impaired functionality and phenotypic profile of dendritic cells from patients with multiple myeloma. Clin. Exp. Immunol. 2006, 144, 76–84. [Google Scholar] [CrossRef]
- Leone, P.; Berardi, S.; Frassanito, M.A.; Ria, R.; De Re, V.; Cicco, S.; Battaglia, S.; Ditonno, P.; Dammacco, F.; Vacca, A.; et al. Dendritic cells accumulate in the bone marrow of myeloma patients where they protect tumor plasma cells from CD8+ T-cell killing. Blood 2015, 126, 1443–1451. [Google Scholar] [CrossRef]
- Schettini, J.; Mukherjee, P. Physiological role of plasmacytoid dendritic cells and their potential use in cancer immunity. Clin. Dev. Immunol. 2008, 2008, 106321. [Google Scholar] [CrossRef]
- Chauhan, D.; Singh, A.V.; Brahmandam, M.; Carrasco, R.; Bandi, M.; Hideshima, T.; Bianchi, G.; Podar, K.; Tai, Y.T.; Mitsiades, C.; et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: A therapeutic target. Cancer Cell 2009, 16, 309–323. [Google Scholar] [CrossRef]
- Bi, E.; Li, R.; Bover, L.C.; Li, H.; Su, P.; Ma, X.; Huang, C.; Wang, Q.; Liu, L.; Yang, M.; et al. E-cadherin expression on multiple myeloma cells activates tumor-promoting properties in plasmacytoid DCs. J. Clin. Investig. 2018, 128, 4821–4831. [Google Scholar] [CrossRef]
- Pessoa de Magalhaes, R.J.; Vidriales, M.B.; Paiva, B.; Fernandez-Gimenez, C.; Garcia-Sanz, R.; Mateos, M.V.; Gutierrez, N.C.; Lecrevisse, Q.; Blanco, J.F.; Hernandez, J.; et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica 2013, 98, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Brimnes, M.K.; Vangsted, A.J.; Knudsen, L.M.; Gimsing, P.; Gang, A.O.; Johnsen, H.E.; Svane, I.M. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR−/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand. J. Immunol. 2010, 72, 540–547. [Google Scholar] [CrossRef]
- Bascones-Martinez, A.; Mattila, R.; Gomez-Font, R.; Meurman, J.H. Immunomodulatory drugs: Oral and systemic adverse effects. Med. Oral. Patol. Oral. Cir. Bucal 2014, 19, e24–e31. [Google Scholar] [CrossRef]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef]
- Saltarella, I.; Altamura, C.; Campanale, C.; Laghetti, P.; Vacca, A.; Frassanito, M.A.; Desaphy, J.F. Anti-Angiogenic Activity of Drugs in Multiple Myeloma. Cancers 2023, 15, 1990. [Google Scholar] [CrossRef]
- Asatsuma-Okumura, T.; Ito, T.; Handa, H. Molecular Mechanisms of the Teratogenic Effects of Thalidomide. Pharmaceuticals 2020, 13, 95. [Google Scholar] [CrossRef]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef]
- Palumbo, A.; Facon, T.; Sonneveld, P.; Blade, J.; Offidani, M.; Gay, F.; Moreau, P.; Waage, A.; Spencer, A.; Ludwig, H.; et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood 2008, 111, 3968–3977. [Google Scholar] [CrossRef]
- Escoubet-Lozach, L.; Lin, I.L.; Jensen-Pergakes, K.; Brady, H.A.; Gandhi, A.K.; Schafer, P.H.; Muller, G.W.; Worland, P.J.; Chan, K.W.; Verhelle, D. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009, 69, 7347–7356. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Chauhan, D.; Shima, Y.; Raje, N.; Davies, F.E.; Tai, Y.T.; Treon, S.P.; Lin, B.; Schlossman, R.L.; Richardson, P.; et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 2000, 96, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Niesvizky, R.; Jayabalan, D.S.; Christos, P.J.; Furst, J.R.; Naib, T.; Ely, S.; Jalbrzikowski, J.; Pearse, R.N.; Zafar, F.; Pekle, K.; et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood 2008, 111, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Lacy, M.Q.; Tefferi, A. Pomalidomide therapy for multiple myeloma and myelofibrosis: An update. Leuk. Lymphoma 2011, 52, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Isatuximab: A Review of Its Use in Multiple Myeloma. Target. Oncol. 2021, 16, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Murai, H.; Saito, D.; Abe, G.; Tokunaga, E.; Iwasaki, T.; Takahashi, H.; Takeda, H.; Suzuki, T.; Shibata, N.; et al. Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF. EMBO J. 2021, 40, e105375. [Google Scholar] [CrossRef] [PubMed]
- Merz, M.; Dechow, T.; Scheytt, M.; Schmidt, C.; Hackanson, B.; Knop, S. The clinical management of lenalidomide-based therapy in patients with newly diagnosed multiple myeloma. Ann. Hematol. 2020, 99, 1709–1725. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Weisel, K.; Moreau, P.; Anderson, L.D., Jr.; White, D.; San-Miguel, J.; Sonneveld, P.; Engelhardt, M.; Jenner, M.; Corso, A.; et al. Pomalidomide, bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide (OPTIMISMM): Outcomes by prior treatment at first relapse. Leukemia 2021, 35, 1722–1731. [Google Scholar] [CrossRef]
- Richardson, P.G.; Trudel, S.; Popat, R.; Mateos, M.V.; Vangsted, A.J.; Ramasamy, K.; Martinez-Lopez, J.; Quach, H.; Orlowski, R.Z.; Arnao, M.; et al. Mezigdomide plus Dexamethasone in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 1009–1022. [Google Scholar] [CrossRef]
- Field-Smith, A.; Morgan, G.J.; Davies, F.E. Bortezomib (Velcadetrade mark) in the Treatment of Multiple Myeloma. Ther. Clin. Risk Manag. 2006, 2, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Kouroukis, T.C.; Baldassarre, F.G.; Haynes, A.E.; Imrie, K.; Reece, D.E.; Cheung, M.C. Bortezomib in multiple myeloma: Systematic review and clinical considerations. Curr. Oncol. 2014, 21, e573–e603. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, S.P.E.; Wanigasinghe Kanakanamge, S.P.; Rajalingam, D.; Silva, G.N. Carfilzomib: A Promising Proteasome Inhibitor for the Treatment of Relapsed and Refractory Multiple Myeloma. Front. Oncol. 2021, 11, 740796. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; Ghazarian, R.N.; Ou, M.; Luderer, M.J.; Kusdono, H.D.; Azab, A.K. Spotlight on ixazomib: Potential in the treatment of multiple myeloma. Drug Des. Dev. Ther. 2016, 10, 217–226. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, F.; Gazze, G.; Hewitt, J.; Kolm, K.; Pollock, D.; Rowland, S.; Crosbie, T. Canadian perspectives in multiple myeloma on the use of steroids in clinical practice based on patient and healthcare provider interviews. Front. Oncol. 2022, 12, 1061417. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.A.; Mouhieddine, T.H.; Ortiz, R.J.; Richter, J. Revisiting the role of alkylating agents in multiple myeloma: Up-to-date evidence and future perspectives. Crit. Rev. Oncol. Hematol. 2023, 187, 104040. [Google Scholar] [CrossRef]
- Lu, X.; Ding, Z.C.; Cao, Y.; Liu, C.; Habtetsion, T.; Yu, M.; Lemos, H.; Salman, H.; Xu, H.; Mellor, A.L.; et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J. Immunol. 2015, 194, 2011–2021. [Google Scholar] [CrossRef]
- Kaiser, M.F.; Hall, A.; Walker, K.; Sherborne, A.; De Tute, R.M.; Newnham, N.; Roberts, S.; Ingleson, E.; Bowles, K.; Garg, M.; et al. Daratumumab, Cyclophosphamide, Bortezomib, Lenalidomide, and Dexamethasone as Induction and Extended Consolidation Improves Outcome in Ultra-High-Risk Multiple Myeloma. J. Clin. Oncol. 2023, 41, 3945–3955. [Google Scholar] [CrossRef]
- Sidana, S.; Hosoya, H.; Jensen, A.; Liu, L.; Goyal, A.; Hovanky, V.; Sahaf, B.; Bharadwaj, S.; Latchford, T.; Arai, S.; et al. Bendamustine vs. fludarabine/cyclophosphamide lymphodepletion prior to BCMA CAR-T cell therapy in multiple myeloma. Blood Cancer J. 2023, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Vigna, E.; Recchia, A.G.; Morabito, L.; Mendicino, F.; Giagnuolo, G.; Morabito, F. Bendamustine in multiple myeloma. Eur. J. Haematol. 2015, 95, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.; Michael, A.; Lesokhin, A. Immunotherapy: A New Approach to Treating Multiple Myeloma with Daratumumab and Elotuzumab. Ann. Pharmacother. 2016, 50, 555–568. [Google Scholar] [CrossRef]
- Jadoon, Y.; Siddiqui, M.A. Immunotherapy in multiple myeloma. Cancer Treat. Res. Commun. 2021, 29, 100468. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Kaufman, J.L.; Hofmeister, C.C.; Joseph, N.S.; Heffner, T.L.; Gupta, V.A.; Sullivan, H.C.; Neish, A.S.; Dhodapkar, M.V.; Lonial, S. Daratumumab in multiple myeloma. Cancer 2019, 125, 2364–2382. [Google Scholar] [CrossRef] [PubMed]
- Phipps, C.; Chen, Y.; Gopalakrishnan, S.; Tan, D. Daratumumab and its potential in the treatment of multiple myeloma: Overview of the preclinical and clinical development. Ther. Adv. Hematol. 2015, 6, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, K.; Gimsing, P.; Gronbaek, K. The role of epigenetics in the biology of multiple myeloma. Blood Cancer J. 2014, 4, e207. [Google Scholar] [CrossRef]
- Pan, D.; Mouhieddine, T.H.; Upadhyay, R.; Casasanta, N.; Lee, A.; Zubizarreta, N.; Moshier, E.; Richter, J. Outcomes with panobinostat in heavily pretreated multiple myeloma patients. Semin. Oncol. 2023, 50, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Corral, L.G.; Haslett, P.A.; Muller, G.W.; Chen, R.; Wong, L.M.; Ocampo, C.J.; Patterson, R.T.; Stirling, D.I.; Kaplan, G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J. Immunol. 1999, 163, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Treon, S.P.; Shima, Y.; Hideshima, T.; Podar, K.; Tai, Y.T.; Lin, B.; Lentzsch, S.; Davies, F.E.; Chauhan, D.; et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: Therapeutic applications. Leukemia 2001, 15, 1950–1961. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Kortuem, K.M.; Stewart, A.K. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk. Lymphoma 2013, 54, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, I.; Raab, M.S.; Vallet, S.; Hideshima, T.; Raje, N.; Mitsiades, C.; Chauhan, D.; Okawa, Y.; Munshi, N.C.; Richardson, P.G.; et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 2008, 22, 1925–1932. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Kaminska, W.; Hus, I.; Dmoszynska, A. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: Detailed characterisation of immune status in multiple myeloma. Br. J. Cancer 2012, 106, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Galustian, C.; Meyer, B.; Labarthe, M.C.; Dredge, K.; Klaschka, D.; Henry, J.; Todryk, S.; Chen, R.; Muller, G.; Stirling, D.; et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol. Immunother. 2009, 58, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Corral, L.G.; Fleming, Y.W.; Stein, B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol. Immunother. 2008, 57, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Adams, M.; Carter, T.; Chen, R.; Muller, G.; Stirling, D.; Schafer, P.; Bartlett, J.B. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin. Cancer Res. 2008, 14, 4650–4657. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Boccadoro, M.; Smith, E.L. Novel Immunotherapies for Multiple Myeloma. Curr. Hematol. Malig. Rep. 2017, 12, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.; Shen, D.; Zhang, W.; He, R.; Jiang, M. Immune Checkpoint Inhibitors in Cancer Therapy—How to Overcome Drug Resistance? Cancers 2022, 14, 3575. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, C.J.; Cho, Y.U.; You, E.; Jang, S.; Seo, E.J.; Lee, J.H.; Yoon, D.H.; Suh, C. Immune Checkpoint Programmed Cell Death Protein-1 (PD-1) Expression on Bone Marrow T Cell Subsets in Patients With Plasma Cell Myeloma. Ann. Lab. Med. 2021, 41, 259–267. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Chambers, C.A.; Kuhns, M.S.; Allison, J.P. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4+ T cell responses. Proc. Natl. Acad. Sci. USA 1999, 96, 8603–8608. [Google Scholar] [CrossRef] [PubMed]
- Braga, W.M.; da Silva, B.R.; de Carvalho, A.C.; Maekawa, Y.H.; Bortoluzzo, A.B.; Rizzatti, E.G.; Atanackovic, D.; Colleoni, G.W. FOXP3 and CTLA4 overexpression in multiple myeloma bone marrow as a sign of accumulation of CD4+ T regulatory cells. Cancer Immunol. Immunother. 2014, 63, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, S.; Nandakumar, B.; Kumar, S. Immune-based therapies in the management of multiple myeloma. Blood Cancer J. 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D.; et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef] [PubMed]
- Atrash, S.; Ali, S.A.; Usmani, S.Z. Chimeric Antigen Receptor T-cell Therapy for Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 21–34. [Google Scholar] [CrossRef]
- Choi, T.; Kang, Y. Chimeric antigen receptor (CAR) T-cell therapy for multiple myeloma. Pharmacol. Ther. 2022, 232, 108007. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Latouche, J.B.; Santos, E.; Marti, F.; Gong, M.C.; Lyddane, C.; King, P.D.; Larson, S.; Weiss, M.; Riviere, I.; et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 2003, 9, 279–286. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Feldman, S.A.; Zhao, Y.; Xu, H.; Black, M.A.; Morgan, R.A.; Wilson, W.H.; Rosenberg, S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009, 32, 689–702. [Google Scholar] [CrossRef]
- Jena, B.; Moyes, J.S.; Huls, H.; Cooper, L.J. Driving CAR-based T-cell therapy to success. Curr. Hematol. Malig. Rep. 2014, 9, 50–56. [Google Scholar] [CrossRef]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Li, M.; Kitto, A.; Li, J.; Wang, C.S.; Kirk, D.T.; Yellin, O.; Nichols, C.M.; Dreyer, M.P.; Ahles, C.P.; et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012, 158, 727–738. [Google Scholar] [CrossRef]
- Moreaux, J.; Legouffe, E.; Jourdan, E.; Quittet, P.; Reme, T.; Lugagne, C.; Moine, P.; Rossi, J.F.; Klein, B.; Tarte, K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004, 103, 3148–3157. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. A Study of Whether Ide-cel (bb2121) Can Be Made from People with Multiple Myeloma Who Have Had a Hematopoietic Cell Transplant [NCT05393804]. Available online: https://www.clinicaltrials.gov/study/NCT05393804?term=NCT05393804&rank=1 (accessed on 25 February 2024).
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.V.; Fernandez de Larrea, C.; Martinez-Lopez, J.; Moreau, P.; Touzeau, C.; et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Yong, K.; Harrison, S.J.; Mateos, M.-V.; Moreau, P.; van de Donk, N.W.C.J.; Sidana, S.; Popat, R.; Lendvai, N.; Lonardi, C.; et al. First phase 3 results from CARTITUDE-4: Cilta-cel versus standard of care (PVd or DPd) in lenalidomide-refractory multiple myeloma. J. Clin. Oncol. 2023, 41, LBA106. [Google Scholar] [CrossRef]
- Zhao, W.H.; Liu, J.; Wang, B.Y.; Chen, Y.X.; Cao, X.M.; Yang, Y.; Zhang, Y.L.; Wang, F.X.; Zhang, P.Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef]
- Mailankody, S.; Htut, M.; Lee, K.P.; Bensinger, W.; Devries, T.; Piasecki, J.; Ziyad, S.; Blake, M.; Byon, J.; Jakubowiak, A. JCARH125, Anti-BCMA CAR T-cell Therapy for Relapsed/Refractory Multiple Myeloma: Initial Proof of Concept Results from a Phase 1/2 Multicenter Study (EVOLVE). Blood 2018, 132, 957. [Google Scholar] [CrossRef]
- Mailankody, S.; Jakubowiak, A.J.; Htut, M.; Costa, L.J.; Lee, K.P.; Ganguly, S.; Kaufman, J.L.; Siegel, D.S.D.; Bensinger, W.; Cota, M.; et al. Orvacabtagene autoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): Update of the phase 1/2 EVOLVE study (NCT03430011). J. Clin. Oncol. 2020, 38, 8504. [Google Scholar] [CrossRef]
- Frigault, M.J.; Bishop, M.R.; Rosenblatt, J.; O’Donnell, E.K.; Raje, N.; Cook, D.; Yee, A.J.; Logan, E.; Avigan, D.E.; Jakubowiak, A.; et al. Phase 1 study of CART-ddBCMA for the treatment of subjects with relapsed and refractory multiple myeloma. Blood Adv. 2023, 7, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Gupta, A.; Dagar, G.; Das, D.; Chakraborty, A.; Haque, S.; Prasad, C.P.; Singh, A.; Bhat, A.A.; Macha, M.A.; et al. CAR-T-Cell Therapy in Multiple Myeloma: B-Cell Maturation Antigen (BCMA) and Beyond. Vaccines 2023, 11, 1721. [Google Scholar] [CrossRef]
- Wang, X.; Pan, B.; Huang, H.; Xu, K. Non-BCMA targeted CAR-T cell therapies for multiple myeloma. ImmunoMedicine 2021, 1, e1030. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Kochi, Y.; Nakai, W.; Mizuno, H.; Baba, T.; Habu, K.; Sawada, N.; Tsunoda, H.; Shima, T.; Miyawaki, K.; et al. Anti-GPRC5D/CD3 Bispecific T-Cell-Redirecting Antibody for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2019, 18, 1555–1564. [Google Scholar] [CrossRef]
- Sun, C.; Mahendravada, A.; Ballard, B.; Kale, B.; Ramos, C.; West, J.; Maguire, T.; McKay, K.; Lichtman, E.; Tuchman, S.; et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget 2019, 10, 2369–2383. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 947. [Google Scholar] [CrossRef]
- Rapoport, A.P.; Stadtmauer, E.A.; Binder-Scholl, G.K.; Goloubeva, O.; Vogl, D.T.; Lacey, S.F.; Badros, A.Z.; Garfall, A.; Weiss, B.; Finklestein, J.; et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015, 21, 914–921. [Google Scholar] [CrossRef]
- Cannons, J.L.; Tangye, S.G.; Schwartzberg, P.L. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011, 29, 665–705. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Curio, S.; Jonsson, G.; Marinovic, S. A summary of current NKG2D-based CAR clinical trials. Immunother. Adv. 2021, 1, ltab018. [Google Scholar] [CrossRef]
- Spear, P.; Wu, M.R.; Sentman, M.L.; Sentman, C.L. NKG2D ligands as therapeutic targets. Cancer Immun. 2013, 13, 8. [Google Scholar]
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef]
- Spear, P.; Barber, A.; Rynda-Apple, A.; Sentman, C.L. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol. Cell Biol. 2013, 91, 435–440. [Google Scholar] [CrossRef]
- Garfall, A.L.; Stadtmauer, E.A.; Hwang, W.T.; Lacey, S.F.; Melenhorst, J.J.; Krevvata, M.; Carroll, M.P.; Matsui, W.H.; Wang, Q.; Dhodapkar, M.V.; et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight 2018, 3, e120505. [Google Scholar] [CrossRef]
- van der Schans, J.J.; van de Donk, N.; Mutis, T. Dual Targeting to Overcome Current Challenges in Multiple Myeloma CAR T-Cell Treatment. Front. Oncol. 2020, 10, 1362. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol. Res. 2019, 7, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; Brayer, J.B.; Poire, X.; Havelange, V.; Awada, A.; Lewalle, P.; Odunsi, K.; Wang, E.S.; Lonez, C.; Lequertier, T.; et al. Results from the Completed Dose-Escalation of the Hematological Arm of the Phase I Think Study Evaluating Multiple Infusions of NKG2D-Based CAR T-Cells As Standalone Therapy in Relapse/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome Patients. Blood 2019, 134, 3826. [Google Scholar] [CrossRef]
- Bal, S.; Kocoglu, M.H.; Nadeem, O.; Htut, M.; Gregory, T.; Anderson, L.D.; Costa, L.J.; Buchholz, T.J.; Ziyad, S.; Li, M.; et al. Clinical Activity of BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-Targeted Chimeric Antigen Receptor (CAR) T Cell Therapy, in Patients with Relapsed and/or Refractory (R/R) Multiple Myeloma (MM): First Results from a Phase 1, Multicenter, Open-Label Study. Blood 2022, 140, 883–885. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, J.; Song, Y.; Liu, D. Recent updates on CAR T clinical trials for multiple myeloma. Mol. Cancer 2019, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Wang, K.; Wei, C.; Feng, D.; Liu, Y.; He, Q.; Xu, X.; Wang, C.; Zhao, S.; Lv, L.; et al. The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Front. Immunol. 2021, 12, 609421. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Safety Study of Anti LewisY Chimeric Antigen Receptor in Myeloma, Acute Myeloid Leukemia or Myelodysplastic Syndrome [NCT01716364]. Available online: https://www.clinicaltrials.gov/study/NCT01716364?term=NCT01716364&rank=1 (accessed on 25 February 2024).
- Clinicaltrials.gov. Study to Evaluate the Safety and Efficacy of Anti-CD38 CAR-T in Relapsed or Refractory Multiple Myeloma Patients [NCT03464916]. Available online: https://www.clinicaltrials.gov/study/NCT03464916?term=NCT03464916&rank=1 (accessed on 25 February 2024).
- Owusu, K.A.; Schiffer, M.; Perreault, S. Chimeric Antigen Receptor T Cells: Toxicity and Management Considerations. AACN Adv. Crit. Care 2022, 33, 301–307. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 10254. [Google Scholar] [CrossRef]
- Xiao, X.; Huang, S.; Chen, S.; Wang, Y.; Sun, Q.; Xu, X.; Li, Y. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J. Exp. Clin. Cancer Res. 2021, 40, 367. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, N.G.; Xie, H.; Bentzen, S.; Kesari, V.; Bukhari, A.; El Chaer, F.; Lutfi, F.; Siglin, J.; Hutnick, E.; Gahres, N.; et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: Predictive biomarkers and clinical outcomes. Neuro Oncol. 2021, 23, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer immunotherapy: Challenges and limitations. Pathol. Res. Pract. 2022, 229, 153723. [Google Scholar] [CrossRef]
- Wu, J.; Cai, J. Dilemma and Challenge of Immunotherapy for Pancreatic Cancer. Dig. Dis. Sci. 2021, 66, 359–368. [Google Scholar] [CrossRef]
- Gupta, S.; Shukla, S. Limitations of Immunotherapy in Cancer. Cureus 2022, 14, e30856. [Google Scholar] [CrossRef]
- Teoh, P.J.; Chng, W.J. CAR T-cell therapy in multiple myeloma: More room for improvement. Blood Cancer J. 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Le Calvez, B.; Moreau, P.; Touzeau, C. Immune checkpoint inhibitors for the treatment of myeloma: Novel investigational options. Expert Opin. Investig. Drugs 2021, 30, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, O.; Tai, Y.T.; Anderson, K.C. Immunotherapeutic and Targeted Approaches in Multiple Myeloma. Immunotargets Ther. 2020, 9, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Vicidomini, R.; Poondla, N.; Tanzadehpanah, H.; Dirbaziyan, A.; Mahaki, H.; Manoochehri, H.; Kalhor, N.; Dama, P. CAR T therapies in multiple myeloma: Unleashing the future. Cancer Gene Ther. 2024. [Google Scholar] [CrossRef]
| Drugs | Mode of Action | Route of Administration | Overall Response Rate (ORR) | References |
|---|---|---|---|---|
| Immunomodulatory Drugs | ||||
| Thalidomide | Binds to CRBN and triggers proteasomal degradation of IKZF1 and IKZF3, leading to direct anti-multiple myeloma activity and immunomodulatory and stromal cell effects. | Oral | 28% | [96,108] |
| Lenalidomide | Bind CRBN, the substrate adaptor for the CRBN-CRL4 E3 ubiquitin ligase, and modulate the enzyme’s substrate specificity. These drugs induce the degradation of two lymphoid transcription factors, IKZF1 and IKZF3, leading to dramatic clinical efficacy in multiple myeloma. | Oral | 25–27% | [109] |
| Pomalidomide | Degrades the IKZF1 and IKZF3 proteins more potentially. | Oral | 30–35% | [110] |
| Mezigdomide | The drug is designed to achieve rapid, potent, and deep degradation of Ikaros and Aiolos, key transcription factors in hematopoietic cell development and differentiation. | Oral | 40% | [111] |
| Proteasome inhibitors | ||||
| Bortezomib | Primarily acts through plasma cell depletion, reducing antibody production to T-dependent antigens. | Intravenous (IV) or subcutaneous injection | 38% | [112,113] |
| Carfilzomib | The drug irreversibly binds with the proteasome and inhibits its chymotrypsin-like activity. | Intravenous infusion | 23% | [114] |
| Ixazomib | Reversibly binds and inhibits the chymotrypsin-like proteolytic (β5) site of the 20S proteasome, reducing the tumor progression. | Oral | 78% | [115] |
| Corticosteroids/Steroids | ||||
| Dexamethasone | Agonist to glucocorticoid receptor. It prevents white blood cells from migrating to locations where malignant myeloma cells are causing damage and reduces swelling and inflammation. | Oral or intravenous | 90% | [116] |
| Prednisone | Agonist to glucocorticoid receptor. | Oral | 10% | [117,118] |
| Alkylating drugs | ||||
| Melphalan | Melphalan induces inter- or infrastructural DNA crosslinks and DNA–protein crosslinks. Increases tumor-specific CD4 + T-cell responses and inhibits BM stromal cell expression of IL-6. | Intravenous (IV) and oral | 82% | [119,120] |
| Cyclophosphamide | The formation of DNA crosslinking alters cytokine expression in the BM microenvironment, causing MM cell death/apoptosis. | Intravenous (IV) and oral | 58.1% | [121] |
| Bendamustine | Formation of DNA crosslinking. Checkpoint controls inhibition, causing a mitotic catastrophe. | Intravenous (IV) | 55% | [122,123] |
| Monoclonal Antibodies | ||||
| Elotuzumab | Drug targets the signaling lymphocytic activation molecule F7 (SLAMF7). It directly activates natural killer cells, enhancing their ability to kill myeloma cells. | Intravenous infusion | 79% | [124,125] |
| Daratumumab | It works by targeting the protein CD38 on myeloma cells. It helps to slow or stop the progression of multiple myeloma. | Intravenous infusion or subcutaneous injection | 81% | [126,127] |
| Isatuximab | Anti-CD38 monoclonal antibody exerts antimyeloma activity through several mechanisms of action, such as antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and direct induction of apoptotic cell death. | Intravenous infusion | 23.8% | [107] |
| Epigenetic inhibitors | ||||
| Panobinostat | It is an HDAC inhibitor used in the treatment of relapsed and refractory multiple myeloma. | Oral | 24.8% | [128,129] |
| Trial Number | Name | Phase | Number of Patients | Overall Response Rate (ORR) | Sponsors | References |
|---|---|---|---|---|---|---|
| NCT03548207 | CARTITUDE-1 | 2 | 113 | 89% | Janssen Research & Development, LLC | [156] |
| NCT03338972 | FCARH143 | 1 | 12 | 100% | Fred Hutchinson Cancer Center | [148] |
| NCT05393804 | bb2121 | 2 | Recruiting | NA | Memorial Sloan Kettering Cancer Center | [157] |
| NCT02658929 | bb2121 or Ide-cel | 1 | 33 | 85% | Bluebird Bio and Celgene | [158] |
| NCT02215967 | CAR-BCMA | 1 | 24 | 81% | National Cancer Institute (NCI) | [155] |
| NCT03361748 | KarMMa | 2 | 140 | 73% | Celgene | [158,159] |
| NCT04181827 | CARTITUDE-4 | 3 | 419 | 76% | Janssen Research & Development, LLC | [160,161] |
| NCT03090659 | LEGEND-2 | 1 | 57 | 88% | Nanjing Legend Biotech Co. | [162] |
| NCT03430011 | Orva-cel | 1/2 | 44 | 82% | Juno Therapeutics, Celgene | [163,164] |
| NCT04155749 | NA | 1 | 13 | 60–75% | Arcellx, Inc. | [165] |
| Trial Number | Name | Phase | Number of Patients | Sponsors | References |
|---|---|---|---|---|---|
| NCT02135406 | CTL019 | 1 | 12 | University of Pennsylvania | [179] |
| NCT03767725 | NA | 1 | Recruiting | Shenzhen Second People’s Hospital | [73] |
| NCT03706547 | NA | 1 | Unknown | Peng Liu | [180] |
| NCT02203825 | NA | 1 | 6 | Celyad Oncology SA | [181] |
| NCT03018405 | THINK | 1 | 16 | Celyad Oncology SA | [182] |
| NCT04674813 | CC-95266 | 1 | 21 | Juno Therapeutics, a Subsidiary of Celgene | [183] |
| NCT03710421 | NA | 1 | Recruiting | City of Hope Medical Center | [184] |
| NCT03778346 | NA | 1 | Recruiting | The Sixth Affiliated Hospital of Wenzhou Medical University | [185] |
| NCT01716364 | NA | 1 | 6 | Peter MacCallum Cancer Centre, Australia | [186] |
| NCT03464916 | CAR2 Anti-CD38 A2 | 1 | Unknown | Sorrento Therapeutics, Inc. | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhakrishnan, V.; Golla, U.; Kudva, A.K. Role of Immune Cells and Immunotherapy in Multiple Myeloma. Life 2024, 14, 461. https://doi.org/10.3390/life14040461
Radhakrishnan V, Golla U, Kudva AK. Role of Immune Cells and Immunotherapy in Multiple Myeloma. Life. 2024; 14(4):461. https://doi.org/10.3390/life14040461
Chicago/Turabian StyleRadhakrishnan, Vijay, Upendarrao Golla, and Avinash Kundadka Kudva. 2024. "Role of Immune Cells and Immunotherapy in Multiple Myeloma" Life 14, no. 4: 461. https://doi.org/10.3390/life14040461
APA StyleRadhakrishnan, V., Golla, U., & Kudva, A. K. (2024). Role of Immune Cells and Immunotherapy in Multiple Myeloma. Life, 14(4), 461. https://doi.org/10.3390/life14040461







