Evolutionary Echoes: A Four-Day Fasting and Low-Caloric Intake Study on Autonomic Modulation and Physiological Adaptations in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ultraendurance Probe
2.3. Experimental Procedures
2.4. Study Variables
- Body Composition: Measurements were taken using the validated InBody 270 bioelectrical impedance analysis (BIA) device, which is renowned for its accuracy and reliability in assessing body composition. This device employs a multi-frequency, segmental BIA method, utilizing eight-point tactile electrodes to provide detailed body composition readings. Participants stood on the device’s platform with their legs slightly apart and arms not touching the torso, in a similar posture required for the previously mentioned device. They were barefoot and wore minimal clothing to ensure precise measurements. The InBody 270’s electrodes, designed to optimize contact and improve the accuracy of the measurement, required no additional preparation of the skin. Participants were instructed to grasp the hand electrodes according to the InBody’s specific protocols [25]. The utilization of the InBody 270 was justified due to its validated methodology for providing precise and segmental body composition analysis, including muscle mass, fat mass, and water distribution. This validated device is widely recognized for its clinical accuracy and reproducibility, making it an ideal choice for our study to ensure high-quality, reliable body composition data [26].
- Body Mass Index (BMI): Calculated as weight in kilograms divided by height in meters squared (kg/m2), following World Health Organization guidelines.
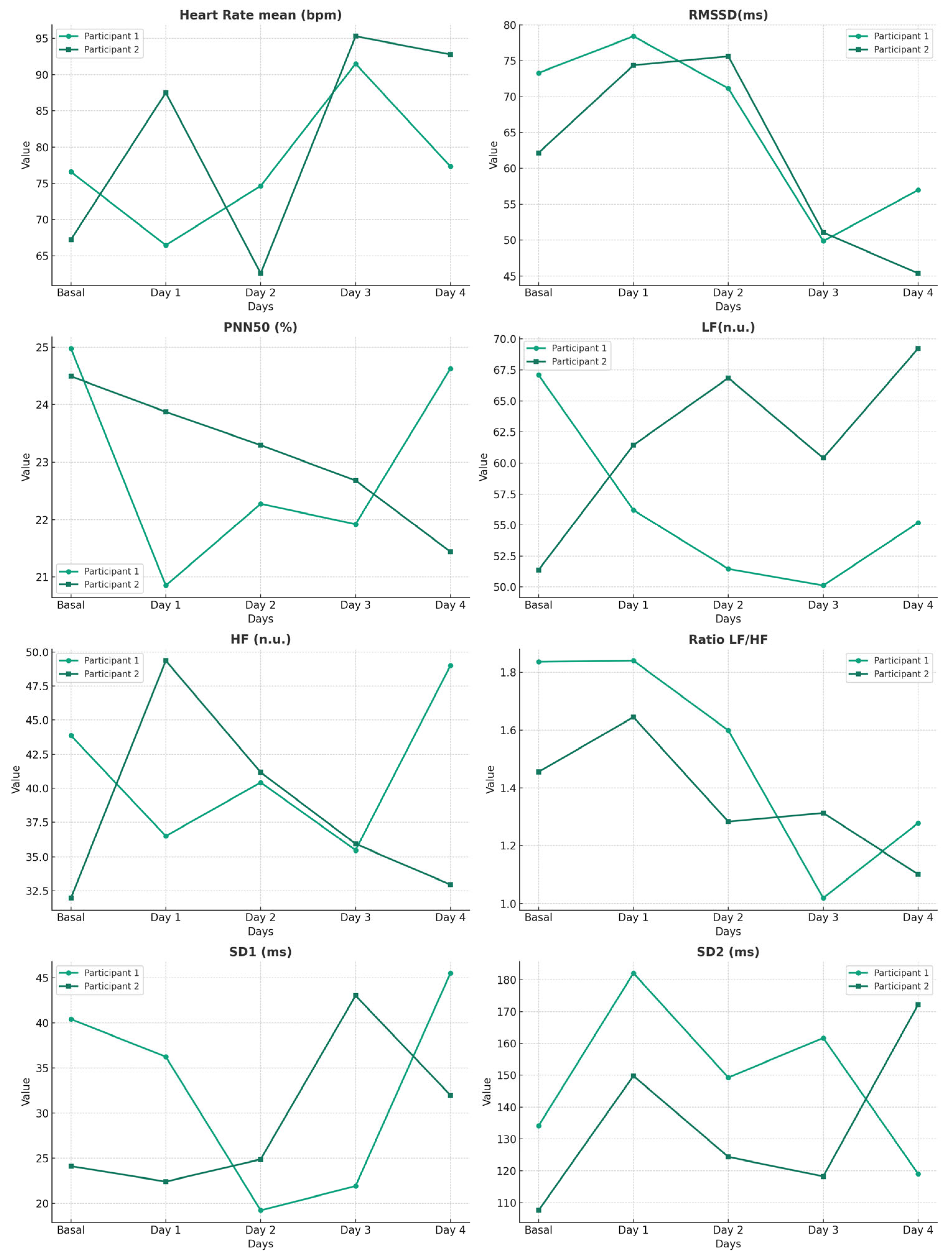
- HRV: Monitored using a Polar V800 HRV monitor (Kempele, Finland). Measurements commenced minutes before the event’s onset and concluded upon its completion.
- Reaction Time: Assessed using a web-based test accessed through a mobile device via the URL https://www.arealme.com/reaction-test/es/ (accessed on 27 November 2023). The test interface displayed on the phone’s screen transitions from white to a randomly chosen color, prompting the participant to touch the screen as quickly as possible. Prior to the actual measurements, participants were given an opportunity to familiarize themselves with the procedure. Three measurements were recorded both before and after the intervention. The final reaction time value was determined by calculating the average of these three measurements [27].
- Handgrip Strength: Isometric handgrip strength was measured using a TKK 5402 dynamometer (Takei Scientific Instruments Co. Ltd., Niigata, Japan). The participant’s dominant hand grip strength was evaluated. The participant sat with 0 degrees of shoulder flexion, 90 degrees of elbow flexion, and the forearm in a neutral position. The highest result of two trials was recorded.
- Jump Height: Lower limb strength was assessed using a horizontal jump test. The participant stood behind a marked line on the ground with feet shoulder-width apart. Three attempts were made, and the highest result was recorded.
- Forced Vital Capacity, Forced Expiratory Volume in 1 Second, Peak Expiratory Flow: These parameters were measured using a QM-SP100 spirometer (Quirumed, Valencia, Spain) during a maximum inhalation–exhalation cycle.
- Body Temperature: Measured using a digital infrared thermometer (Temp Touch; Xilas Medical, San Antonio, TX, USA).
- Blood Glucose Levels: Determined by analyzing 5 μL of capillary blood from the finger using a portable analyzer (One Touch Basic, LifeScan Inc., Madrid, Spain).
- Hydration Status: Immediately after the event, hydration status was evaluated using a colorimetry procedure with a urine color chart (UCC), assisting in identifying pH status and the presence of glucose, nitrites, proteins, and urine specific gravity (USG), following established protocols [19,20,21].
- Rate of Perceived Exertion (RPE): Based on the 6 to 20 scale [28].
- Subjective Pain Level, Leg Pain Level, Hunger Level: Each assessed on a self-reported scale from 0 to 100, with 0 being the lowest value and 100 the highest for the level.
3. Results and Discussion
3.1. Practical Applications
3.2. Limitation of the Study and Future Research
4. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Vogel, R.; Lavie, C.J.; Cordain, L. Exercise Like a Hunter-Gatherer: A Prescription for Organic Physical Fitness. Prog. Cardiovasc. Dis. 2011, 53, 471–479. [Google Scholar] [CrossRef]
- Wheeler, P. The evolution of bipedality and loss of functional body hair in hominids. J. Hum. Evol. 1984, 13, 91–98. [Google Scholar] [CrossRef]
- Bramble, D.M.; Lieberman, D.E. Endurance running and the evolution of Homo. Nature 2004, 432, 345–352. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J. Multidisciplinary intervention in the treatment of mixed anxiety and depression disorder. Physiol. Behav. 2020, 219, 112858. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.D.; Watson, K.B.; Carlson, S.A.; Fulton, J.E.; Dorn, J.M.; Elam-Evans, L. Adult Participation in Aerobic and Muscle-Strengthening Physical Activities—United States, 2011. Morb. Mortal. Wkly. Rep. 2013, 62, 326–330. [Google Scholar]
- Sponheimer, M.; Alemseged, Z.; Cerling, T.E.; Grine, F.E.; Kimbel, W.H.; Leakey, M.G.; Lee-Thorp, J.A.; Manthi, F.K.; Reed, K.E.; Wood, B.A.; et al. Isotopic evidence of early hominin diets. Proc. Natl. Acad. Sci. USA 2013, 110, 10513–10518. [Google Scholar] [CrossRef]
- Ungar, P.S.; Sponheimer, M. The Diets of Early Hominins. Science 2011, 334, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Carvalho, S.; Marean, C.W.; Alemseged, Z. Origins of the Human Predatory Pattern: The Transition to Large-Animal Exploitation by Early Hominins. Curr. Anthr. 2019, 60, 1–23. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef]
- Dishman, R.K.; Berthoud, H.; Booth, F.W.; Cotman, C.W.; Edgerton, V.R.; Fleshner, M.R.; Gandevia, S.C.; Gomez-Pinilla, F.; Greenwood, B.N.; Hillman, C.H.; et al. Neurobiology of Exercise. Obesity 2006, 14, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Monda, M.; Valenzano, A.; Messina, G.; Villano, I.; Moscatelli, F.; Cibelli, G.; Marsala, G.; Polito, R.; Ruberto, M.; et al. Functional Changes Induced by Orexin A and Adiponectin on the Sympathetic/Parasympathetic Balance. Front. Physiol. 2018, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Polito, R.; Valenzano, A.; Scarinci, A.; Villano, I.; Monda, M.; Messina, A.; Cibelli, G.; Porro, C.; La Torre, E.; Pisanelli, D.; et al. Very Low-Calorie Ketogenic Diet Modulates the Autonomic Nervous System Activity through Salivary Amylase in Obese Population Subjects. Int. J. Environ. Res. Public Health 2021, 18, 8475. [Google Scholar] [CrossRef] [PubMed]
- Mammen, G.; Faulkner, G. Physical activity and the prevention of depression: A systematic review of prospective studies. Am. J. Prev. Med. 2013, 45, 649–657. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef]
- Clael, S.; Campos, L.F.; Correia, K.L.; de Lucena, J.M.S.; Gentil, P.; Durigan, J.L.; Ribeiro, A.L.d.A.; Martins, W.R. Exercise interventions can improve muscle strength, endurance, and electrical activity of lumbar extensors in individuals with non-specific low back pain: A systematic review with meta-analysis. Sci. Rep. 2021, 11, 16842. [Google Scholar] [CrossRef]
- Belinchón-Demiguel, P.; Ramos-Campo, D.J.; Clemente-Suárez, V.J. Exploring the Evolutionary Disparities: A Case Study on the Psychophysiological Response to Recreating the Hunter–Gatherer Lifestyle through Physical Activity and Caloric Restriction. Appl. Sci. 2023, 13, 11140. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Ponce-González, J.G.; Pérez-Suárez, I.; Herrero, J.d.l.C.; Holmberg, H. A time-efficient reduction of fat mass in 4 days with exercise and caloric restriction. Scand. J. Med. Sci. Sports 2015, 25, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Hosono, A.; Shibata, K.; Ghadimi, R.; Fuku, M.; Goto, C.; Imaeda, N.; Tokudome, Y.; Hoshino, H.; Marumoto, M.; et al. Changes in blood biochemical markers before, during, and after a 2-day ultramarathon. Open Access J. Sports Med. 2016, 7, 43–50. [Google Scholar] [CrossRef]
- Korobeynikov, G.; Korobeynikova, L.; Iermakov, S.; Nosko, M. Reaction of Heart Rate Regulation to Extreme Sport Activity in Elite Athletes. 2016. Available online: http://erpub.chnpu.edu.ua:8080/jspui/bitstream/123456789/194/1/Reaction%20of%20heart%20rate%20regulation%20to%20extreme%20sport%20activity%20in%20elite%20athletes.pdf (accessed on 27 November 2023).
- Kajaia, T.; Maskhulia, L.; Chelidze, K.; Akhalkatsi, V.; Kakhabrishvili, Z. The effects of non-functional overreaching and overtraining on autonomic nervous system function in highly trained Georgian athletes. Georgian Med. Newa 2017, 3, 97–101. [Google Scholar]
- Belinchón-Demiguel, P.; Tornero-Aguilera, J.F.; Dalamitros, A.A.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B.; Clemente-Suárez, V.J. Multidisciplinary Analysis of Differences Between Finisher and Non-finisher Ultra-Endurance Mountain Athletes. Front. Physiol. 2019, 10, 1507. [Google Scholar] [CrossRef]
- Belinchon-Demiguel, P.; Clemente-Suárez, V.J. Psychophysiological, Body Composition, Biomechanical and Autonomic Modulation Analysis Procedures in an Ultraendurance Mountain Race. J. Med. Syst. 2018, 42, 32. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.R.; Manimaleth, R.; Czartoryski, P.; Napolitano, P.; Watters, H.; Weber, C.; Alvarez-Beaton, A.; Nieto, A.C.; Patel, A.; Peacock, C. A Comparative Study of Body Composition Assessment Techniques: DXA and InBody 270. J. Exerc. Nutr. 2020, 3, 3. [Google Scholar]
- Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Cognitive and psychophysiological impact of surgical mask use during university lessons. Physiol. Behav. 2021, 234, 113342. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef]
- Willis, E.A.; Creasy, S.A.; Honas, J.J.; Melanson, E.L.; Donnelly, J.E. The effects of exercise session timing on weight loss and components of energy balance: Midwest exercise trial 2. Int. J. Obes. 2020, 44, 114–124. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Lavie, C.J.; Earnest, C.P.; Church, T.S. The Role of Exercise and Physical Activity in Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2014, 56, 441–447. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr. Fuel Metabolism in Starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef]
- Phinney, S.; Bistrian, B.; Evans, W.; Gervino, E.; Blackburn, G. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef]
- Most, J.; Redman, L.M. Impact of calorie restriction on energy metabolism in humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Ismail, I.; Keating, S.E.; Baker, M.K.; Johnson, N.A. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes. Rev. 2012, 13, 68–91. [Google Scholar] [CrossRef]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef]
- You, T.; Murphy, K.M.; Lyles, M.F.; Demons, J.L.; Lenchik, L.; Nicklas, B.J. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int. J. Obes. 2006, 30, 1211–1216. [Google Scholar] [CrossRef]
- Basile, A.J.; Renner, M.W.; Hidaka, B.H.; Sweazea, K.L. An evolutionary mismatch narrative to improve lifestyle medicine: A patient education hypothesis. Evol. Med. Public Health 2021, 9, 157–163. [Google Scholar] [CrossRef]
- Tiller, N.B.; Chiesa, S.T.; Roberts, J.D.; Turner, L.A.; Jones, S.; Romer, L.M. Physiological and Pathophysiological Consequences of a 25-Day Ultra-Endurance Exercise Challenge. Front. Physiol. 2019, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Heydenreich, J.; Kayser, B.; Schutz, Y.; Melzer, K. Total Energy Expenditure, Energy Intake, and Body Composition in Endurance Athletes Across the Training Season: A Systematic Review. Sports Med. Open 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mitch, W.E.; Price, S.R. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat. Rev. Nephrol. 2021, 18, 138–152. [Google Scholar] [CrossRef]
- Kinrade, E.J.; Galloway, S.D.R. Dietary Observations of Ultra-Endurance Runners in Preparation for and During a Continuous 24-h Event. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN exercise & sport nutrition review: Research & recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef]
- Hoffman, M.D. Proper Hydration During Ultra-endurance Activities. Sports Med. Arthrosc. Rev. 2019, 27, 8–14. [Google Scholar] [CrossRef]
- Vicente-Salar, N.; Otegui, A.U.; Collado, E.R. Endurance Training in Fasting Conditions: Biological Adaptations and Body Weight Management. Nutr. Hosp. 2015, 32, 2409–2420. [Google Scholar]
- Clemente-Suárez, V.J. Psychophysiological response and energy balance during a 14-h ultraendurance mountain running event. Appl. Physiol. Nutr. Metab. 2015, 40, 269–273. [Google Scholar] [CrossRef]
- Moore, R.D.; Romine, M.W.; O’Connor, P.J.; Tomporowski, P.D. The influence of exercise-induced fatigue on cognitive function. J. Sports Sci. 2012, 30, 841–850. [Google Scholar] [CrossRef]
- Wollseiffen, P.; Schneider, S.; Martin, L.A.; Kerhervé, H.A.; Klein, T.; Solomon, C. The effect of 6 h of running on brain activity, mood, and cognitive performance. Exp. Brain Res. 2016, 234, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H. Economy and Endurance in Human Evolution. Curr. Biol. 2017, 27, R613–R621. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, S.D.; Aslaksen, P.M.; Pettersen, S.A. Pain processing in elite and high-level athletes compared to non-athletes. Front. Psychol. 2020, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Fogard, K. Factors Related to Successful Completion of a 161-km Ultramarathon. Int. J. Sports Physiol. Perform. 2011, 6, 25–37. [Google Scholar] [CrossRef]
- Berger, N.J.A.; Best, R.; Best, A.W.; Lane, A.M.; Millet, G.Y.; Barwood, M.; Marcora, S.; Wilson, P.; Bearden, S. Limits of Ultra: Towards an Interdisciplinary Understanding of Ultra-Endurance Running Performance. Sports Med. 2023, 54, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Best, R.; Barwick, B.; Best, A.; Berger, N.; Harrison, C.; Wright, M.; Sparrow, J. Changes in Pain and Nutritional Intake Modulate Ultra-Running Performance: A Case Report. Sports 2018, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.M.; Pollatos, O. Attenuated interoceptive sensitivity in overweight and obese individuals. Eat. Behav. 2014, 15, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, I.; Sanchez-Gómez, J.M.; Aparicio, I.; Priego-Quesada, J.I.; Pérez-Soriano, P.; Collado, E.; Hernando, B.; Hernando, C. Effect of mountain ultramarathon distance competition on biochemical variables, respiratory and lower-limb fatigue. PLoS ONE 2020, 15, e0238846. [Google Scholar] [CrossRef]
| Distance Covered (km; m) | Duration (h; min) | Positive Elevation (m) | Negative Elevation (m) | Cumulative Elevation (m) | Maximum Temperature (°C) | Minimum Temperature (°C) | |
|---|---|---|---|---|---|---|---|
| Day 1 | 40 | 8:00 | 755 | 745 | 1500 | 16 | 5 |
| Day 2 | 36.31 | 7:53 | 250 | 205 | 455 | 12 | 3 |
| Day 3 | 32.94 | 8:25 | 405 | 385 | 790 | 14 | 5 |
| Day 4 | 30.55 | 7:53 | 300 | 300 | 600 | 16 | 3 |
| Total | 139.8 | 32:11 | 1710 | 1635 | 3345 | 14.5 medium | 4 medium |
| Nutrient | 40 g | Per 100 g |
|---|---|---|
| Energy (kJ) | 628.4 kJ | 1571 kJ |
| Energy (kcal) | 151.2 kcal | 378 kcal |
| Lipids (Fats) | 7.2 g | 18 g |
| Saturated Fats | 4.3 g | 10.8 g |
| Carbohydrates | 14.35 g | 35.9 g |
| Sugars | 0.2 g | 0.47 g |
| Polyalcohols | 12.55 g | 31.4 g |
| Fiber | 1.3 g | 3.3 g |
| Proteins | 11.2 g | 28 g |
| Salt | 0.35 g | 0.83 g |
| Participant | Evaluation Moment | Weight | Total Body Water | Protein | Body Fat Mass | Fat Free Mass | Skeletal Muscle Mass | Body Mass Index | Percent Body Fat | Fat Free Mass of Right Arm | Fat Free Mass of Left Arm | Fat Free Mass of Trunk | Fat Free Mass of Right Leg | Fat Free Mass of Left Leg | Body Fat Mass of Right Arm | Body Fat Mass of Left Arm | Body Fat Mass of Trunk | Body Fat Mass of Right Leg | Body Fat Mass of Left Leg | Visceral Fat Level | Obesity Degree 90–110 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 72.2 | 42.1 | 11.6 | 14.6 | 57.6 | 32.8 | 23.8 | 20.2 | 3.00 | 3.01 | 24.9 | 9.68 | 9.45 | 0.8 | 0.8 | 7.2 | 2.4 | 2.3 | Level 5 | 108 |
| 2 | 71.2 | 42.6 | 11.7 | 13.0 | 58.2 | 33.3 | 23.5 | 18.3 | 3.11 | 3.03 | 25.2 | 9.84 | 9.63 | 0.6 | 0.7 | 6.4 | 2.2 | 2.1 | Level 4 | 107 | |
| 3 | 70.1 | 42.1 | 11.7 | 12.4 | 57.7 | 33.0 | 23.2 | 17.7 | 3.04 | 2.98 | 25.0 | 9.75 | 9.61 | 0.6 | 0.6 | 6.0 | 2.1 | 2.1 | Level 4 | 105 | |
| 4 | 70.1 | 41.8 | 11.6 | 12.8 | 57.3 | 32.8 | 23.2 | 18.3 | 2.96 | 3.02 | 24.9 | 9.75 | 9.50 | 0.6 | 0.6 | 6.2 | 2.2 | 2.1 | Level 4 | 105 | |
| 5 | 69.0 | 41.3 | 11.3 | 12.6 | 56.4 | 32.3 | 22.8 | 18.2 | 2.91 | 2.98 | 24.7 | 9.67 | 9.47 | 0.6 | 0.6 | 6.0 | 2.1 | 2.1 | Level 4 | 104 | |
| 6 | 68.9 | 42.1 | 11.6 | 11.4 | 57.5 | 33.0 | 22.8 | 16.5 | 3.08 | 3.11 | 25.4 | 9.61 | 9.47 | 0.5 | 0.5 | 5.5 | 1.9 | 1.9 | Level 3 | 103 | |
| 7 | 68.1 | 41.0 | 11.3 | 12.0 | 56.1 | 32.1 | 22.5 | 17.7 | 2.88 | 2.96 | 24.5 | 9.56 | 9.38 | 0.6 | 0.6 | 5.8 | 2.0 | 2.0 | Level 3 | 102 | |
| 8 | 68.3 | 41.1 | 11.2 | 12.1 | 56.2 | 32.1 | 22.6 | 17.7 | 2.89 | 2.84 | 24.2 | 9.55 | 9.42 | 0.6 | 0.6 | 5.8 | 2.1 | 2.0 | Level 3 | 103 | |
| 9 | 67.4 | 40.6 | 11.2 | 11.8 | 55.6 | 31.8 | 22.3 | 17.5 | 2.87 | 2.93 | 24.5 | 9.50 | 9.34 | 0.6 | 0.6 | 5.6 | 2.0 | 2.0 | Level 3 | 101 | |
| 10 | 69.4 | 42.6 | 11.7 | 11.1 | 58.3 | 33.2 | 22.9 | 16.0 | 2.99 | 3.04 | 24.8 | 9.79 | 9.66 | 0.5 | 0.5 | 5.3 | 1.9 | 1.9 | Level 3 | 104 | |
| 11 | 69.7 | 41.8 | 11.4 | 12.6 | 57.1 | 32.5 | 23.0 | 18.0 | 2.93 | 2.98 | 24.5 | 9.74 | 9.55 | 0.6 | 0.6 | 6.0 | 2.1 | 2.1 | Level 4 | 105 | |
| 2 | 1 | 80.7 | 44.3 | 12.2 | 20.1 | 60.6 | 34.7 | 26.4 | 25.0 | 3.49 | 3.56 | 28.0 | 9.23 | 9.32 | 1.1 | 1.1 | 11.2 | 2.7 | 2.7 | Level 8 | 120 |
| 2 | 78.9 | 43.3 | 12.0 | 19.5 | 59.4 | 34.1 | 25.8 | 24.8 | 3.32 | 3.26 | 26.9 | 9.38 | 9.43 | 1.1 | 1.1 | 10.5 | 2.8 | 2.8 | Level 7 | 117 | |
| 3 | 77.7 | 43.2 | 11.9 | 18.5 | 59.2 | 33.9 | 25.4 | 23.9 | 3.24 | 3.31 | 26.7 | 9.32 | 9.40 | 1.0 | 1.0 | 9.9 | 2.7 | 2.7 | Level 7 | 115 | |
| 4 | 77.0 | 43.2 | 12.0 | 17.8 | 59.2 | 34.0 | 25.1 | 23.1 | 3.24 | 3.26 | 26.5 | 9.34 | 9.44 | 1.0 | 1.0 | 9.4 | 2.6 | 2.6 | Level 6 | 114 | |
| 5 | 76.8 | 42.8 | 11.8 | 18.2 | 58.6 | 33.6 | 25.1 | 23.6 | 3.21 | 3.18 | 26.3 | 9.41 | 9.59 | 1.0 | 1.0 | 9.5 | 2.7 | 2.7 | Level 6 | 114 | |
| 6 | 76.2 | 42.6 | 11.7 | 17.9 | 58.3 | 33.4 | 24.9 | 23.5 | 3.19 | 3.12 | 26.1 | 9.37 | 9.53 | 1.0 | 1.0 | 9.3 | 2.7 | 2.7 | Level 6 | 113 | |
| 7 | 76.3 | 42.5 | 11.7 | 18.1 | 58.2 | 33.4 | 24.9 | 23.7 | 3.20 | 3.16 | 26.2 | 9.40 | 9.64 | 1.0 | 1.0 | 9.5 | 2.7 | 2.7 | Level 6 | 113 | |
| 8 | 77.1 | 43.9 | 12.1 | 17.0 | 60.1 | 34.3 | 25.2 | 22.1 | 3.24 | 3.25 | 26.3 | 9.65 | 9.88 | 0.9 | 0.9 | 8.8 | 2.6 | 2.6 | Level 6 | 114 | |
| 9 | 77.9 | 43.7 | 12.0 | 18.1 | 59.8 | 34.3 | 25.4 | 23.2 | 3.31 | 3.43 | 27.2 | 9.17 | 9.23 | 1.0 | 1.0 | 9.9 | 2.5 | 2.5 | Level 7 | 116 | |
| 10 | 78.5 | 44.6 | 12.3 | 17.4 | 61.1 | 34.8 | 25.6 | 22.2 | 3.29 | 3.29 | 26.5 | 9.60 | 9.92 | 0.9 | 1.0 | 9.1 | 2.6 | 2.7 | Level 6 | 117 | |
| 11 | 78.8 | 43.7 | 12.0 | 19.0 | 59.8 | 34.3 | 25.7 | 24.1 | 3.40 | 3.40 | 27.3 | 9.21 | 9.25 | 1.1 | 1.1 | 10.4 | 2.6 | 2.6 | Level 7 | 117 |
| 1—Pre Day 1 | 2—Post Day 1 | 3—Pre Day 2 | 4—Post Day 2 | 5—Pre Day 3 | 6—Post Day 3 | 7—Pre Day 4 | 8—Post Day 4 | 9—Day 5 | 10—Day 6 | 11—Day 7 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants | Unit | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Reaction time | (ms) | 292 | 294 | 317 | 314 | 258 | 310 | 282 | 284 | 309 | 258 | 298 | 289 | 289 | 293 | 283 | 231 | 280 | 259 | 311 | 287 | 262 | 276 |
| Handgrip | kg | 54.8 | 43.8 | 50.9 | 54 | 54.2 | 44.4 | 56.9 | 51.4 | 50.1 | 45.6 | 54.3 | 47.6 | 50.5 | 46.7 | 52.5 | 48.1 | 51.1 | 47.7 | 50.3 | 43.8 | 50 | 42.8 |
| Horizontal Jump | cm | 130 | 135 | 100 | 105 | 115 | 99 | 85 | 67 | 110 | 101 | 92 | 55 | 110 | 80 | 94 | 68 | 110 | 94 | 110 | 90 | 111 | 91 |
| FVC | 3.78 | 4.16 | 3.88 | 4.08 | 4.34 | 4.16 | 4.14 | 4.1 | 4.15 | 4.19 | 4.36 | 4.02 | 4.11 | 4.1 | 4.34 | 4.12 | 4.34 | 4.11 | 4.12 | 4.2 | 4.22 | 4.7 | |
| FEV1 | 3.29 | 3.72 | 3.4 | 3.47 | 3.58 | 3.79 | 3.63 | 3.86 | 3.66 | 3.75 | 3.6 | 3.71 | 3.55 | 3.76 | 3.66 | 3.79 | 3.79 | 3.75 | 3.64 | 3.7 | 3.63 | 3.7 | |
| PEF | 11.8 | 12.43 | 6.68 | 12.2 | 11.88 | 9.78 | 11.67 | 9.85 | 12.09 | 11.98 | 12.31 | 12.43 | 11.37 | 11.37 | 12.43 | 12.2 | 11.98 | 11.77 | 12.31 | 12.31 | 11.98 | 12.2 | |
| Tª | °C | 31.4 | 29.5 | 31.2 | 32.2 | 31.5 | 30.9 | 31.9 | 31.7 | 31.2 | 31.6 | 32.2 | 32 | 31.3 | 31 | 32.2 | 32.2 | 31.6 | 30.4 | 31.9 | 32.1 | 31.9 | 31.5 |
| Glucose | mmol/L | 7.7 | 7.7 | 5.7 | 7.7 | 7.1 | 6.8 | 5.1 | 6.1 | 7.1 | 7.6 | 5.4 | 6.7 | 7.3 | 8.2 | 5.1 | 6.8 | 6.5 | 7.6 | 7.4 | 7.9 | 7.2 | 7.2 |
| USG | 1.02 | 1.02 | 1.01 | 1.02 | 1.02 | 1.03 | 1.00 | 1.03 | 1.01 | 1.02 | 1.00 | 1.03 | 1.02 | 1.02 | 1.00 | 1.03 | 1.01 | 1.02 | 1.01 | 1.01 | 1.02 | 1.02 | |
| UCC | 2 | 2 | 4 | 3 | 3 | 3 | 1 | 6 | 4 | 4 | 2 | 5 | 4 | 5 | 1 | 5 | 4 | 3 | 3 | 3 | 4 | 4 | |
| Nitrites urine | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | |
| pH urine | 6 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | 5 | 5 | 5 | 6 | 5 | 6 | 6 | 6 | 7 | 7 | 6 | 6 | 7 | 6 | |
| Protein urine | N | N | N | N | 1 | 1 | N | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | |
| Glucose urine | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | |
| RPE (6–20) | 6 | 6 | 15 | 18 | 8 | 10 | 15 | 16 | 7 | 8 | 18 | 18 | 9 | 11 | 16 | 15 | 9 | 10 | 7 | 10 | 7 | 8 | |
| Pain (0–100) | 0 | 10 | 50 | 30 | 15 | 30 | 50 | 51 | 30 | 40 | 55 | 80 | 30 | 40 | 50 | 65 | 20 | 45 | 20 | 30 | 15 | 30 | |
| Pain leg (0–100) | 0 | 10 | 60 | 40 | 25 | 50 | 70 | 65 | 40 | 60 | 65 | 90 | 40 | 60 | 65 | 70 | 30 | 65 | 25 | 40 | 20 | 40 | |
| Hunger | 0 | 7 | 5 | 17 | 10 | 25 | 30 | 42 | 15 | 20 | 25 | 30 | 25 | 45 | 20 | 52 | 15 | 40 | 0 | 40 | 0 | 35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belinchón-deMiguel, P.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Clemente-Suárez, V.J. Evolutionary Echoes: A Four-Day Fasting and Low-Caloric Intake Study on Autonomic Modulation and Physiological Adaptations in Humans. Life 2024, 14, 456. https://doi.org/10.3390/life14040456
Belinchón-deMiguel P, Navarro-Jiménez E, Laborde-Cárdenas CC, Clemente-Suárez VJ. Evolutionary Echoes: A Four-Day Fasting and Low-Caloric Intake Study on Autonomic Modulation and Physiological Adaptations in Humans. Life. 2024; 14(4):456. https://doi.org/10.3390/life14040456
Chicago/Turabian StyleBelinchón-deMiguel, Pedro, Eduardo Navarro-Jiménez, Carmen Cecilia Laborde-Cárdenas, and Vicente Javier Clemente-Suárez. 2024. "Evolutionary Echoes: A Four-Day Fasting and Low-Caloric Intake Study on Autonomic Modulation and Physiological Adaptations in Humans" Life 14, no. 4: 456. https://doi.org/10.3390/life14040456
APA StyleBelinchón-deMiguel, P., Navarro-Jiménez, E., Laborde-Cárdenas, C. C., & Clemente-Suárez, V. J. (2024). Evolutionary Echoes: A Four-Day Fasting and Low-Caloric Intake Study on Autonomic Modulation and Physiological Adaptations in Humans. Life, 14(4), 456. https://doi.org/10.3390/life14040456









