Towards Lipid from Microalgae: Products, Biosynthesis, and Genetic Engineering
Abstract
1. Introduction
2. Application of Lipids from Microalgae
| Genus | Lipid Yield (% DW ***) | Bioactive Lipid | Market Price (** $/g) | Application | References |
|---|---|---|---|---|---|
| Chlorococcum sp. | 20–24 | Phosphatidylcholine | 50–8000 | Anti-inflammatory, anti-thrombotic activities | [21] |
| Nannochloropsis spp. | 37–60 | Eicosapentaenoic acid | 40–23,000 | Reduce heart attack and cardiovascular death | [22] |
| Crypthecodinium cohnii, Schizochytrium spp. | 14–33 | Docosahexaenoic acid | 2–4000 | Improved vision, brain, and memory development | [23] |
| Chlamynodomonas reinhardtii | 25–51 | 1,3-dioleolyl-2-palmitate | 4–16,000 | Proper infant growth and development | [15,17] |
| Nannochloropsis oceanica | 23–68 | Medium-chain triglyceride | 3–16,000 | Anti-atherosclerosis, anti-obesity | [14,16] |
| Phaeodactylum tricornutum | 10–32 | Fucoxanthin | 1000–43,000 | Ophthalmic, cerebrovascular and hepatic health | [24,25] |
| Euglena gracilis | 9–17 | Lycopene | 2–5300 | Antioxidant, cerebrovascular health | [26] |
| Coelastrella terrestris | 11–23 | Canthaxanthin | 1–20,000 | Antioxidant, visual health | [27] |
| Heterosigma akashiwo | N/A * | Zeaxanthin | 1–110,000 | Anti-Inflammatory, anticancer | [28] |
| Chlamydomonadales sp. | 15–23 | Neoxanthin | 5000–120,000 | Antioxidant, cardiovascular health | [29] |
| Haematococcus pluvialis, Chlorella zofingiensis | 30–50 | Astaxanthin | 3–4500 | Anti-oxidation, anti-inflammation | [30,31] |
| Rhodophyte, Chlorophyte, Bacillariophyte, etc. | 12–33 | Oxylipins | 1–50,000 | Anti-inflammatory, tissue regeneration | [32] |
| Chlorella protothecoides | 10–30 | Lutein | 1–27,000 | Immune stimulant, anti-inflammatory, antioxidant | [33] |
| Dunaliella salina | 12–44 | β-carotene | 1–12,000 | Antioxidant, anti-allergic, anti-inflammatory | [33,34] |
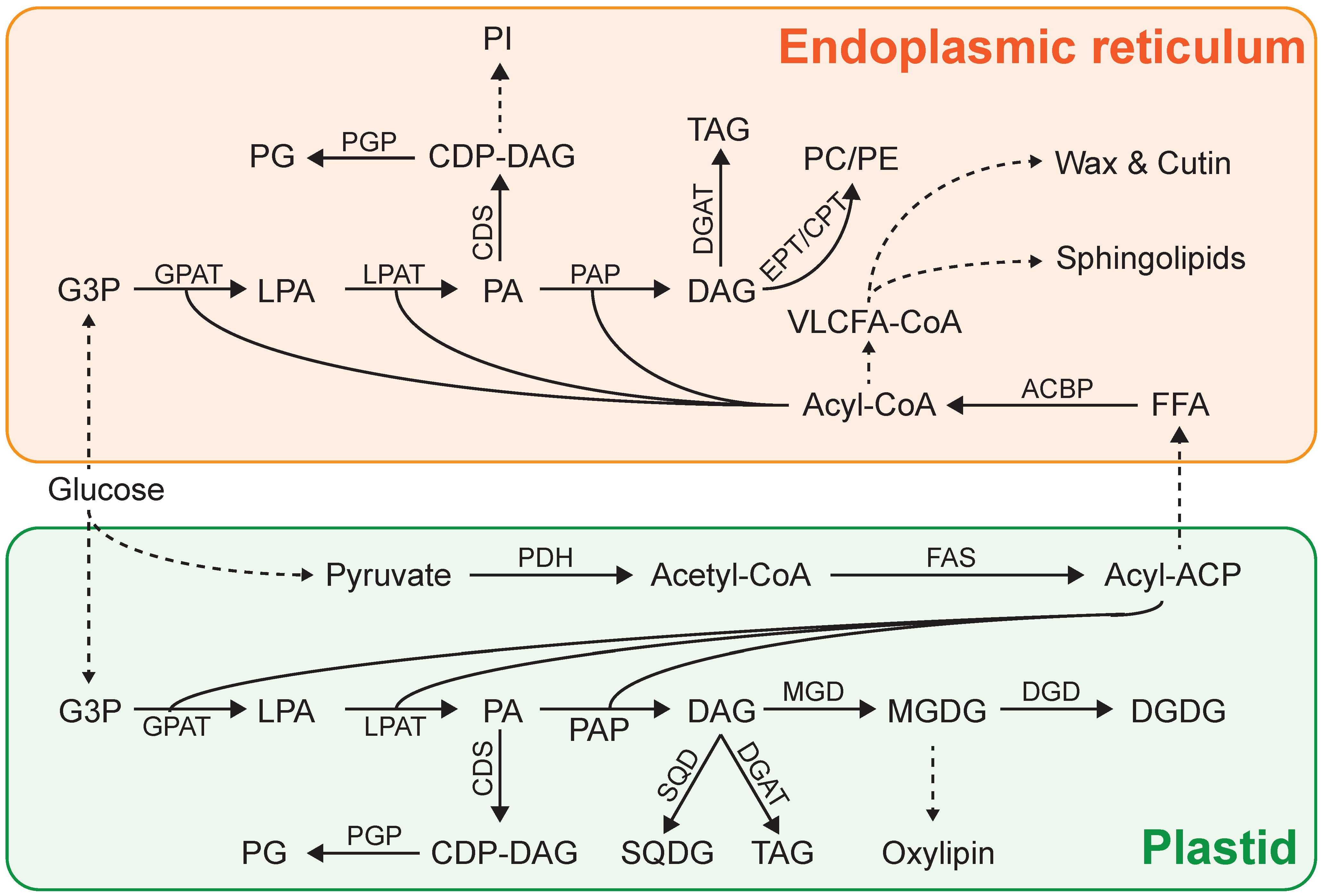
3. Lipid Biosynthesis in Microalgae
4. Lipid Induction Strategies in Microalgae
| Genus | Affecting Factor | Effect to Lipid Production | Effect to Biomass | Reference |
|---|---|---|---|---|
| Acutodesmus obliquus | Blue–green light | Higher percentage of PUFAs | N/A * | [61] |
| Haematococcus pluvialis | Gluconate plus white–blue LED | Increased astaxanthin content to 3.3% | Increase to 4.5 g/L | [62] |
| Scenedesmus sp. | Microwave | Increased lipid content by 1.4 g/L | 1.5-fold increase | [63] |
| Nannochloropsis oceanica | Salicylic acid | Increased lipid and EPA contents | N/A | [64] |
| Graesiella emersonii | Indole acetic acid plus kinetin | Increased lipid yield by 2.5-fold | 2.3-fold increase | [65] |
| Phaeodactylum tricornutum | Marinobacter | Increased lipid content by 30 mg/L | Increase to 0.2 g/L | [66] |
| Nannochloropsis oceanica | Probiotic bacteria | Increased EPA content by 2.3-fold | 1.6-fold increase | [67] |
| Monoraphidium sp. | Strigolactone | Increased lipid productivity by 55% | Increased | [68] |
| Euglena gracilis | Phenolic compounds | Increased carotenoids and lipids | 2.3-fold increase | [69] |
| Chlorella sp. | Magnesium aminoclay nanoparticles | Increased lipid content by 18% | N/A | [70] |
| Chlamydomonas reinhardtii | Salt stress with NaCl and KCl | Increased saturated fatty acids | N/A | [71] |
| Neochloris oleoabundans | High light plus CaCO3 crystal | Increased lipid productivity by 32% | Increase to 3.1 g/L | [72] |
| Scenedesmus sp. | Oxidative stress plus nanoparticles | Increased lipid content to 40% | Increase to 3.2 g/L | [73] |
| Chlorella pyrenoidosa | Salt stress plus abscisic acid | Increased lipid productivity by 3.7-fold | 1.5-fold increase | [74] |
| Monoraphidium sp. | Cu2+ induction plus γ-aminobutyric acid | Increased lipid content to 58% | Increase to 1.3 g/L | [75,76] |
| Chlamydomonas sp. | 5% CO2 concentration | Increased lipid content (65%) and productivity (169 mg/L/day) | [82] | |
| Chlorella vulgaris | 30% CO2 | Increased lipid content (46%) and productivity (86 mg/L/day) | [58] | |
| Chlorella vulgaris | Nanoscale MgSO4 | Increased lipid productivity by 185% | [83] | |
| Nannochloropsis maritima | Fe3O4 nanoparticles | More total lipid amount | Increase to 1 g/L | [84] |
| Nannochloropsis sp. | High-light (700 μmol photons/m2/s) | Increased lipid content to 47% | N/A | [85] |
| Scenedesmus sp. | High-light (400 μmol photons/m2/s) | Increased lipid content by 11-folds | N/A | [86] |
| Heterochlorella luteoviridis | High temperature (27 °C) | Increased SFA content to 53% | N/A | [87] |
| Microcystis aeruginosa | High nitrogen (ten times higher) | Increased lipid content (34%) and productivity (47 mg/L/day) | [88] | |
| Chlamydomonas reinhardtii | Limited mixotrophic conditions | 66% increase in lipid production (0.08 g/L) | [58] | |
| Chlorella vulgaris | MnCl2 (10 μM) | Increased lipid content by 16% | N/A | [89] |
5. Genetic Engineering of Microalgae for Enhanced Lipid Production
| Genus | Targeted Genes | Strategy * | Effect on Lipid Synthesis | References |
|---|---|---|---|---|
| Chlamydomonas reinhardtii | GroELS, PEPC1 | OE, KD | Boosted lipids and lutein with 893 and 23.5 mg/L | [92] |
| SAMS | OE | Two-fold increased lipid content | [93] | |
| HpWS | HE | 150% and 39% increased astaxanthin and TAG content | [94] | |
| FAX1, FAX2, ABCA2 | CE | 2.4-fold increased TAG content | [95,96,97,98,99] | |
| DOF, LACS2, CIS | OE, KD | Lipids and FA content increased by 142% and 52% | [100,101] | |
| MYB1 | OE | 3.2-fold increased TAG content | [102] | |
| FAT1 | OE | Increased lipid production | [103] | |
| CpZF_CCCH1 | HE | Increased PUFA content by 16% | [104] | |
| CrPrp19 | KD | 1.3-fold increased TAG content | [105] | |
| CrGPATer | OE | Increased yield of OPO and galactolipids | [17] | |
| ApACBP3, ApDGAT1 | HE | Increased C18:1 content by 59% | [107] | |
| HpDGTT2 | HE | Enhanced TAG accumulation | [106] | |
| Nannochloropsis spp. | CrCAO | HE | Increased lipid productivity | [109] |
| AtDXS | HE | Lipids and TAG content increased by 111% and 149% | [110] | |
| mCpTE | HE | Elevated C12:0 content by 6.6-fold | [111] | |
| FAD12 | OE | 1.5-fold increase in EPA | [112] | |
| NoΔ6-FAE | OE | Higher contents of FA, TAG and EPA | [50] | |
| NoGPAT, AoGPAT | OE | TAG, FA and PUFA increase by 51%, 42%, and 24% | [113] | |
| NoPDAT | OE | 33% increased TAG content | [114,115] | |
| NobZIP1 | OE | Elevation of lipid accumulation and lipid secretion | [116] | |
| NsbHLH2 | OE | Increased FA production | [117] | |
| NobZIP77 | KO | Double the peak productivity of TAG | [118] | |
| NoDGAT2D, AtWRI1, etc. | CE | Elevated MCT productivity by 64.8-fold | [16] | |
| Phaeodactylum tricornutum | PtDGAT2B, OtElo5 | CE | Higher lipid yields and TAG-associated DHA level | [124] |
| PAP | OE | 51% increased fucoxanthin content | [123] | |
| G6PDH | OE | Much higher of lipid and EPA content | [121] | |
| Δ9-DES | KO | 1.4-fold increased EPA content | [122] | |
| PtME, PtD5b | OE | 2.4-fold increased TAG content | [125] | |
| PtPPT | OE | 30% increased lipid content | [120] | |
| Chlorella spp. | HSbZIP1 | OE | 113% increased FA content | [127] |
| AtLEC1 | HE | Lipids and FA content increased by 30% and 33% | [128] | |
| CvarLOG1 | OE | 20% increased lipid yield | [129] | |
| Ostreococcus tauri | pω3-Des | OE | Higher TAG-associated ALA | [130] |
| Δ6-DES | OE | Increased TAG content | [131] | |
| Neochloris oleoabundans | NeoLPAAT1, NeoDGAT2 | CE | 2.1- and 1.6-fold increased TAG and lipid content | [132,133] |
| LPAT, GPAT, DGAT | CE | 1.2-folds increase in FA content | [134] | |
| Cyanidioschyzon merolae | LPAT1 | OE | Increased TAG accumulation | [135] |
| Schizochytrium spp. | AACT4419 | OE | 1.8- and 2.4-fold increased β-carotene and astaxanthin | [136] |
| CcME, MaELO3 | CE | 1.4-fold increased DHA content | [137] | |
| Dunaliella salina | DsME1, DsME2 | OE | 36% higher lipid production | [138] |
| Scenedesmus sp. Z-4 | ACCase | OE | 29% increased lipid content | [139] |
| Synechocystis sp. | ACCase | HE | 3.6-fold increased lipid content | [140] |
6. Challenges and Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Environmental science. rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594–1257605. [Google Scholar] [CrossRef] [PubMed]
- Novoveska, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-properties and health benefits of fish lipid bioactives; the effects of thermal processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.R. Microalgal lipids: Biochemistry and biotechnology. Curr. Opin. Biotechnol. 2022, 74, 1–7. [Google Scholar] [CrossRef]
- Ruiz-López, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-chain polyunsaturated fatty acids (LCPUFAs) and the developing immune system: A narrative review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800–101814. [Google Scholar] [CrossRef]
- Gupta, J.; Gupta, R. Nutraceutical status and scientific strategies for enhancing production of omega-3 fatty acids from microalgae and their role in healthcare. Curr. Pharm. Biotechnol. 2020, 21, 1616–1631. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. Cell Mol. Biol. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Ghide, M.K.; Yan, Y.J. 1,3-Dioleoyl-2-palmitoyl glycerol (OPO)-enzymatic synthesis and use as an important supplement in infant formulas. J. Food Biochem. 2021, 45, 13799–13811. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wang, Q.T.; Shen, C.; Hu, C.X.; Shi, X.Z.; Lv, N.N.; Du, X.F.; Xu, G.W.; Xu, J. Medium-chain triglyceride production in Nannochloropsis via a fatty acid chain length discriminating mechanism. Plant Physiol. 2022, 190, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Lu, Y.C.; Ma, H.Y.; Li, Y.H.; Chen, G.Q.; Han, D.X.; Hu, Q. Microalgal glycerol-3-phosphate acyltransferase role in galactolipids and high-value storage lipid biosynthesis. Plant Physiol. 2023, 192, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Paliwal, C.; Ghosh, T.; Pancha, I.; Chokshi, K.; Mitra, M.; Ghosh, A.; Mishra, S. Applications of de-oiled microalgal biomass towards development of sustainable biorefinery. Bioresour. Technol. 2016, 214, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Sun, H.; Deng, J.Q.; Huang, J.C.; Chen, F. Carotenoid production from microalgae: Biosynthesis, salinity responses and novel biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef] [PubMed]
- de-Morais, M.G.; Vaz, B.S.; de-Morais, E.G.; Costa, J.A. Biologically active metabolites synthesized by microalgae. Biomed Res. Int. 2015, 2015, 835761–835775. [Google Scholar] [CrossRef] [PubMed]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive lipids of marine microalga Chlorococcum sp. SABC 012504 with anti-inflammatory and anti-thrombotic activities. Mar. Drugs 2021, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Riaz, S.; Mazhar, S.; Essa, R.; Maryam, M.; Saleem, Y.; Syed, Q.; Perveen, I.; Bukhari, B.; Ashfaq, S.; et al. Microbial production of docosahexaenoic acid (DHA): Biosynthetic pathways, physical parameter optimization, and health benefits. Arch. Microbiol. 2023, 205, 321. [Google Scholar] [CrossRef] [PubMed]
- Nagao, R.; Kato, K.; Suzuki, T.; Ifuku, K.; Uchiyama, I.; Kashino, Y.; Dohmae, N.; Akimoto, S.; Shen, J.R.; Miyazaki, N.; et al. Structural basis for energy harvesting and dissipation in a diatom PSII-FCPII supercomplex. Nat. Plants 2019, 5, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, S.F.; Zhao, W.Y.; Kong, Q.; Zhu, C.L.; Fu, X.D.; Zhang, F.; Liu, Z.M.; Zhan, Y.M.; Mou, H.J.; et al. Fucoxanthin from marine microalgae: A promising bioactive compound for industrial production and food application. Crit. Rev. Food Sci. Nutr. 2023, 63, 7996–8012. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Fu, W.; Du, M.; Chen, Z.X.; Lei, A.P.; Wang, J.X. Carotenoids biosynthesis, accumulation, and applications of a model microalga Euglena gracilis. Mar. Drugs 2022, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- Doppler, P.; Kriechbaum, R.; Kafer, M.; Kopp, J.; Remias, D.; Spadiut, O. Coelastrella terrestris for adonixanthin production: Physiological characterization and evaluation of secondary carotenoid productivity. Mar. Drugs 2022, 20, 175. [Google Scholar] [CrossRef]
- Sun, K.M.; Gao, C.L.; Zhang, J.; Tang, X.X.; Wang, Z.L.; Zhang, X.L.; Li, Y. Rapid formation of antheraxanthin and zeaxanthin in seconds in microalgae and its relation to non-photochemical quenching. Photosynth. Res. 2020, 144, 317–326. [Google Scholar] [CrossRef]
- Osterrothova, K.; Culka, A.; Nemeckova, K.; Kaftan, D.; Nedbalova, L.; Prochazkova, L.; Jehlicka, J. Analyzing carotenoids of snow algae by raman microspectroscopy and high-performance liquid chromatography. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2019, 212, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Linares-Maurizi, A.; Reversat, G.; Awad, R.; Bultel-Ponce, V.; Oger, C.; Galano, J.M.; Balas, L.; Durbec, A.; Bertrand-Michel, J.; Durand, T.; et al. Bioactive oxylipins profile in marine microalgae. Mar. Drugs 2023, 21, 136. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Koketsu, M. Isolation and analysis of polysaccharide showing high hyaluronidase inhibitory activity in Nostochopsis lobatus MAC0804NAN. J. Biosci. Bioeng. 2016, 121, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J. Eukaryotic microalgae as hosts for light-driven heterologous isoprenoid production. Planta 2019, 249, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Seth, K.; Maheshwari, K.; Baroliya, P.K.; Meena, M.; Kumar, A.; Vinayak, V.; Harish. Biosynthesis and extraction of high-value carotenoid from algae. Front. Biosci. 2021, 26, 171–190. [Google Scholar]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2010, 8, 133–197. [Google Scholar] [CrossRef]
- Qiu, X. Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7,10,13,16,19): Two distinct pathways. Prostaglandins Leukot. Essental Fat. Acids 2003, 68, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Meesapyodsuk, D.; Qiu, X. The front-end desaturase: Structure, function, evolution and biotechnological use. Lipids 2012, 47, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Uttaro, A.D. Biosynthesis of polyunsaturated fatty acids in lower eukaryotes. IUBMB Life 2006, 58, 563–571. [Google Scholar] [CrossRef]
- Meesapyodsuk, D.; Qiu, X. Structure determinants for the substrate specificity of acyl-CoA delta9 desaturases from a marine copepod. ACS Chem. Biol. 2014, 9, 922–934. [Google Scholar] [CrossRef]
- Abe, T.; Sakuradani, E.; Asano, T.; Kanamaru, H.; Shimizu, S. Functional characterization of delta9 and omega9 desaturase genes in Mortierella alpina 1S-4 and its derivative mutants. Appl. Microbiol. Biotechnol. 2006, 70, 711–719. [Google Scholar] [CrossRef]
- Kajikawa, M.; Yamato, K.T.; Kohzu, Y.; Nojiri, M.; Sakuradani, E.; Shimizu, S.; Sakai, Y.; Fukuzawa, H.; Ohyama, K. Isolation and characterization of delta(6)-desaturase, an ELO-like enzyme and delta(5)-desaturase from the liverwort Marchantia polymorpha and production of arachidonic and eicosapentaenoic acids in the methylotrophic yeast Pichia pastoris. Plant Mol. Biol. 2004, 54, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hong, H.; MacKenzie, S.L. Identification of a delta 4 fatty acid desaturase from Thraustochytrium sp. involved in the biosynthesis of docosahexanoic acid by heterologous expression in Saccharomyces cerevisiae and Brassica juncea. J. Biol. Chem. 2001, 276, 31561–31566. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, R.; Napier, J.A.; Sayanova, O. Identification and functional characterization of genes encoding omega-3 polyunsaturated fatty acid biosynthetic activities from unicellular microalgae. Mar. Drugs 2013, 11, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Madrigal, J.; Navarro, J.C.; Hontoria, F.; Tocher, D.R.; Martinez-Palacios, C.A.; Monroig, O. Diversification of substrate specificities in teleostei fads2: Characterization of delta4 and delta6delta5 desaturases of Chirostoma estor. J. Lipid Res. 2014, 55, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Tonon, T.; Harvey, D.; Larson, T.R.; Graham, I.A. Identification of a very long chain polyunsaturated fatty acid delta4-desaturase from the microalga Pavlova lutheri. FEBS Lett. 2003, 553, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, M.J.; Pan, Y.F.; Hu, H.H.; Liu, J. Delta6 fatty acid elongase is involved in eicosapentaenoic acid biosynthesis via the omega6 pathway in the marine alga Nannochloropsis oceanica. J. Agric. Food Chem. 2021, 69, 9837–9848. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, H. The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Mena, P.; Rojas, C. Essential fatty acids in early life: Structural and functional role. Proc. Nutr. Soc. 2000, 59, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.G.; Roessler, P.; Facciotti, D.; Levering, C.; Dittrich, F.; Lassner, M.; Valentine, R.; Lardizabal, K.; Domergue, F.; Yamada, A.; et al. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 2001, 293, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Harwood, J.L.; Lemieux, M.J.; Stone, S.J.; Weselake, R.J. Acyl-CoA:diacylglycerol acyltransferase: Properties, physiological roles, metabolic engineering and intentional control. Prog. Lipid Res. 2022, 88, 101181. [Google Scholar] [PubMed]
- Young, D.Y.; Pang, N.; Shachar-Hill, Y. 13C-labeling reveals how membrane lipid components contribute to triacylglycerol accumulation in Chlamydomonas. Plant Physiol. 2022, 189, 1326–1344. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, H.; Hu, C. Effects of temperature and its combination with high light intensity on lipid production of Monoraphidium dybowskii Y2 from semi-arid desert areas. Bioresour. Technol. 2018, 265, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramirez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Factories 2019, 18, 178. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef]
- Dupre, C.; Burrows, H.D.; Campos, M.G.; Delattre, C.; Encarnacao, T. Microalgal biomass of industrial interest: Methods of characterization. In Handbook on Characterization of Biomass, Biowaste and Related By-Products; Springer Science: Berlin/Heidelberg, Germany, 2020; pp. 537–639. [Google Scholar]
- Helamieh, M.; Reich, M.; Bory, S.; Rohne, P.; Riebesell, U.; Kerner, M.; Kummerer, K. Blue-green light is required for a maximized fatty acid unsaturation and pigment concentration in the microalga Acutodesmus obliquus. Lipids 2022, 57, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.; Fu, X.; Fernandez, J.S.M.; Chen, S.L. Multilevel heuristic LED regime for stimulating lipid and bioproducts biosynthesis in Haematococcus pluvialis under mixotrophic conditions. Bioresour. Technol. 2019, 288, 121525–121532. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Suresh, S.; Pugazhendhi, A.; Pauline, J.M.N.; Incharoensakdi, A. Response of Scenedesmus sp. to microwave treatment: Enhancement of lipid, exopolysaccharide and biomass production. Bioresour. Technol. 2020, 312, 123562–123569. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Sabapathy, H.; Arumugam, M. Stress hormones mediated lipid accumulation and modulation of specific fatty acids in Nannochloropsis oceanica CASA CC201. Bioresour. Technol. 2020, 310, 123437–123445. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.K.; Chanu, N.K.; Chaurasia, N. Exogenous addition of indole acetic acid and kinetin under nitrogen-limited medium enhances lipid yield and expression of glycerol-3-phosphate acyltransferase & diacylglycerol acyltransferase genes in indigenous microalgae: A potential approach for biodiesel production. Bioresour. Technol. 2020, 297, 122439–122453. [Google Scholar] [PubMed]
- Chorazyczewski, A.M.; Huang, I.S.; Abdulla, H.; Mayali, X.; Zimba, P.V. The influence of bacteria on the growth, lipid production, and extracellular metabolite accumulation by Phaeodactylum Tricornutum (bacillariophyceae). J. Phycol. 2021, 57, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Eltanahy, E.E.; Liu, H.W.; Chua, E.T.; Thomas-Hall, S.R.; Wass, T.J.; Pan, K.; Schenk, P.M. Growth-promoting bacteria double eicosapentaenoic acid yield in microalgae. Bioresour. Technol. 2020, 316, 123916–123925. [Google Scholar] [CrossRef] [PubMed]
- Song, X.T.; Zhao, Y.T.; Li, T.; Han, B.Y.; Zhao, P.; Xu, J.W.; Yu, X.Y. Enhancement of lipid accumulation in Monoraphidium sp. QLY-1 by induction of strigolactone. Bioresour. Technol. 2019, 288, 121607–121614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Tan, X.M.; Hafid, H.S.; Wakisaka, M. Enhancement of biomass yield and lipid accumulation of freshwater microalga Euglena gracilis by phenolic compounds from basic structures of lignin. Bioresour. Technol. 2021, 321, 124441–124451. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kim, Y.E.; Lee, N.; Yu, H.; Lee, J.; Lee, S.Y.; Lee, Y.C.; Oh, Y.K. Simultaneous enhancement of lipid biosynthesis and solvent extraction of Chlorella using aminoclay nanoparticles. Bioresour. Technol. 2023, 384, 129314–129323. [Google Scholar] [CrossRef] [PubMed]
- Atikij, T.; Syaputri, Y.; Iwahashi, H.; Praneenararat, T.; Sirisattha, S.; Kageyama, H.; Waditee-Sirisattha, R. Enhanced lipid production and molecular dynamics under salinity stress in green microalga Chlamydomonas reinhardtii (137C). Mar. Drugs 2019, 17, 484. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.E.; Yu, B.S.; Patel, A.K.; Choi, H.I.; Song, S.; Sung, Y.J.; Chang, W.S.; Sim, S.J. Enhanced biomass and lipid production of Neochloris oleoabundans under high light conditions by anisotropic nature of light-splitting CaCO(3) crystal. Bioresour. Technol. 2019, 287, 121483–121490. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Dai, Y.Q.; Kong, F.; Xing, D.F.; Zhao, L.; Ren, N.Q.; Ma, J.; Liu, B.F. Enhanced microalgal growth and lipid accumulation by addition of different nanoparticles under xenon lamp illumination. Bioresour. Technol. 2020, 297, 122409–122413. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Huang, K.X.; Zhang, Y.R.; Yang, L.; Zhou, J.L.; Yang, Q.; Gao, F. Efficient microalgal lipid production driven by salt stress and phytohormones synergistically. Bioresour. Technol. 2023, 367, 128270–128278. [Google Scholar] [CrossRef]
- Li, X.M.; Gu, D.; You, J.K.; Qiao, T.S.; Yu, X.Y. Gamma-aminobutyric acid coupled with copper ion stress stimulates lipid production of green microalga Monoraphidium sp. QLY-1 through multiple mechanisms. Bioresour. Technol. 2022, 352, 127091–127100. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Song, X.T.; Zhong, D.B.; Yu, L.; Yu, X.Y. Gamma-aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Bioresour. Technol. 2020, 297, 122500–122510. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Q. Regulatory mechanisms of lipid biosynthesis in microalgae. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2373–2391. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Pienkos, P.T. Algal omics: Unlocking bioproduct diversity in algae cell factories. Photosynth. Res. 2015, 123, 255–263. [Google Scholar] [CrossRef]
- Rai, V.; Muthuraj, M.; Gandhi, M.N.; Das, D.; Srivastava, S. Real-time iTRAQ-based proteome profiling revealed the central metabolism involved in nitrogen starvation induced lipid accumulation in microalgae. Sci. Rep. 2017, 7, 45732. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Sakata, M.; Ohnishi, K.; Tokumaru, Y.; Kato, Y.; Tokutsu, R.; Sakamoto, W.; Minagawa, J.; Matsuda, F.; Shimizu, H. Targeted proteome analysis of microalgae under high-light conditions by optimized protein extraction of photosynthetic organisms. J. Biosci. Bioeng. 2019, 127, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhou, Z.; Huang, Y.; Chen, L.; Cong, W. Multi-omics insights into the mechanism of the high-temperature tolerance in a thermotolerant Chlorella sorokiniana. Bioresour. Technol. 2023, 390, 129859. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Aikawa, S.; Ho, S.H.; Chen, C.Y.; Chang, J.S.; Hasunuma, T.; Kondo, A. Development of lipid productivities under different CO2 conditions of marine microalgae Chlamydomonas sp. JSC4. Bioresour. Technol. 2014, 152, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.J.; Das, R.K.; Brar, S.K.; Le Bihan, Y.; Buelna, G.; Verma, M.; Soccol, C.R. Application of magnesium sulfate and its nanoparticles for enhanced lipid production by mixotrophic cultivation of algae using biodiesel waste. Energy 2014, 78, 16–22. [Google Scholar] [CrossRef]
- Hu, Y.R.; Wang, F.; Wang, S.K.; Liu, C.Z.; Guo, C. Efficient harvesting of marine microalgae Nannochloropsis maritima using magnetic nanoparticles. Bioresour. Technol. 2013, 138, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, C.; Hu, G.; Li, F. Effects of light intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11-1 under nitrogen limitation. Appl. Biochem. Biotechnol. 2012, 166, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Menegol, T.; Diprat, A.B.; Rodrigues, E.; Rech, R. Effect of temperature and nitrogen concentration on biomass composition of Heterochlorella luteoviridis. Food Sci. Technol. 2017, 37, 28–37. [Google Scholar] [CrossRef]
- Mata, T.M.; Almeida, R.; Caetano, N.S. Effect of the culture nutrients on the biomass and lipid productivities of microalgae Dunaliella tertiolecta. Bioresour. Technol. 2013, 102, 1649–1655. [Google Scholar]
- Battah, M.; El-Ayoty, Y.; Abomohra, E.F.; El-Ghany, S.A.; Esmael, A. Effect of Mn2+, Co2+ and H2O2 on biomass and lipids of the green microalga Chlorella vulgaris as a potential candidate for biodiesel production. Ann. Microbiol. 2015, 65, 155–162. [Google Scholar] [CrossRef]
- Munoz, C.F.; Sudfeld, C.; Naduthodi, M.I.S.; Weusthuis, R.A.; Barbosa, M.J.; Wijffels, R.H.; D’Adamo, S. Genetic engineering of microalgae for enhanced lipid production. Biotechnol. Adv. 2021, 52, 107836. [Google Scholar] [CrossRef] [PubMed]
- Rochaix, J.D. Chlamydomonas reinhardtii as the photosynthetic yeast. Annu. Rev. Genet. 1995, 29, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Ng, I. Enhanced carbon capture, lipid and lutein production in Chlamydomonas reinhardtii under meso-thermophilic conditions using chaperone and CRISPRi system. Bioresour. Technol. 2023, 384, 129340–129349. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, J.W.; Park, E.J.; Choi, J.I. Overexpression of s-adenosylmethionine synthetase in recombinant Chlamydomonas for enhanced lipid production. J. Microbiol. Biotechnol. 2023, 33, 310–318. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, X.; Liang, L.M.; Liu, K.; Ye, H.Y.; Liu, Z.Y.; Liu, Y.F.; Huang, L.Q.; He, W.J.; Chen, Y.Q.; et al. Overexpression of acetyl-CoA carboxylase increases fatty acid production in the green alga Chlamydomonas reinhardtii. Biotechnol. Lett. 2019, 41, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; Huleux, M.; Spaniol, B.; Sommer, F.; Neunzig, J.; Schroda, M.; Li-Beisson, Y.; Philippar, K. Fatty acid export (FAX) proteins contribute to oil production in the green microalga Chlamydomonas reinhardtii. Front. Mol. Biosci. 2022, 9, 939834–939849. [Google Scholar] [CrossRef] [PubMed]
- Li, N.N.; Zhang, Y.; Meng, H.J.; Li, S.T.; Wang, S.F.; Xiao, Z.C.; Chang, P.; Zhang, X.H.; Li, Q.; Guo, L.; et al. Characterization of fatty acid exporters involved in fatty acid transport for oil accumulation in the green alga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2019, 12, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Kong, F.T.; Lee, J.; Choi, B.Y.; Wang, P.F.; Gao, P.; Yamano, T.; Fukuzawa, H.; Kang, B.H.; Lee, Y. CrABCA2 facilitates triacylglycerol accumulation in Chlamydomonas reinhardtii under nitrogen starvation. Mol. Cells 2020, 43, 48–57. [Google Scholar] [PubMed]
- Chen, R.; Yang, M.; Li, M.J.; Zhang, H.; Lu, H.; Dou, X.T.; Feng, S.Q.; Xue, S.; Zhu, C.B.; Chi, Z.Y.; et al. Enhanced accumulation of oil through co-expression of fatty acid and ABC transporters in Chlamydomonas under standard growth conditions. Biotechnol. Biofuels Bioprod. 2022, 15, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yamaoka, Y.; Feng, Y.B.; Chi, Z.Y.; Xue, S.; Kong, F.T. Co-expression of lipid transporters simultaneously enhances oil and starch accumulation in the green microalga Chlamydomonas reinhardtii under nitrogen starvation. Metabolites 2023, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Xie, X.F.; Wu, M.; Lin, Z.J.; Yin, J.B.; Lou, S.L.; Huang, Y.; Hu, Z.L. Understanding the functions of endogenous DOF transcript factor in Chlamydomonas reinhardtii. Biotechnol. Biofuels 2019, 12, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Yin, J.B.; Li, X.L.; Li, Y.L.; Yang, X.C.; Lan, C.X.; Huang, Y. Increased lipids in Chlamydomonas reinhardtii by multiple regulations of DOF, LACS2, and CIS1. Int. J. Mol. Sci. 2022, 23, 10176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Ge, Y.L.; Liu, K.Q.; Yamaoka, Y.; Zhang, D.; Chi, Z.Y.; Akkaya, M.; Kong, F.T. Overexpression of a MYB1 transcription factor enhances triacylglycerol and starch accumulation and biomass production in the green microalga Chlamydomonas reinhardtii. J. Agric. Food Chem. 2023, 71, 17833–17841. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Shim, D.; Kong, F.; Auroy, P.; Lee, Y.; Yamaoka, Y. The Chlamydomonas transcription factor MYB1 mediates lipid accumulation under nitrogen depletion. New Phytol. 2022, 235, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, J.H.; Zhang, F.; Miao, X.L. Non-tandem CCCH-type Zinc-finger protein CpZF_CCCH1 improves fatty acid desaturation and stress tolerance in Chlamydomonas reinhardtii. J. Agric. Food Chem. 2023, 45, 17188–17201. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.L.; Zhu, H.; Wang, C.G.; Li, Y.J.; Zou, X.H.; Hu, Z.L. A u-box type e3 ubiquitin ligase prp19-like protein negatively regulates lipid accumulation and cell size in Chlamydomonas reinhardtii. Front. Microbiol. 2022, 13, 860024–860036. [Google Scholar] [CrossRef]
- Ma, H.Y.; Wu, X.Y.; Wei, Z.W.; Zhao, L.; Li, Z.Z.; Liang, Q.; Zheng, J.; Wang, Y.; Li, Y.H.; Huang, L.F.; et al. Functional divergence of diacylglycerol acyltransferases in the unicellular green alga Haematococcus pluvialis. J. Exp. Bot. 2021, 72, 510–524. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.Y.; Xing, C.; Yuan, H.L.; Yang, J.S. Characterization of Auxenochlorella protothecoides acyltransferases and potential of their protein interactions to promote the enrichment of oleic acid. Biotechnol. Biofuels Bioprod. 2023, 16, 69–84. [Google Scholar] [CrossRef]
- Poliner, E.; Farre, E.M.; Benning, C. Advanced genetic tools enable synthetic biology in the oleaginous microalgae Nannochloropsis sp. Plant Cell Rep. 2018, 37, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.G.; Kang, N.K.; Jeon, S.; Shin, S.E.; Jeong, B.R.; Chang, Y.K. Heterologous synthesis of chlorophyll b in Nannochloropsis salina enhances growth and lipid production by increasing photosynthetic efficiency. Biotechnol. Biofuels 2019, 12, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Song, X.J.; Li, F.L.; Lu, Y.D. Improving lipid productivity by engineering a control-knob gene in the oleaginous microalga Nannochloropsis oceanica. Metab. Eng. Commun. 2020, 11, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.T.; Feng, Y.B.; Lu, Y.D.; Xin, Y.; Shen, C.; Wei, L.; Liu, Y.X.; Lv, N.N.; Du, X.F.; Zhu, W.Q.; et al. Manipulating fatty-acid profile at unit chain-length resolution in the model industrial oleaginous microalgae Nannochloropsis. Metab. Eng. 2021, 66, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ryu, A.J.; Jeong, B.R.; Kang, N.K.; Jeon, S.; Sohn, M.G.; Yun, H.J.; Lim, J.M.; Jeong, S.W.; Park, Y.I.; Jeong, W.J.; et al. Safe-harboring based novel genetic toolkit for Nannochloropsis salina CCMP1776: Efficient overexpression of transgene via CRISPR/Cas9-mediated knock-in at the transcriptional hotspot. Bioresour. Technol. 2021, 340, 125676–125685. [Google Scholar] [CrossRef] [PubMed]
- Sudfeld, C.; Kiyani, A.; Wefelmeie, K.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Expression of glycerol-3-phosphate acyltransferase increases non-polar lipid accumulation in Nannochloropsis oceanica. Microb. Cell Factories 2023, 22, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Fattore, N.; Bucci, F.; Bellan, A.; Bossi, S.; Maffei, M.E.; Morosinotto, T. An increase in the membrane lipids recycling by PDAT overexpression stimulates the accumulation of triacylglycerol in Nannochloropsis gaditana. J. Biotechnol. 2022, 357, 28–37. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Pan, Y.F.; Marechal, E.; Amato, A.; Liu, M.J.; Gong, Y.M.; Li, Y.T.; Hu, H.H. PDAT regulates PE as transient carbon sink alternative to triacylglycerol in Nannochloropsis. Plant Physiol. 2022, 189, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Balamurugan, S.; Yang, Y.F.; Zheng, J.W.; Huang, D.; Zou, L.G.; Yang, W.D.; Liu, J.S.; Guan, Y.F.; Li, H.Y. Transcriptional regulation of microalgae for concurrent lipid overproduction and secretion. Sci. Adv. 2019, 5, 3795–3804. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.K.; Kim, E.K.; Sung, M.G.; Kim, Y.U.; Jeong, B.R.; Chang, Y.K. Increased biomass and lipid production by continuous cultivation of Nannochloropsis salina transformant overexpressing a bHLH transcription factor. Biotechnol. Bioeng. 2019, 116, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xin, Y.; He, Y.H.; Tang, X.F.; Shen, C.; Wang, Q.T.; Lv, N.N.; Li, Y.; Hu, Q.; Xu, J. Exploring a blue-light-sensing transcription factor to double the peak productivity of oil in Nannochloropsis oceanica. Nat. Commun. 2022, 13, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A diatom cell factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Kim, J.; Lee, J.W.; Nam, O.; Chang, K.S.; Jin, E. Enhanced pyruvate metabolism in plastids by overexpression of putative plastidial pyruvate transporter in Phaeodactylum tricornutum. Biotechnol. Biofuels 2020, 13, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Gu, W.H.; Huang, A.Y.; Li, Y.X.; Kumar, M.; Lim, P.E.; Huan, L.; Gao, S.; Wang, G.C. Elevated CO(2) improves both lipid accumulation and growth rate in the glucose-6-phosphate dehydrogenase engineered Phaeodactylum tricornutum. Microb. Cell Factories 2019, 18, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Jouhet, J.; Gandini, C.; Nekrasov, V.; Marechal, E.; Napier, J.A.; Sayanova, O. Plastidial acyl carrier protein delta9-desaturase modulates eicosapentaenoic acid biosynthesis and triacylglycerol accumulation in Phaeodactylum tricornutum. Plant J. Cell Mol. Biol. 2021, 106, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.Y.; Fan, Y.; Phung, N.; Xia, W.Y.; Hu, G.; Li, F.L. Overexpression of plastid lipid-associated protein in marine diatom enhances the xanthophyll synthesis and storage. Front. Microbiol. 2023, 14, 1143017–1143029. [Google Scholar] [CrossRef] [PubMed]
- Haslam, R.P.; Hamilton, M.L.; Economou, C.K.; Smith, R.; Hassall, K.L.; Napier, J.A.; Sayanova, O. Overexpression of an endogenous type 2 diacylglycerol acyltransferase in the marine diatom Phaeodactylum tricornutum enhances lipid production and omega-3 long-chain polyunsaturated fatty acid content. Biotechnol. Biofuels 2020, 13, 87–103. [Google Scholar] [CrossRef]
- Zou, L.G.; Balamurugan, S.; Zhou, T.B.; Chen, J.W.; Li, D.W.; Yang, W.D.; Liu, J.S.; Li, H.Y. Potentiation of concurrent expression of lipogenic genes by novel strong promoters in the oleaginous microalga Phaeodactylum tricornutum. Biotechnol. Bioeng. 2019, 116, 3006–3015. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, J.; Jiang, Y.; Chen, F. Chlorella species as hosts for genetic engineering and expression of heterologous proteins: Progress, challenge and perspective. Biotechnol. J. 2016, 11, 1244–1261. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, W.S.; Kim, Y.U.; Jeon, S.; Kim, M.; Kang, N.K.; Chang, Y.K. Enhancement of lipid production under heterotrophic conditions by overexpression of an endogenous bZIP transcription factor in Chlorella sp. HS2. J. Microbiol. Biotechnol. 2020, 30, 1597–1606. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Zhang, J.H.; Chen, Y.H.; Liu, X.L.; Fan, C.M.; Wang, R.R.; Hou, Y.Y.; Hu, Z.M. Overexpression of the transcription factor AtLEC1 significantly improved the lipid content of Chlorella ellipsoidea. Front. Bioeng. Biotechnol. 2021, 9, 626162–626176. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S. Exploring the role of a cytokinin-activating enzyme LONELY GUY in unicellular microalga Chlorella variabilis. Front. Plant Sci. 2020, 11, 611871–611886. [Google Scholar] [CrossRef] [PubMed]
- Degraeve-Guilbault, C.; Pankasem, N.; Gueirrero, M.; Lemoigne, C.; Domergue, F.; Kotajima, T.; Suzuki, I.; Joubes, J.; Corellou, F. Temperature acclimation of the picoalga Ostreococcus tauri triggers early fatty-acid variations and involves a plastidial omega3-desaturase. Front. Plant Sci. 2021, 12, 639330–639346. [Google Scholar] [CrossRef] [PubMed]
- Degraeve-Guilbault, C.; Gomez, R.E.; Lemoigne, C.; Pankansem, N.; Morin, S.; Tuphile, K.; Joubes, J.; Jouhet, J.; Gronnier, J.; Suzuki, I.; et al. Plastidic delta6 fatty-acid desaturases with distinctive substrate specificity regulate the pool of C18-PUFAs in the ancestral picoalga Ostreococcus tauri. Plant Physiol. 2020, 184, 82–96. [Google Scholar] [CrossRef]
- Chungjatupornchai, W.; Areerat, K.; Fa-Aroonsawat, S. Increased triacylglycerol production in oleaginous microalga Neochloris oleoabundans by overexpression of plastidial lysophosphatidic acid acyltransferase. Microb. Cell Factories 2019, 18, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Chungjatupornchai, W.; Fa-Aroonsawat, S. Enhanced triacylglycerol production in oleaginous microalga Neochloris oleoabundans by co-overexpression of lipogenic genes: Plastidial LPAAT1 and ER-located DGAT2. J. Biosci. Bioeng. 2021, 131, 124–130. [Google Scholar] [CrossRef]
- Munoz, C.F.; Weusthuis, R.A.; D’Adamo, S.; Wijffels, R.H. Effect of single and combined expression of lysophosphatidic acid acyltransferase, glycerol-3-phosphate acyltransferase, and diacylglycerol acyltransferase on lipid accumulation and composition in Neochloris oleoabundans. Front. Plant Sci. 2019, 10, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Okubo, R.; Kanesaki, Y.; Zhou, B.; Takaya, K.; Watanabe, S.; Tanaka, K.; Imamura, S. Identification of transcription factors and the regulatory genes involved in triacylglycerol accumulation in the unicellular red alga Cyanidioschyzon merolae. Plants 2021, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.W.; Xu, Y.S.; Sun, X.M.; Shi, T.Q.; Gu, Y.; Ye, C.; Huang, H. Development of an efficient gene editing tool in Schizochytrium sp. and improving its lipid and terpenoid biosynthesis. Front. Nutr. 2021, 8, 795651–795660. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Bi, Y.L.; Diao, J.J.; Lv, M.M.; Cui, J.Y.; Chen, L.; Zhang, W.W. Metabolic engineering to enhance biosynthesis of both docosahexaenoic acid and odd-chain fatty acids in Schizochytrium sp. S31. Biotechnol. Biofuels 2019, 12, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.L.; He, Y.J.; Chen, H.H.; Jiang, J.G. Dual roles of two malic enzymes in lipid biosynthesis and salt stress response in Dunaliella salina. J. Agric. Food Chem. 2023, 71, 17067–17079. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ren, H.Y.; Xing, D.F.; Xie, G.J.; Ren, N.Q.; Liu, B.F. Mechanistic understanding towards the effective lipid production of a microalgal mutant strain Scenedesmus sp. Z-4 by the whole genome bioinformation. J. Hazard. Mater. 2019, 375, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Fathy, W.; Essawy, E.; Tawfik, E.; Khedr, M.; Abdelhameed, M.S.; Hammouda, O.; Elsayed, K. Recombinant overexpression of the Escherichia coli acetyl-CoA carboxylase gene in Synechocystis sp. boosts lipid production. J. Basic Microbiol. 2021, 61, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Kasi, I.K.; Islary, P.; Bhatti, S.K. Nutritional and health-promoting effects of lichens used in food applications. Curr. Nutr. Rep. 2023, 12, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kselikova, V.; Singh, A.; Bialevich, V.; Cizkova, M.; Bisova, K. Improving microalgae for biotechnology—From genetics to synthetic biology—Moving forward but not there yet. Biotechnol. Adv. 2022, 58, 107885. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, J.A.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in microalgae engineering and synthetic biology applications for biofuel production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Li, M.; Hu, C. Algal biomass valorisation to high-value chemicals and bioproducts: Recent advances, opportunities and challenges. Bioresour. Technol. 2022, 344, 126371. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Wu, S.; Miao, C.; Xu, T.; Lu, Y. Towards Lipid from Microalgae: Products, Biosynthesis, and Genetic Engineering. Life 2024, 14, 447. https://doi.org/10.3390/life14040447
Xin Y, Wu S, Miao C, Xu T, Lu Y. Towards Lipid from Microalgae: Products, Biosynthesis, and Genetic Engineering. Life. 2024; 14(4):447. https://doi.org/10.3390/life14040447
Chicago/Turabian StyleXin, Yi, Shan Wu, Congcong Miao, Tao Xu, and Yandu Lu. 2024. "Towards Lipid from Microalgae: Products, Biosynthesis, and Genetic Engineering" Life 14, no. 4: 447. https://doi.org/10.3390/life14040447
APA StyleXin, Y., Wu, S., Miao, C., Xu, T., & Lu, Y. (2024). Towards Lipid from Microalgae: Products, Biosynthesis, and Genetic Engineering. Life, 14(4), 447. https://doi.org/10.3390/life14040447







