Could Life Have Started on Mars? Planetary Conditions That Assemble and Destroy Protocells
Abstract
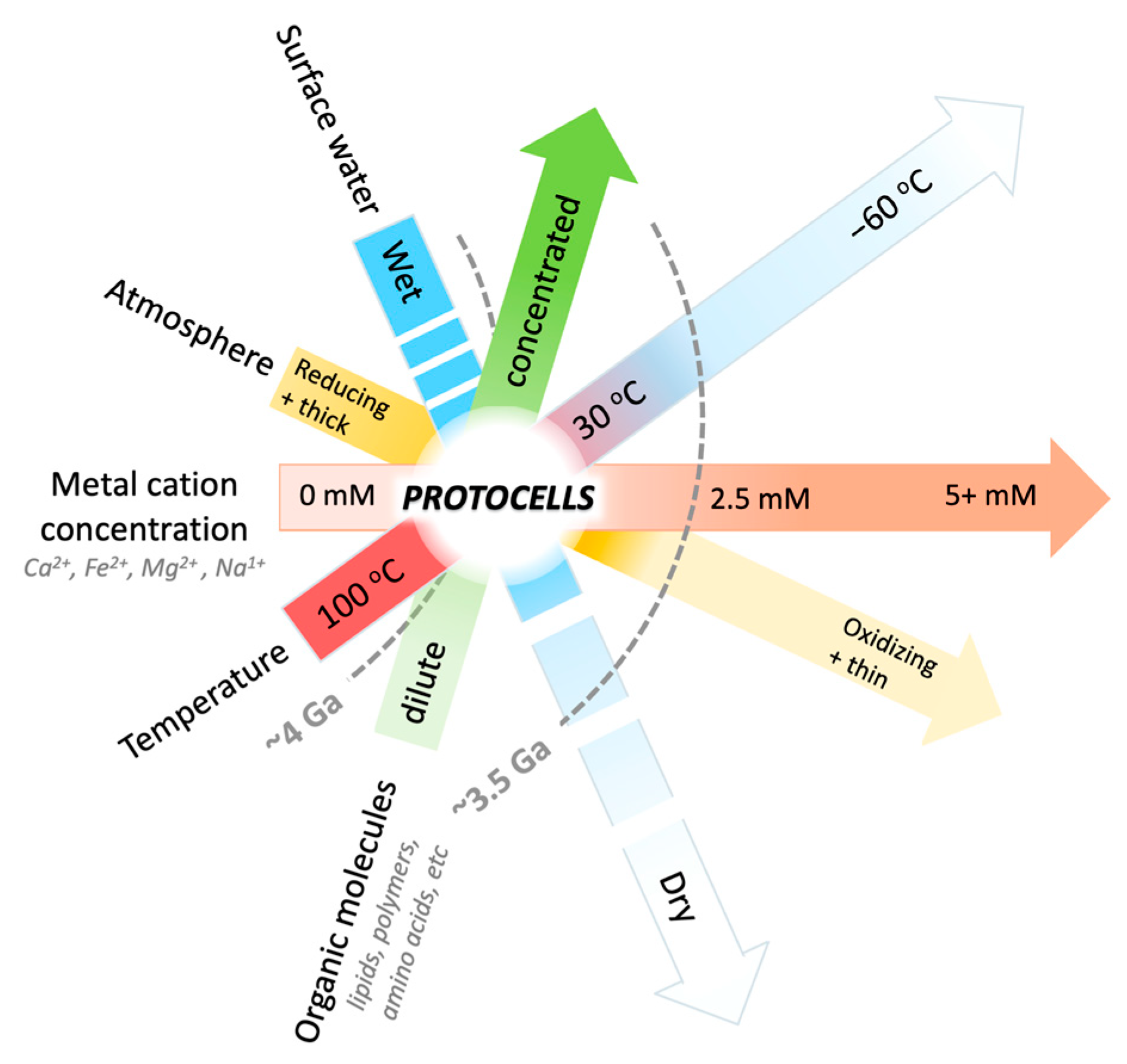
1. Introduction
2. Materials and Methods
2.1. Anaerobic Conditions
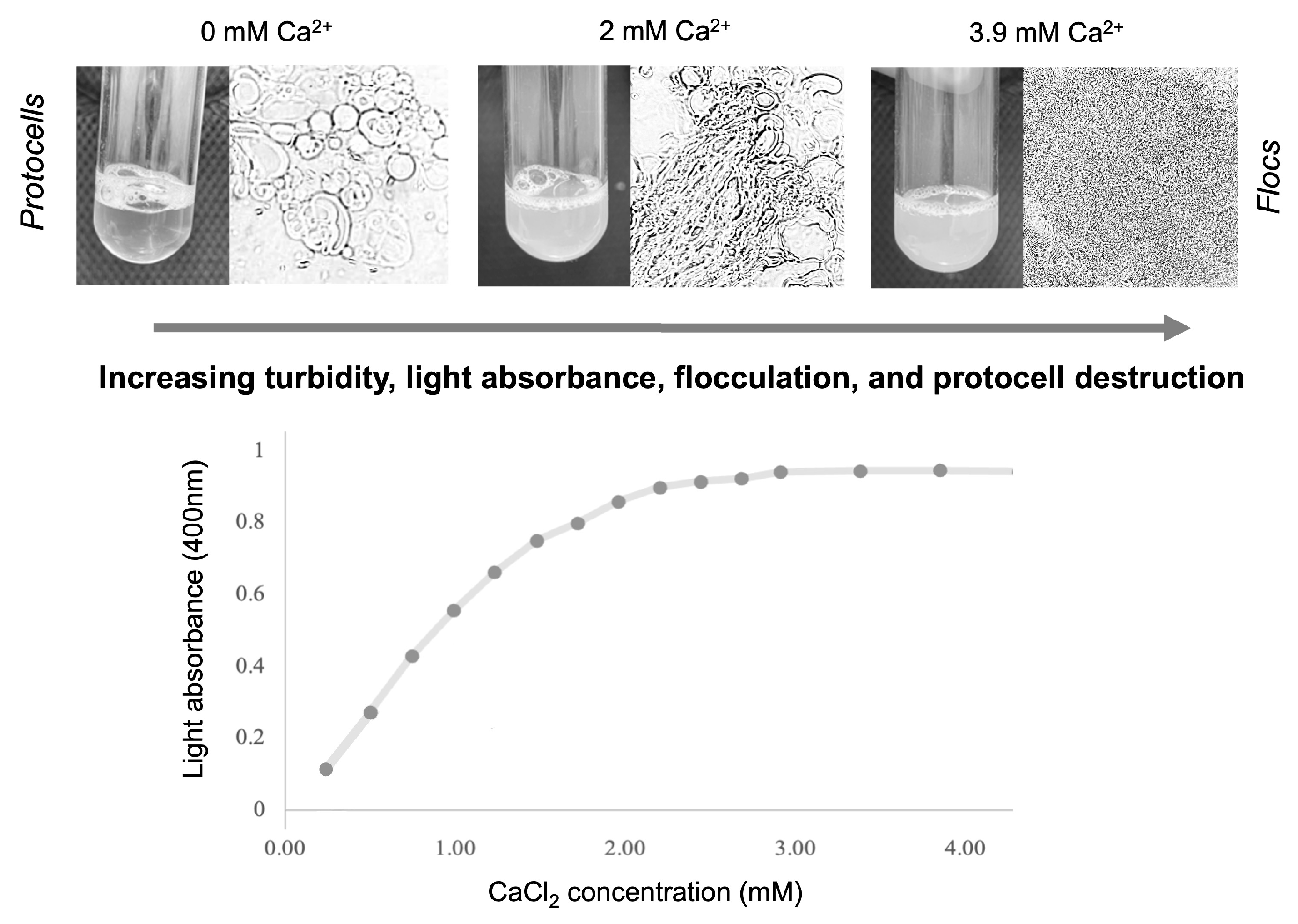
2.2. Flocculation of Lipid Vesicles in the Presence of Cations
2.3. Flocculation of Lipid Vesicles with Encapsulated RNA in the Presence of Cations
2.4. Buffer Choice
3. Results and Interpretations
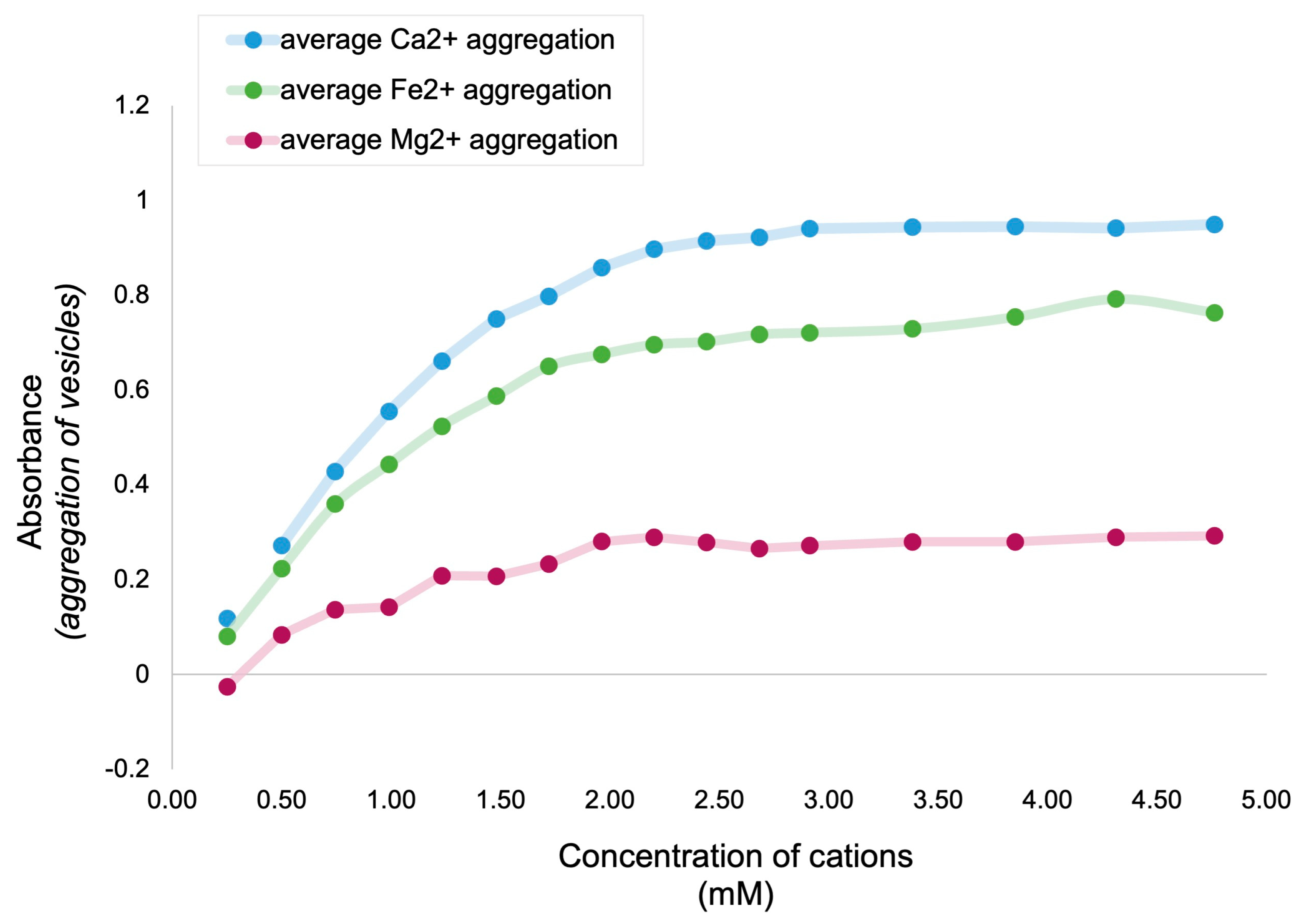
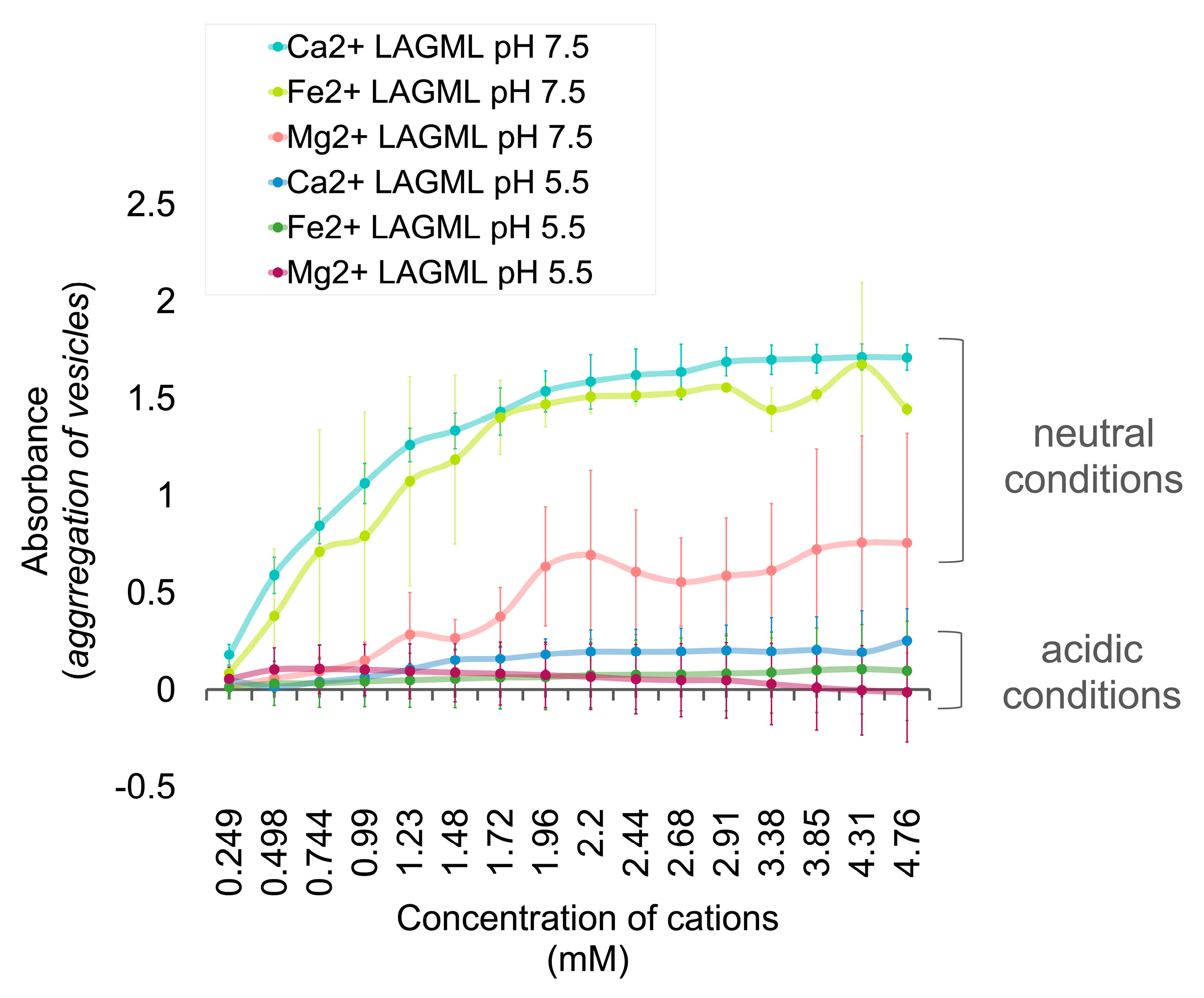
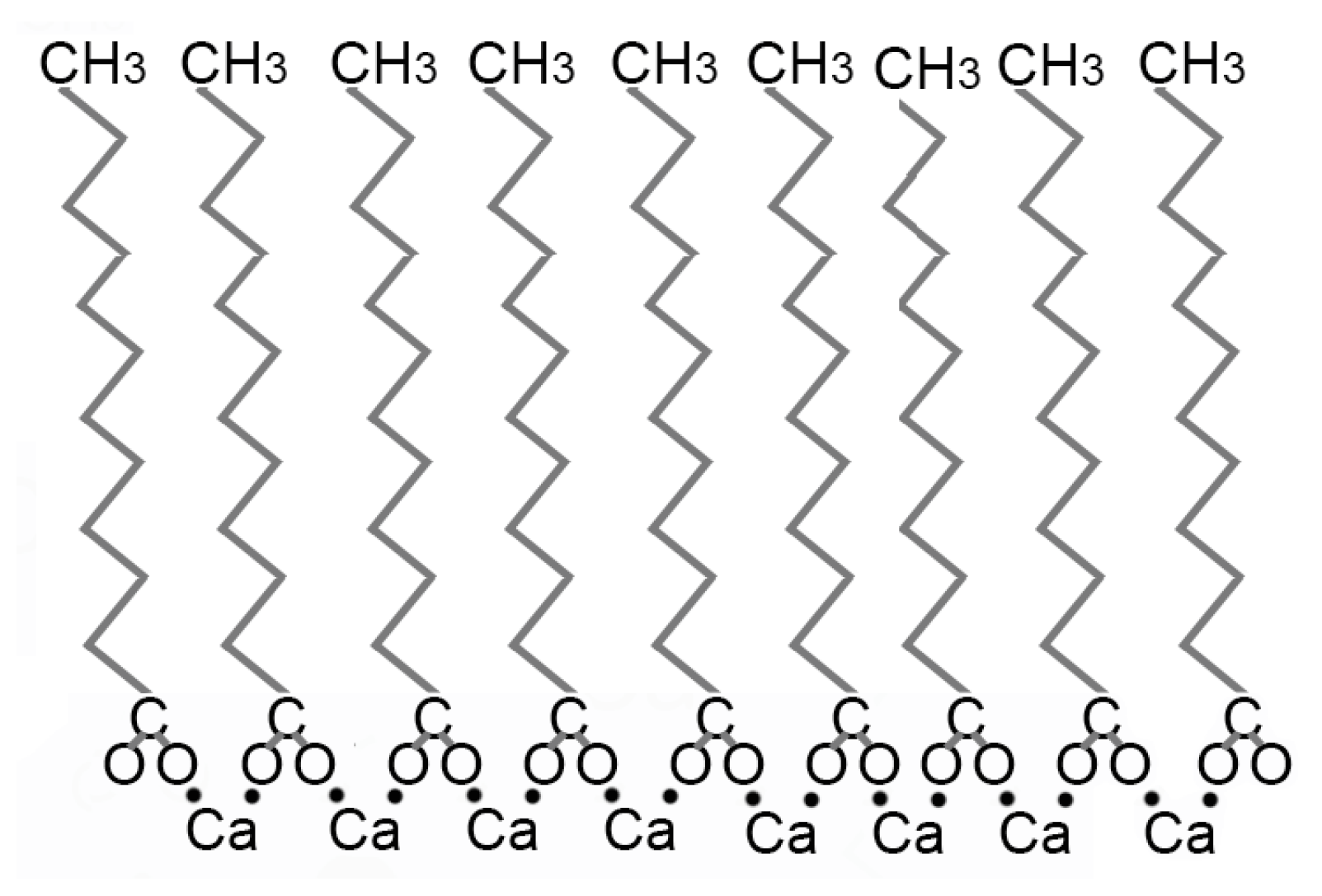
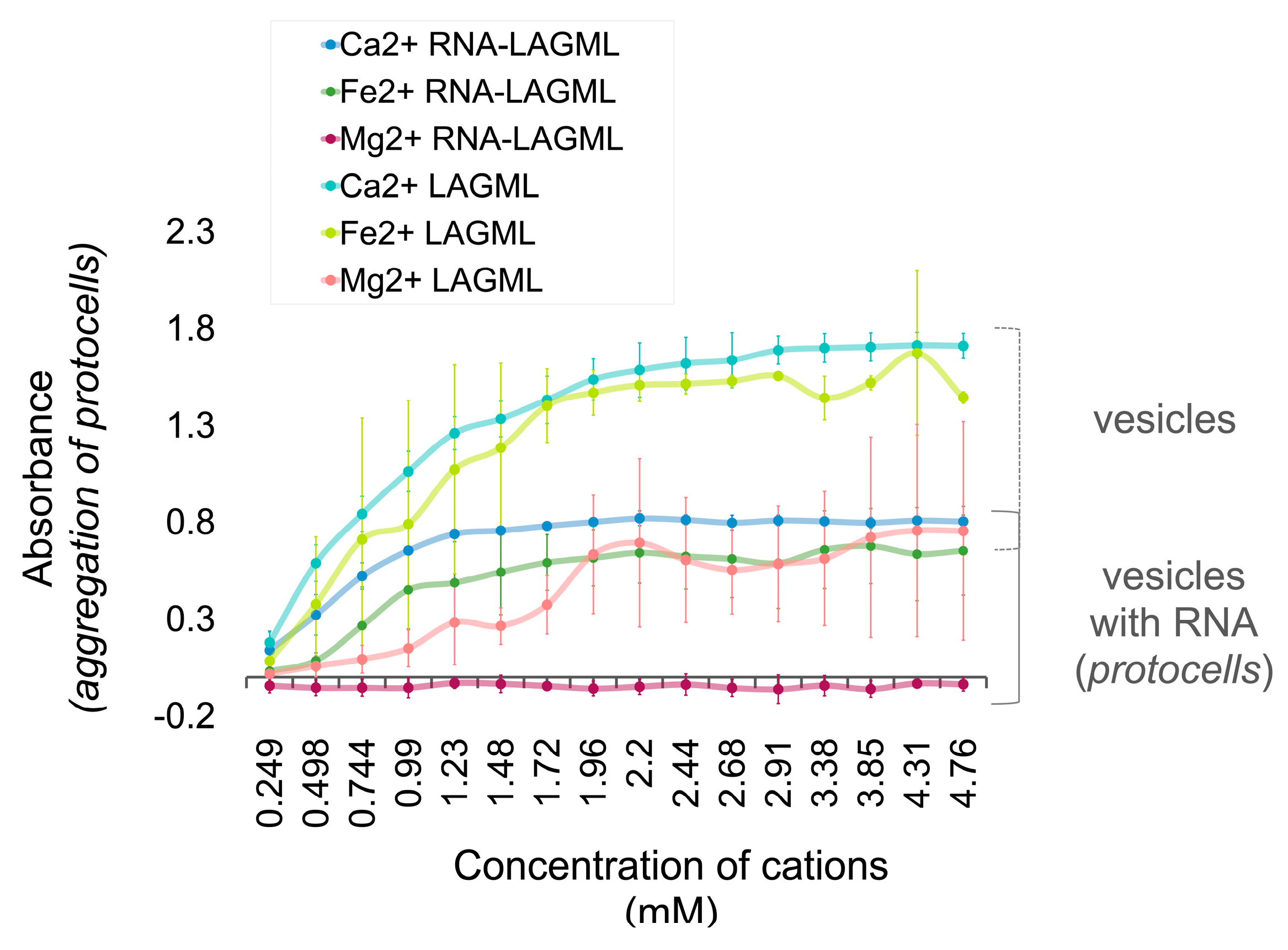
3.1. Relative Impact of Ca, Fe, and Mg on Vesicle Stability
Interpretation
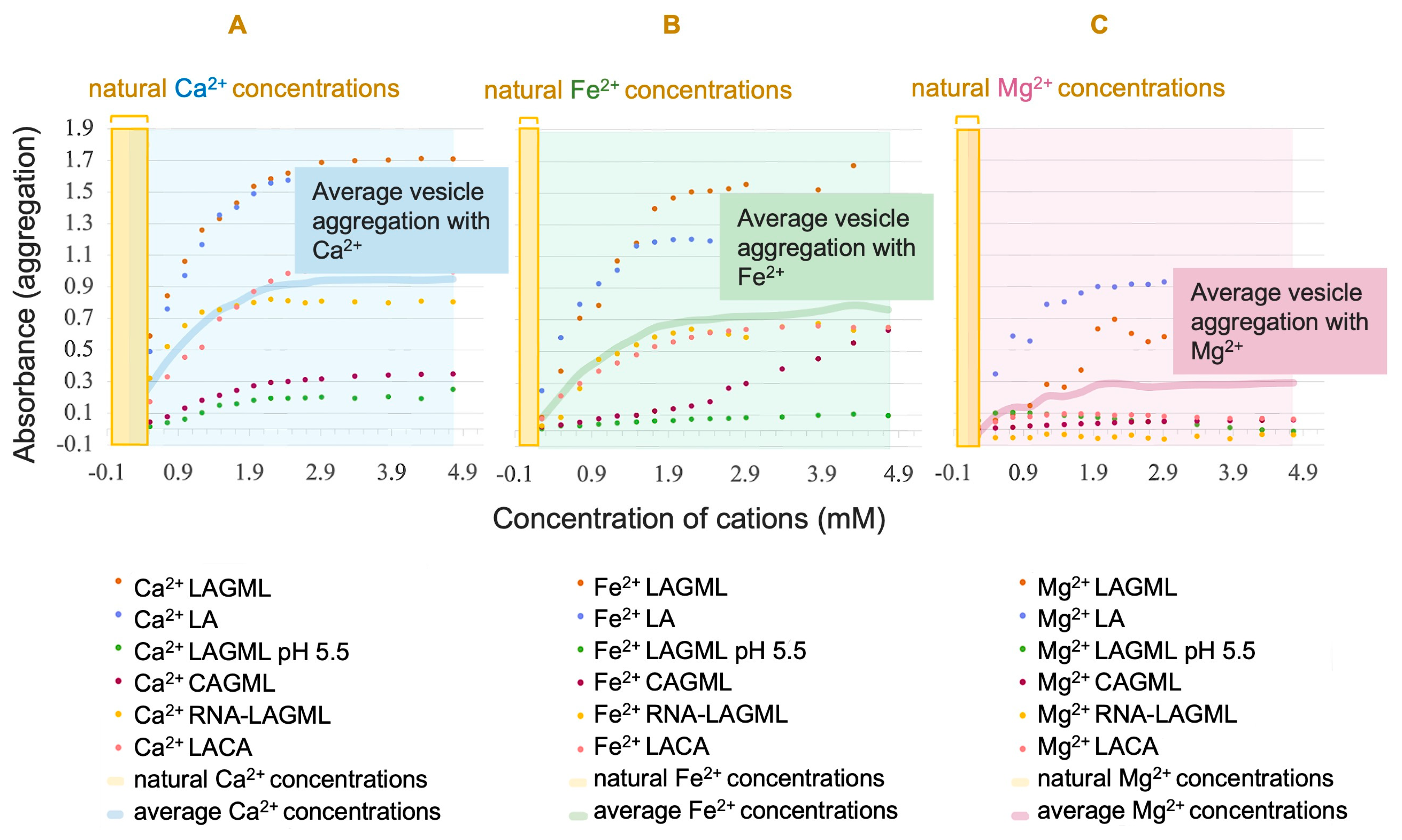
3.2. Concentration of Cations in Natural Settings on Earth
Interpretation
3.3. Effect of Low pH on Vesicle Stability
Interpretation
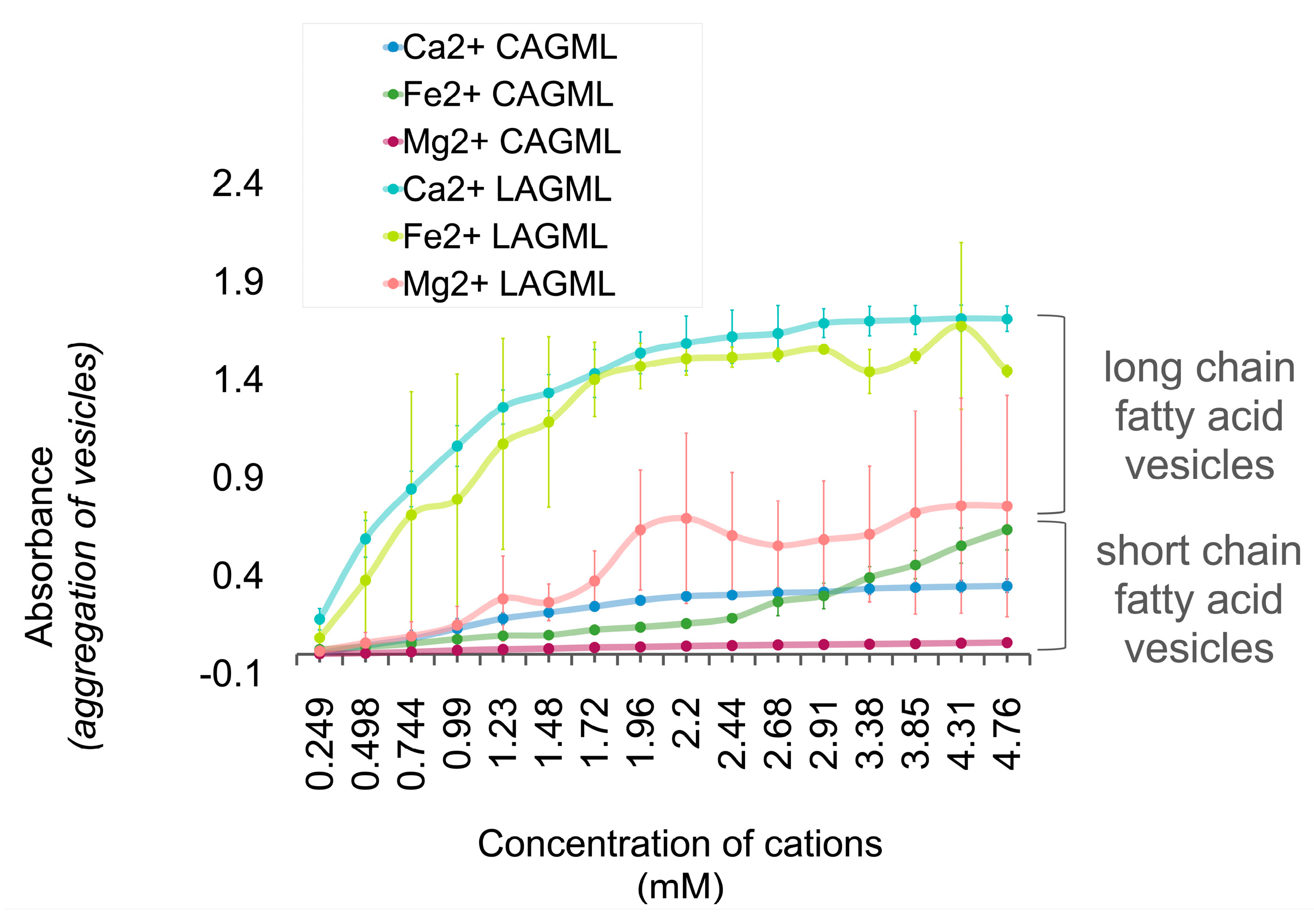
3.4. Effect of Fatty Acid Chain Length on Vesicle Stability
Interpretation
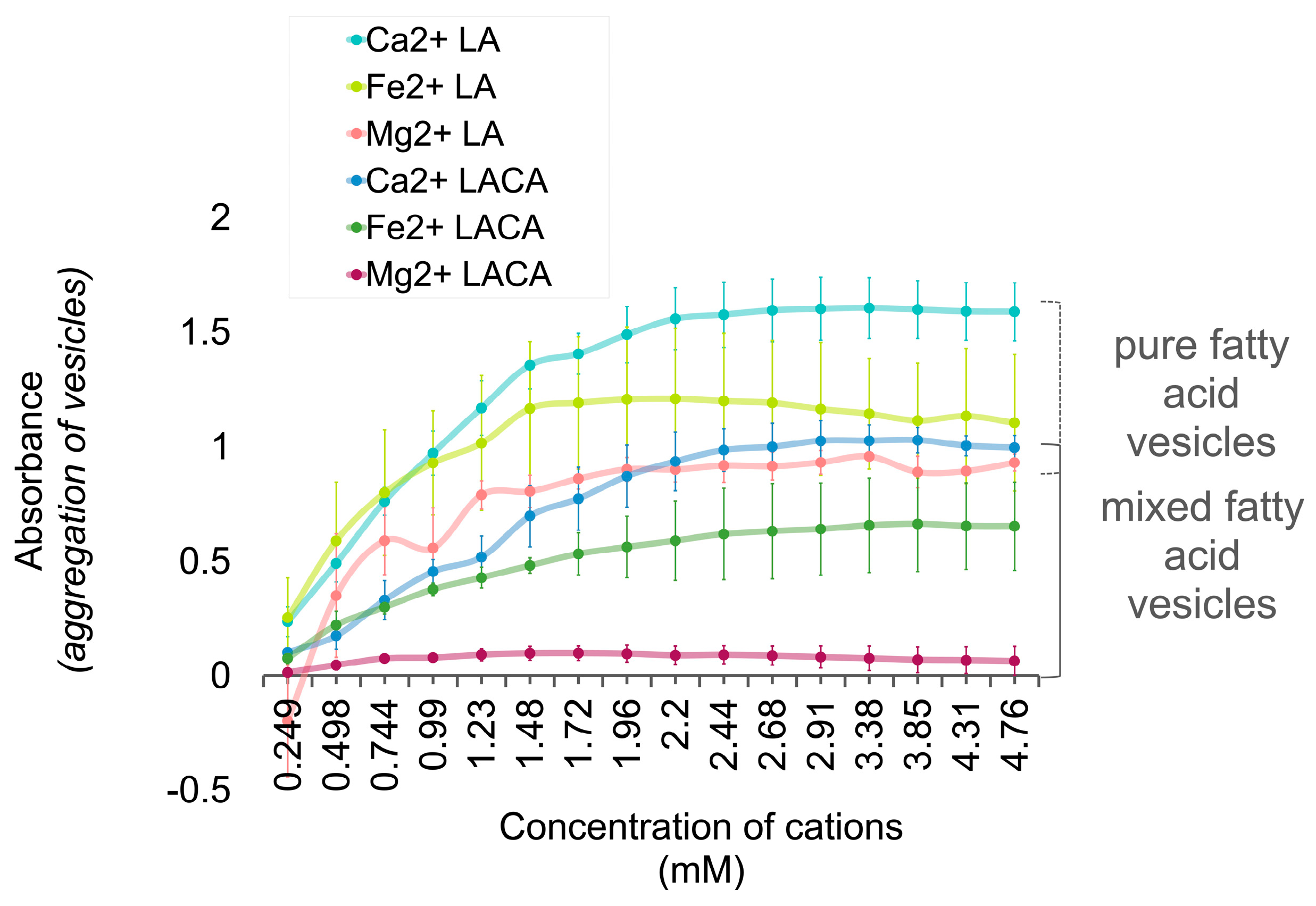
3.5. Fatty Acids Combined in a ‘Mosaic’ Vesicle
Interpretation
3.6. Polymer and Lipid Co-Localization
3.6.1. Interpretation
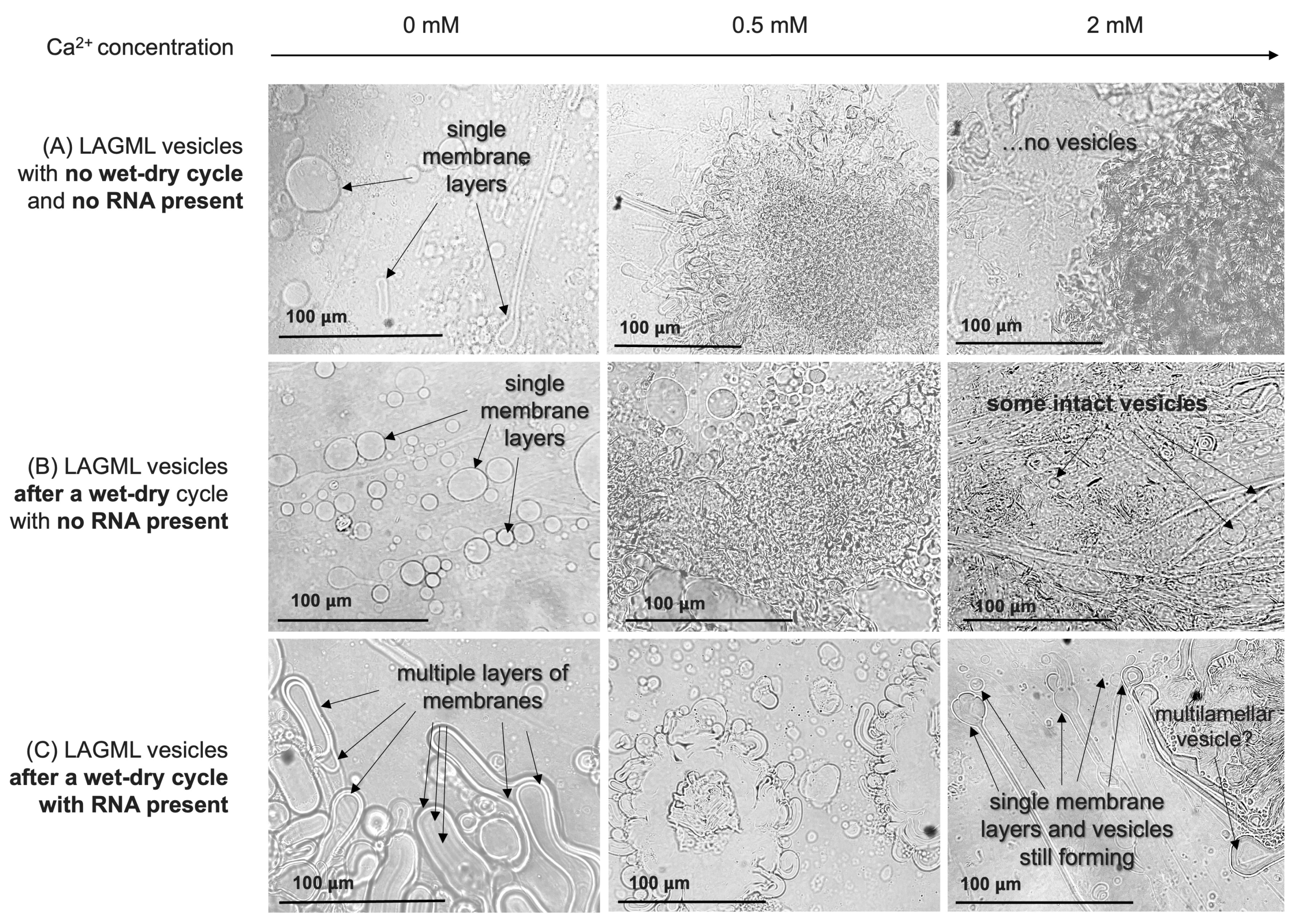
RNA–Lipid Interactions during Wet–Dry Cycling
Morphological Changes/Multilamellar Structures
Effect of Morphological Differences on Light Absorbance
3.7. Summary of Experimental Findings
4. Discussion
4.1. Iron-Rich Geobiochemistry on Mars? Implications of the Relative Impact of Ca2+ Fe2+ and Mg2+ on Vesicle Stability
4.2. The Dehydration of Mars: Implications from the Concentration of Cations in Natural Settings on Earth and Mars
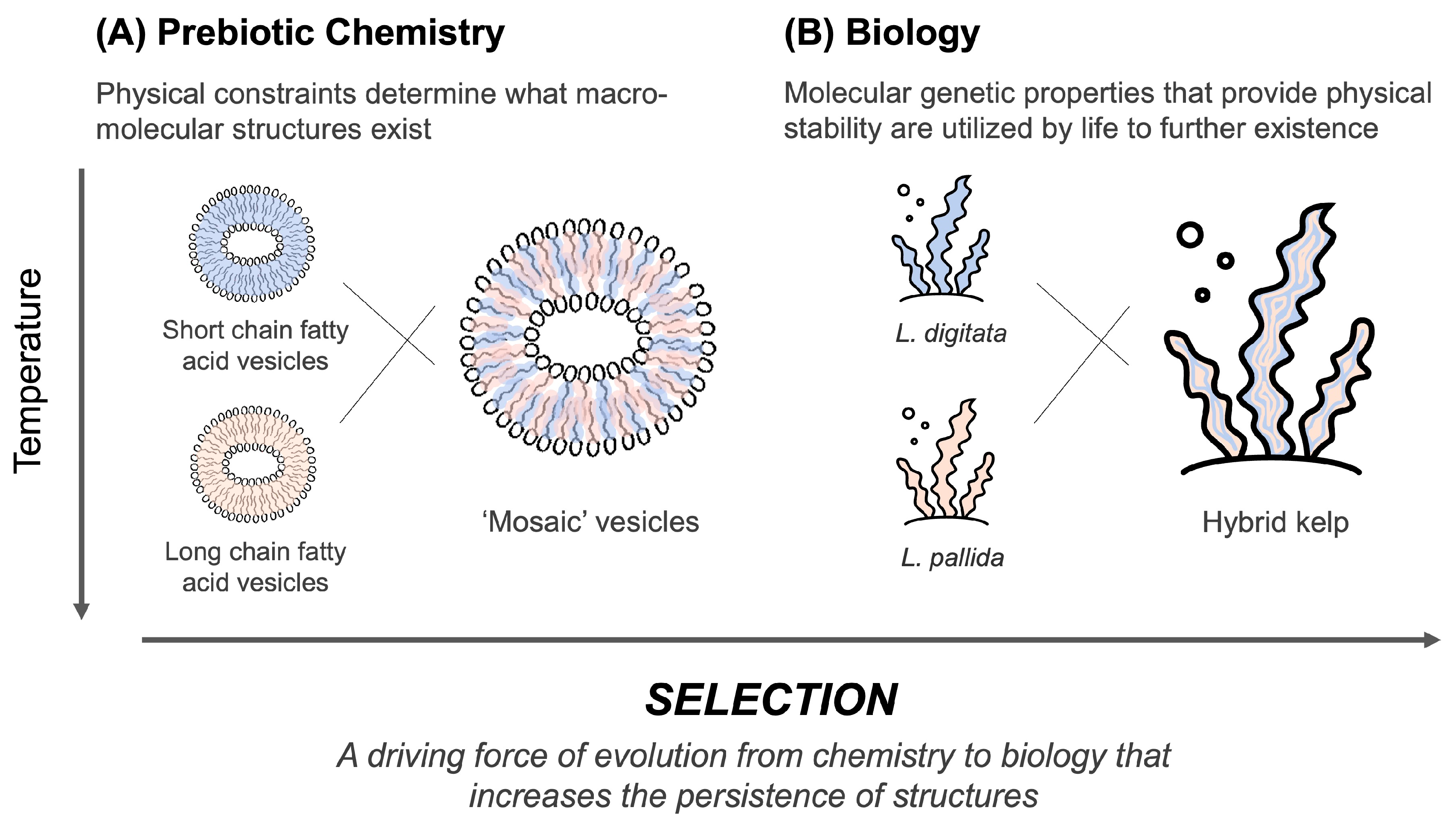
4.3. Selection of Cell Membranes on Earth-like Planets Such as Mars
4.3.1. The Origin of Chemical and Biophysical Selection Processes
4.3.2. pH
4.3.3. Membrane Composition
4.4. Shift from Passive Selection to Functional Selection: Implications of RNA–Lipid Stabalising Interactions
4.5. Searching for Life on Mars and Other Earth-like Planets: An Origin-of-Life-Informed Space-Exploration Strategy
4.6. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deamer, D.; Cary, F.; Damer, B. Urability: A property of planetary bodies that can support an origin of life. Astrobiology 2022, 22, 889–900. [Google Scholar] [CrossRef]
- Clark, B.C.; Kolb, V.M.; Steele, A.; House, C.H.; Lanza, N.L.; Gasda, P.J.; VanBommel, S.J.; Newsom, H.E.; Martínez-Frías, J. Origin of life on Mars: Suitability and opportunities. Life 2021, 11, 539. [Google Scholar] [CrossRef]
- Pelegrín, J.; Guerrero, A. Life estimates in the universe: Outstanding the importance of astrochemical and astrobiological parameters. III Lat. Am. Congr. Astrobiol. (3CLA) 2023, 55, 59–61. [Google Scholar] [CrossRef]
- Cockell, C.S. Habitable worlds with no signs of life. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130082. [Google Scholar] [CrossRef] [PubMed]
- Scharf, C.; Cronin, L. Quantifying the origins of life on a planetary scale. Proc. Natl. Acad. Sci. USA 2016, 113, 8127–8132. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Bartlett, S.; Chen, S.; Tierney, L. Searching for life, mindful of lyfe’s possibilities. Life 2022, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. The hot spring hypothesis for an origin of life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Russell, M.J. On the origin of biochemistry at an alkaline hydrothermal vent. Philos. Trans. R. Soc. B Biol. Sci. 2006, 362, 1887–1926. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Pudritz, R.E.; Semenov, D.A.; Henning, T.K. Origin of the RNA world: The fate of nucleobases in warm little ponds. Proc. Natl. Acad. Sci. USA 2017, 114, 11327–11332. [Google Scholar] [CrossRef]
- Bywater, R.P.; Conde-Frieboes, K. Did life begin on the beach? Astrobiology 2005, 5, 568–574. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hartman, H. Clays and the origin of life: The experiments. Life 2022, 12, 259. [Google Scholar] [CrossRef]
- Kauffman, S.A. Origins of Order in Evolution: Self-Organization and Selection. In Understanding Origins. Boston Studies in the Philosophy and History of Science; Varela, F.J., Dupuy, J.P., Eds.; Springer: Dordrecht, The Netherlands, 1992; Volume 130. [Google Scholar] [CrossRef]
- Deamer, D.W. Chapter 515: Self-Assembly Processes Were Essential for Life’s Origin. In Assembling Life: How Can Life Begin on Earth and Other Habitable Planets? Oxford Academic: Essay, NY, USA, 2019; pp. 51–69. [Google Scholar]
- Root-Bernstein, R.; Brown, A.W. Novel apparatuses for incorporating natural selection processes into origins-of-life experiments to produce adaptively evolving chemical ecosystems. Life 2022, 12, 1508. [Google Scholar] [CrossRef]
- Vincent, L.; Berg, M.; Krismer, M.; Saghafi, S.T.; Cosby, J.; Sankari, T.; Vetsigian, K.; Cleaves, H.J.; Baum, D.A. Chemical ecosystem selection on mineral surfaces reveals long-term dynamics consistent with the spontaneous emergence of mutual catalysis. Life 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. Coupled phases and combinatorial selection in fluctuating hydrothermal pools: A scenario to guide experimental approaches to the origin of Cellular Life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, T.A.; Joyce, G.F. Self-sustained replication of an RNA enzyme. Science 2009, 323, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Duim, H.; Otto, S. Towards open-ended evolution in self-replicating molecular systems. Beilstein J. Org. Chem. 2017, 13, 1189–1203. [Google Scholar] [CrossRef]
- Otto, S. An approach to the de novo synthesis of life. Acc. Chem. Res. 2021, 55, 145–155. [Google Scholar] [CrossRef]
- Ameta, S.; Matsubara, Y.J.; Chakraborty, N.; Krishna, S.; Thutupalli, S. Self-Reproduction and Darwinian Evolution in Autocatalytic Chemical Reaction Systems. Life 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, J.; Geerts, M.; Markovitch, O.; Pappas, C.G.; Liu, K.; Otto, S. Out-of-Equilibrium self-replication allows selection for dynamic kinetic stability in a system of competing Replicators. Angew. Chem. Int. Ed. 2022, 61, e202117605. [Google Scholar] [CrossRef]
- Pascal, R.; Pross, A.; Sutherland, J.D. Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics. Open Biol. 2013, 3, 130156. [Google Scholar] [CrossRef]
- Walker, S.I.; Davies, P.C. The algorithmic origins of life. J. R. Soc. Interface 2013, 10, 20120869. [Google Scholar] [CrossRef]
- Adami, C. Information-theoretic considerations concerning the origin of life. Orig. Life Evol. Biosph. 2015, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Dupré, J. The metaphysics of evolution. Interface Focus 2017, 7, 20160148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Czégel, D.; Lachmann, M.; Kempes, C.P.; Walker, S.I.; Cronin, L. Assembly theory explains and quantifies selection and evolution. Nature 2023, 622, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Milshteyn, D.; Damer, B.; Havig, J.; Deamer, D. Amphiphilic compounds assemble into membranous vesicles in hydrothermal hot spring water but not in seawater. Life 2018, 8, 11. [Google Scholar] [CrossRef]
- Michalski, J.; Onstott, T.; Mojzsis, S.; Mustard, J.; Chan, Q.; Niles, P.; Johnson, S. The Martian subsurface as a potential window into the origin of life. Nat. Geosci. 2017, 11, 21–26. [Google Scholar] [CrossRef]
- Longo, A.; Damer, B. Factoring origin of life hypotheses into the search for life in the solar system and beyond. Life 2020, 10, 52. [Google Scholar] [CrossRef]
- Sasselov, D.; Grotzinger, J.; Sutherland, J. The origin of life as a planetary phenomenon. Sci. Adv. 2020, 6, eaax3419. [Google Scholar] [CrossRef]
- Fornari, D.; Embley, R. Tectonic and volcanic controls on hydrothermal processes at the mid-ocean ridge: An overview based on near-bottom and submersible studies. Seafloor hydrothermal systems: Physical, chemical, biological, and geological interactions. Geophys. Monogr. Ser. 2013, 91, 1–46. [Google Scholar] [CrossRef]
- Langmuir, C.; Humphris, S.; Fornari, D.; Van Dover, C.; Von Damm, K.; Tivey, M.; Colodner, D.; Charlou, J.-L.; Desonie, D.; Wilson, C.; et al. Hydrothermal vents near a mantle hot spot: The Lucky Strike vent field at 37°N on the Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 1997, 148, 69–91. [Google Scholar] [CrossRef]
- Furukawa, H.; Walker, S. Major Transitions in Planetary Evolution. In Proceedings of the 2018 Conference on Artificial Life, Tokyo, Japan, 22–28 July 2018. [Google Scholar] [CrossRef]
- Frank, A.; Grinspoon, D.; Walker, S. Intelligence as a planetary scale process. Int. J. Astrobiol. 2022, 21, 47–61. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Tupper, A.S.; Pudritz, R.E.; Higgs, P.G. Constraining the Time Interval for the Origin of Life on Earth. Astrobiology 2018, 18, 343–364. [Google Scholar] [CrossRef]
- McMahon, S.; Bosak, T.; Grotzinger, J.P.; Milliken, R.E.; Summons, R.E.; Daye, M.; Newman, S.A.; Fraeman, A.; Williford, K.H.; Briggs, D.E.G. A field guide to finding fossils on Mars. J. Geophys. Res. Planets 2018, 123, 1012–1040. [Google Scholar] [CrossRef]
- Kite, E.S.; Mischna, M.A.; Fan, B.; Morgan, A.M.; Wilson, S.A.; Richardson, M.I. Changing spatial distribution of water flow charts major change in Mars’s greenhouse effect. Sci. Adv. 2022, 8, eabo5894. [Google Scholar] [CrossRef]
- Stevenson, D. Mars’ core and magnetism. Nature 2001, 412, 214–219. [Google Scholar] [CrossRef]
- Liu, J.; Michalski, J.; Tan, W.; He, H.; Ye, B.; Xiao, L. Anoxic chemical weathering under a reducing greenhouse on early Mars. Nat. Astron. 2021, 5, 503–509. [Google Scholar] [CrossRef]
- King, P.; Lescinsky, D.; Nesbitt, H. The composition and evolution of primordial solutions on Mars, with application to other planetary bodies. Geochim. Cosmochim. Acta 2004, 68, 4993–5008. [Google Scholar] [CrossRef]
- Halliday, A.; Wänke, H.; Birck, J.; Clayton, R. The accretion, composition, and early differentiation of Mars. Space Sci. Rev. 2001, 96, 197–230. [Google Scholar] [CrossRef]
- Zuber, M. The crust and mantle of Mars. Nature 2001, 412, 220–227. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S. Planetary Crusts: Their Composition, Origin, and Evolution; Cambridge University Press: Cambridge, UK, 2009; Volume 10. [Google Scholar]
- Earle, S. Physical Geology, 2nd ed.; Chapter 5, Weathering and Soil; BCcampus Open Education: Victoria, BC, Canada, 2020; pp. 156–159. [Google Scholar]
- Dehouck, E.; Gaudin, A.; Chevrier, V.; Mangold, N. Mineralogical record of the redox conditions on early Mars. Icarus 2016, 271, 67–75. [Google Scholar] [CrossRef]
- Deamer, D.; Dworkin, J. Chemistry and physics of primitive membranes. In Prebiotic Chemistry; Springer: Cham, Switzerland, 2005; pp. 1–27. [Google Scholar] [CrossRef]
- Hsiao, C.; Chou, I.-C.; Okafor, C.D.; Bowman, J.C.; O’Neill, E.B.; Athavale, S.S.; Petrov, A.S.; Hud, N.V.; Wartell, R.M.; Harvey, S.C.; et al. RNA with iron(II) as a cofactor catalyses electron transfer. Nat. Chem. 2013, 5, 525–528. [Google Scholar] [CrossRef]
- Okafor, C.; Lanier, K.; Petrov, A.; Athavale, S.; Bowman, J.; Hud, N.; Williams, L. Iron mediates catalysis of nucleic acid processing enzymes: Support for Fe(II) as a cofactor before the great oxidation event. Nucleic Acids Res. 2017, 45, 3634–3642. [Google Scholar] [CrossRef]
- Guth-Metzler, R.; Bray, M.; Frenkel-Pinter, M.; Suttapitugsakul, S.; Montllor-Albalate, C.; Bowman, J.; Wu, R.; Reddi, A.R.; Okafor, C.D.; Glass, J.B.; et al. Cutting in-line with iron: Ribosomal function and non-oxidative RNA cleavage. Nucleic Acids Res. 2020, 48, 8663–8674. [Google Scholar] [CrossRef]
- Gözen, I.; Köksal, E.; Põldsalu, I.; Xue, L.; Spustova, K.; Pedrueza-Villalmanzo, E.; Ryskulov, R.; Meng, F.; Jesorka, A. Protocells: Milestones and recent advances. Small 2022, 18, 2106624. [Google Scholar] [CrossRef]
- Black, R.; Blosser, M.; Stottrup, B.; Tavakley, R.; Deamer, D.; Keller, S. Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells. Proc. Natl. Acad. Sci. USA 2013, 110, 13272–13276. [Google Scholar] [CrossRef]
- Deamer, D.; Damer, B.; Kompanichenko, V. Hydrothermal chemistry and the origin of cellular life. Astrobiology 2019, 19, 1523–1537. [Google Scholar] [CrossRef]
- Cary, F.; Fagents, S.; Ruttenberg, K.; Jarrett, J.; Deamer, D.; Damer, B. Assembling Primitive Cells under Martian Geochemical Conditions: Implications for the Origin and Survivability of Life on Early Mars. Master’s Thesis, University of Hawai’i at Manoa, ProQuest Dissertations Publishing, Honolulu, HI, USA, 2022. Available online: https://www.proquest.com/docview/2776681794/ (accessed on 30 December 2023).
- Monnard, P.-A.; Apel, C.L.; Kanavarioti, A.; Deamer, D.W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2002, 2, 139–152. [Google Scholar] [CrossRef]
- Apel, C.L.; Deamer, D.W. The formation of glycerol monodecanoate by a dehydration condensation reaction: Increasing the chemical complexity of amphiphiles on the early Earth. Orig. Life Evol. Biosph. 2005, 35, 323–332. [Google Scholar] [CrossRef]
- Morris, R.; Ruff, S.; Gellert, R.; Ming, D.; Arvidson, R.; Clark, B.; Golden, D.C.; Siebach, K.; Klingelhöfer, G.; Schröder, C.; et al. Identification of carbonate-rich outcrops on Mars by the Spirit rover. Science 2010, 329, 421–424. [Google Scholar] [CrossRef]
- Audic, J.-L.; Fourcade, F.; Chaufer, B. Biodegradable material obtained from renewable resource: Plasticized sodium caseinate films. In Thermodynamics, Solubility and Environmental Issues; Elsevier: Amsterdam, The Netherlands, 2007; pp. 369–382. [Google Scholar] [CrossRef]
- Mohan, C. Buffers: A Guide for the Preparation and Use of Buffers in Biological Systems; Calibochem; EMD: San Diego, CA, USA, 2006. [Google Scholar]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. BioMetals 2002, 15, 203–210. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Jiménez Correa, M.M.; Aliprandini, P.; Soares Tenório, J.A.; Romano Espinosa, D.C. Precipitation of metals from liquor obtained in Nickel Mining. In REWAS 2016; Springer: Cham, Switzerland, 2016; pp. 333–338. [Google Scholar] [CrossRef]
- Kielland, J. Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar] [CrossRef]
- Amira, S.; Spångberg, D.; Probst, M.; Hermansson, K. Molecular dynamics simulation of Fe2+(aq) and Fe3+(aq). J. Phys. Chem. B 2003, 108, 496–502. [Google Scholar] [CrossRef]
- Rayner-Canham, G.; Overton, T. Descriptive Inorganic Chemistry, 5th ed.; Appendix 2, Charge Densities of Selected Ions; W.H. Freeman: New York, NY, USA, 2010. [Google Scholar]
- Guskov, A.; Nordin, N.; Reynaud, A.; Engman, H.; Lundbäck, A.-K.; Jong, A.J.O.; Cornvik, T.; Phua, T.; Eshaghi, S. Structural insights into the mechanisms of Mg 2+ uptake, transport, and gating by Cora. Proc. Natl. Acad. Sci. USA 2012, 109, 18459–18464. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.; Gooden, D.M.; Kung, J.E.; Toone, E.J. Additivity and the physical basis of multivalency effects: A thermodynamic investigation of the calcium EDTA interaction. J. Am. Chem. Soc. 2003, 125, 7357–7366. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Liu, C.; Qi, R.; Ren, P. Many-body effect determines the selectivity for Ca 2+ and Mg 2+ in proteins. Proc. Natl. Acad. Sci. USA 2018, 115, E7495–E7501. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W.; Meek, D.W.; Cornwell, D.G. Properties, composition, and structure of stearic acid-stearate monolayers on Alkaline Earth Solutions. J. Lipid Res. 1967, 8, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Brucker, R.; Beard, B.; Roden, E.; Johnson, C. Iron isotope characteristics of hot springs at Chocolate Pots, Yellowstone National Park. Astrobiology 2013, 13, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed; Chapter 6 & 9; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Sóvágó, I.; Kállay, C.; Várnagy, K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complexes. Coord. Chem. Rev. 2012, 256, 2225–2233. [Google Scholar] [CrossRef]
- Hock, C.; Straßburg, S.; Haberland, H.; Issendorff, B.V.; Aguado, A.; Schmidt, M. Melting-point depression by insoluble impurities: A finite size effect. Phys. Rev. Lett. 2008, 101, 023401. [Google Scholar] [CrossRef]
- Jordan, S.F.; Rammu, H.; Zheludev, I.N.; Hartley, A.M.; Maréchal, A.; Lane, N. Promotion of protocell self-assembly from mixed amphiphiles at the origin of life. Nat. Ecol. Evol. 2019, 3, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Structure of the Plasma Membrane, in The Cell—A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000; ISBN 0-87893-106-6. [Google Scholar]
- Silhavy, T.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Willdigg, J.; Helmann, J. Mini review: Bacterial membrane composition and its modulation in response to stress. Front. Mol. Biosci. 2021, 8, 634438. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.S.; Colbow, K. Light scattering and turbidity measurements on lipid vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 1976, 436, 260–282. [Google Scholar] [CrossRef]
- Wang, A.; Miller, C.C.; Szostak, J.W. Core-shell modeling of light scattering by vesicles: Effect of size, contents, and lamellarity. Biophys. J. 2019, 116, 659–666. [Google Scholar] [CrossRef]
- Schrum, J.; Zhu, T.; Szostak, J. The origins of cellular life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002212. [Google Scholar] [CrossRef]
- Shock, E.L.; Boyd, E.S. Principles of geobiochemistry. Elements 2015, 11, 395–401. [Google Scholar] [CrossRef]
- Gellert, R.; Rieder, R.; Anderson, R.; Brückner, J.; Clark, B.; Dreibus, G.; Economou, T.; Klingelhöfer, G.; Lugmair, G.W.; Ming, D.W.; et al. Chemistry of rocks and soils in Gusev Crater from the alpha particle x-ray spectrometer. Science 2004, 305, 829–832. [Google Scholar] [CrossRef]
- Deng, Z.; Moynier, F.; Villeneuve, J.; Jensen, N.; Liu, D.; Cartigny, P.; Mikouchi, T.; Siebert, J.; Agranier, A.; Chaussidon, M.; et al. Early oxidation of the Martian crust triggered by impacts. Sci. Adv. 2020, 6, eabc4941. [Google Scholar] [CrossRef]
- Cohen, I.; Marron, A. The evolution of universal adaptations of life is driven by universal properties of matter: Energy, entropy, and interaction. F1000Research 2020, 9, 626. [Google Scholar] [CrossRef]
- Martins, N.; Pearson, G.A.; Gouveia, L.; Tavares, A.I.; Serrão, E.A.; Bartsch, I. Hybrid vigour for thermal tolerance in hybrids between the allopatric kelps Laminaria digitata and L. pallida (Laminariales, Phaeophyceae) with contrasting thermal affinities. Eur. J. Phycol. 2019, 54, 548–561. [Google Scholar] [CrossRef]
- Wong, M.L.; Cleland, C.E.; Arend, D.; Bartlett, S.; Cleaves, H.J.; Demarest, H.; Prabhu, A.; Lunine, J.I.; Hazen, R.M. On the roles of function and selection in evolving systems. Proc. Natl. Acad. Sci. USA 2023, 120, e2310223120. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Jovanovic, G.; Ces, O.; Buck, M. Membrane stored curvature elastic stress modulates recruitment of maintenance proteins PspA and Vipp1. mBio 2015, 6, e01188-15. [Google Scholar] [CrossRef] [PubMed]
- Koyiloth, M.; Gummadi, S. Regulation and functions of membrane lipids: Insights from Caenorhabditis elegans. BBA Adv. 2022, 2, 100043. [Google Scholar] [CrossRef] [PubMed]
- Van Kranendonk, M.; Baumgartner, R.; Cady, S.; Campbell, K.; Damer, B.; Deamer, D.; Djokic, T.; Gangidine, A.; Ruff, S.; Walter, M.R. Terrestrial hydrothermal fields and the search for life in the solar system. Bull. Am. Astron. Soc. 2021, 53, 272. [Google Scholar] [CrossRef]
- Ruff, S.W.; Campbell, K.A.; Van Kranendonk, M.J.; Rice, M.S.; Farmer, J.D. The case for ancient Hot Springs in Gusev Crater, Mars. Astrobiology 2020, 20, 475–499. [Google Scholar] [CrossRef]
- Reichhardt, T. Going underground. Nature 2005, 435, 266–267. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Origins, Worlds, and Life: A Decadal Strategy for Planetary Science and Astrobiology 2023–2032; The National Academies Press: Washington, DC, USA, 2023. [Google Scholar]
- Guttenberg, N.; Virgo, N.; Chandru, K.; Scharf, C.; Mamajanov, I. Bulk measurements of messy chemistries are needed for a theory of the origins of life. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160347. [Google Scholar] [CrossRef]



| ‘LAGML’ 10 mM | ‘CAGML’ 10 mM |
|---|---|
| Lauric acid (LA, 12 carbons) and glycerol monolaurate (GML) (1:1 by weight) in 10 mM TEA pH 7.5 | Capric acid (CA, 10 carbons) and glycerol monolaurate (GML) (1:1 by weight) in 10 mM TEA pH 7.5 |
| ‘LAGML (acidic)’ 10 mM | ‘LA’ 10 mM |
| LAGML in 10 mM MES pH 5.5 | Pure LA in 10 mM TEA pH 7.5 |
| ‘LACA’ 10 mM | ‘RNA-LAGML’ |
| LA and CA (1:1 by weight) in 10 mM TEA pH 7.5 | LAGML and yeast RNA in a 4:1 ratio in 10 mM TEA pH 7.5, subjected to 1 wet–dry–wet cycle. |
 |  |  | |
| Ionic Property | Calcium (Ca2+) | Iron (Fe2+) | Magnesium (Mg2+) |
D-orbital ( ) configuration ) configuration | empty (0) | half (6) | empty (0) |
| Bond type | electrostatic | covalent | electrostatic |
| Ionic radius (Å) | 0.99 [54], 1.12 [55] | 0.74 [56], 0.78 [55], 1 [57] | 0.65 [54], 0.76 [55] |
| Coordination number | 6 | 6 | 6 |
| Bond length of cation-O in H2O | 2.46 [55] | 2.12 [55], 1.98–2.05 [58] | 2.10 [55] |
| Charge density | 52 [59] | 98–181 [59] | 120 [59] |
| Previous Work | This Work | |
|---|---|---|
| Overall Trends | Novelty | |
|
|
|
|
|
|
| Methods for assessing vesicle stability and flocculation: fluorescent microscopy, dye permeability, UV-Vis spectrophotometry. | Flocculation and stability quantified by measuring light absorbance with spectrophotometry. | Using spectrophotometry for evaluating the degree of flocculation exceeded expectations and proved a useful approach for quantitatively comparing impact of cations on flocculation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cary, F.C.A.; Deamer, D.W.; Damer, B.F.; Fagents, S.A.; Ruttenberg, K.C.; Donachie, S.P. Could Life Have Started on Mars? Planetary Conditions That Assemble and Destroy Protocells. Life 2024, 14, 415. https://doi.org/10.3390/life14030415
Cary FCA, Deamer DW, Damer BF, Fagents SA, Ruttenberg KC, Donachie SP. Could Life Have Started on Mars? Planetary Conditions That Assemble and Destroy Protocells. Life. 2024; 14(3):415. https://doi.org/10.3390/life14030415
Chicago/Turabian StyleCary, Francesca C. A., David W. Deamer, Bruce F. Damer, Sarah A. Fagents, Kathleen C. Ruttenberg, and Stuart P. Donachie. 2024. "Could Life Have Started on Mars? Planetary Conditions That Assemble and Destroy Protocells" Life 14, no. 3: 415. https://doi.org/10.3390/life14030415
APA StyleCary, F. C. A., Deamer, D. W., Damer, B. F., Fagents, S. A., Ruttenberg, K. C., & Donachie, S. P. (2024). Could Life Have Started on Mars? Planetary Conditions That Assemble and Destroy Protocells. Life, 14(3), 415. https://doi.org/10.3390/life14030415









