Vernal Keratoconjunctivitis: Immunopathological Insights and Therapeutic Applications of Immunomodulators
Abstract
1. Introduction
2. Epidemiology of VKC
3. Immunopathophysiology of VKC
4. Differential Diagnosis
5. Ocular Involvement: Signs and Symptoms
6. Management of VKC
7. Future Directions
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S. Vernal keratoconjunctivitis: A major review. Acta Ophthalmol. 2009, 87, 133–147. [Google Scholar] [CrossRef]
- Leonardi, A.; Busca, F.; Motterle, L.; Cavarzeran, F.; Fregona, I.; Plebani, M.; Secchi, A. Case series of 406 vernal keratoconjunctivitis patients: A demographic and epidemiological study. Acta Ophthalmol. Scand. 2006, 84, 406–410. [Google Scholar] [CrossRef]
- Chigbu, D.I.; Sandrasekaramudaly-Brown, S. Ocular surface disease: A case of vernal keratoconjunctivitis. Contact Lens Anterior Eye 2011, 34, 39–44. [Google Scholar] [CrossRef]
- Chigbu, D.I.; Labib, B.A. Immunopharmacology in Vernal Keratoconjunctivitis: Current and Future Perspectives. Pharmaceuticals 2021, 14, 658. [Google Scholar] [CrossRef]
- Singhal, D.; Sahay, P.; Maharana, P.K.; Raj, N.; Sharma, N.; Titiyal, J.S. Vernal Keratoconjunctivitis. Surv. Ophthalmol. 2019, 64, 289–311. [Google Scholar] [CrossRef]
- Bruschi, G.; Ghiglioni, D.G.; Cozzi, L.; Osnaghi, S.; Viola, F.; Marchisio, P. Vernal Keratoconjunctivitis: A Systematic Review. Clin. Rev. Allergy Immunol. 2023, 65, 277–329. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, D.I.; Jain, P.; Khan, Z.K. Immune Mechanisms, Pathology, and Management of Allergic Ocular Diseases. In Advanced Concepts in Human Immunology: Prospects for Disease Control; Jain, P., Ndhlovu, L.C., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 229–277. [Google Scholar]
- Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Yamamoto, K.; Nishida, T. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog. Retin. Eye Res. 2006, 25, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Javadi, M.A.; Alemzadeh-Ansari, M.; Arabi, A.; Shahraki, T.; Kheirkhah, A. Management of corneal complications in vernal keratoconjunctivitis: A review. Ocul. Surf. 2021, 19, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C. Immunosuppressive drugs: The first 50 years and a glance forward. Immunopharmacology 2000, 47, 63–83. [Google Scholar] [CrossRef]
- McMonnies, C.W. Dry eye disease immune responses and topical therapy. Eye Vis. 2019, 6, 12. [Google Scholar] [CrossRef]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef]
- Erdinest, N.; Ben-Eli, H.; Solomon, A. Topical tacrolimus for allergic eye diseases. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.S.; Choi, J.; Cheung, C.M.G. Pediatric Uveitis. Asia Pac. J. Ophthalmol. 2018, 7, 192–199. [Google Scholar]
- Abdel-Aty, A.; Gupta, A.; Del Priore, L.; Kombo, N. Management of noninfectious scleritis. Ther. Adv. Ophthalmol. 2022, 14, 25158414211070879. [Google Scholar] [CrossRef]
- Katz, E.A.; Sunshine, S.; Mun, C.; Sarwar, M.; Surenkhuu, B.; Pradeep, A.; Jain, S. Combinatorial therapy with immunosuppressive, immunomodulatory and tear substitute eyedrops (“Triple Play”) in Recalcitrant Immunological Ocular Surface Diseases. Ocul. Surf. 2022, 23, 1–11. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Bonini, S.; Fernandes, M. Adult vernal keratoconjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 501–506. [Google Scholar] [CrossRef]
- Bonini, S.; Coassin, M.; Aronni, S.; Lambiase, A. Vernal keratoconjunctivitis. Eye 2004, 18, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Lazzarini, D.; Motterle, L.; Bortolotti, M.; Deligianni, V.; Curnow, S.J.; Bonini, S.; Fregona, I.A. Vernal keratoconjunctivitis-like disease in adults. Am. J. Ophthalmol. 2013, 155, 796–803. [Google Scholar] [CrossRef]
- Chigbu, D.I. The pathophysiology of ocular allergy: A review. Contact Lens Anterior Eye 2009, 32, 3–15, quiz 43-4. [Google Scholar] [CrossRef]
- Saboo, U.S.; Jain, M.; Reddy, J.C.; Sangwan, V.S. Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J. Ophthalmol. 2013, 61, 486–489. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Micera, A.; De Piano, M.; Coassin, M.; Sharma, S.; Bonini, S.; Fernandes, M. Adult Vernal Keratoconjunctivitis: Clinical and biochemical profile of a rare disease. Ocul. Surf. 2019, 17, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Micera, A.; Di Zazzo, A.; De Piano, M.; Sharma, S.; Mori, T.; De Gregorio, C.; Coassin, M.; Fernandes, M. Tissue remodeling in adult vernal keratoconjunctivitis. Exp. Eye Res. 2022, 225, 109301. [Google Scholar] [CrossRef] [PubMed]
- Eskandarpour, M.; Zhang, X.; Micera, A.; Zaher, S.; Larkin, F.D.P.; Nunn, M.; Bonini, S.; Weston-Davies, W.; Calder, V.L. Allergic eye disease: Blocking LTB4/C5 in vivo suppressed disease and Th2 & Th9 cells. Allergy 2022, 77, 660–664. [Google Scholar]
- Leonardi, A. Vernal keratoconjunctivitis: Pathogenesis and treatment. Prog. Retin. Eye Res. 2002, 21, 319–339. [Google Scholar] [CrossRef]
- Goswami, R.; Kaplan, M.H. A brief history of IL-9. J. Immunol. 2011, 186, 3283–3288. [Google Scholar] [CrossRef]
- Pajulas, A.; Fu, Y.; Cheung, C.C.L.; Chu, M.; Cannon, A.; Alakhras, N.; Zhang, J.; Ulrich, B.J.; Nelson, A.S.; Zhou, B.; et al. Interleukin-9 promotes mast cell progenitor proliferation and CCR2-dependent mast cell migration in allergic airway inflammation. Mucosal Immunol. 2023, 16, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.Y.; Kita, H. IL-33: Biological properties, functions, and roles in airway disease. Immunol. Rev. 2017, 278, 173–184. [Google Scholar] [CrossRef]
- Labib, B.A.; Chigbu, D.I. Therapeutic Targets in Allergic Conjunctivitis. Pharmaceuticals 2022, 15, 547. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Hu, J.; Gao, N.; Zhang, Y.; Chen, X.; Li, J.; Bian, F.; Chi, W.; Liu, Z.; de Paiva, C.S.; Pflugfelder, S.C.; et al. IL-33/ST2/IL-9/IL-9R signaling disrupts ocular surface barrier in allergic inflammation. Mucosal Immunol. 2020, 13, 919–930. [Google Scholar] [CrossRef]
- Singh, N.; Diebold, Y.; Sahu, S.K.; Leonardi, A. Epithelial barrier dysfunction in ocular allergy. Allergy 2022, 77, 1360–1372. [Google Scholar] [CrossRef]
- Enriquez-de-Salamanca, A.; Calder, V.; Gao, J.; Galatowicz, G.; Garcia-Vazquez, C.; Fernandez, I.; Stern, M.E.; Diebold, Y.; Calonge, M. Cytokine responses by conjunctival epithelial cells: An in vitro model of ocular inflammation. Cytokine 2008, 44, 160–167. [Google Scholar] [CrossRef]
- Leonardi, A.; De Dominicis, C.; Motterle, L. Immunopathogenesis of ocular allergy: A schematic approach to different clinical entities. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 429–435. [Google Scholar] [CrossRef]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef]
- Cherry, W.B.; Yoon, J.; Bartemes, K.R.; Iijima, K.; Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 2008, 121, 1484–1490. [Google Scholar] [CrossRef]
- Williams, C.M.; Rahman, S.; Hubeau, C.; Ma, H.L. Cytokine pathways in allergic disease. Toxicol. Pathol. 2012, 40, 205–215. [Google Scholar] [CrossRef]
- Ninomiya, I.; Yamatoya, K.; Mashimo, K.; Matsuda, A.; Usui-Ouchi, A.; Araki, Y.; Ebihara, N. Role of Oncostatin M in the Pathogenesis of Vernal Keratoconjunctivitis: Focus on the Barrier Function of the Epithelium and Interleukin-33 Production by Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2022, 63, 26. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Okayama, Y.; Terai, N.; Yokoi, N.; Ebihara, N.; Tanioka, H.; Kawasaki, S.; Inatomi, T.; Katoh, N.; Ueda, E.; et al. The Role of Interleukin-33 in Chronic Allergic Conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2009, 63, 26. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Ebihara, N.; Yokoi, N.; Kawasaki, S.; Tanioka, H.; Inatomi, T.; de Waal Malefyt, R.; Hamuro, J.; Kinoshita, S.; Murakami, A. Functional role of thymic stromal lymphopoietin in chronic allergic keratoconjunctivitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Comeau, M.R.; Ziegler, S.F. The influence of TSLP on the allergic response. Mucosal Immunol. 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Katelaris, C. Ocular allergy: Implications for the clinical immunologist. Ann. Allergy Asthma Immunol. 2003, 90 (Suppl. S3), 23–27. [Google Scholar] [CrossRef]
- Solimando, A.G.; Desantis, V.; Ribatti, D. Mast Cells and Interleukins. Int. J. Mol. Sci. 2022, 23, 14004. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Xia, H.Z.; Sanchez-Munoz, L.B.; Dellinger, A.L.; Escribano, L.; Schwartz, L.B. Generation of anaphylatoxins by human beta-tryptase from C3, C4, and C5. J. Immunol. 2008, 180, 6307–6316. [Google Scholar] [CrossRef]
- DiScipio, R.G.; Schraufstatter, I.U. The role of the complement anaphylatoxins in the recruitment of eosinophils. Int. Immunopharmacol. 2007, 7, 1909–1923. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, Y.; Hite, M.R.; Dellinger, A.L.; Schwartz, L.B. Human skin mast cells express complement factors C3 and C5. J. Immunol. 2013, 191, 1827–1834. [Google Scholar] [CrossRef]
- Katz, Y.; Stav, D.; Barr, J.; Passwell, J.H. IL-13 results in differential regulation of the complement proteins C3 and factor B in tumour necrosis factor (TNF)-stimulated fibroblasts. Clin. Exp. Immunol. 1995, 101, 150–156. [Google Scholar] [CrossRef]
- Zhang, X.; Kohl, J. A complex role for complement in allergic asthma. Expert Rev. Clin. Immunol. 2010, 6, 269–277. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Koehl, J. New insights into the role of the complement pathway in allergy and asthma. Curr. Allergy Asthma Rep. 2005, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Maruo, K.; Akaike, T.; Ono, T.; Okamoto, T.; Maeda, H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J. Allergy Clin. Immunol. 1997, 100, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ballow, M.; Donshik, P.C.; Mendelson, L. Complement proteins and C3 anaphylatoxin in the tears of patients with conjunctivitis. J. Allergy Clin. Immunol. 1985, 76, 473–476. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef]
- Hirakata, T.; Lee, H.C.; Ohba, M.; Saeki, K.; Okuno, T.; Murakami, A.; Matsuda, A.; Yokomizo, T. Dietary omega-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating T(h)2 immune responses. FASEB J. 2019, 33, 3392–3403. [Google Scholar] [CrossRef]
- Luna-Gomes, T.; Bozza, P.T.; Bandeira-Melo, C. Eosinophil recruitment and activation: The role of lipid mediators. Front. Pharmacol. 2013, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Kimani, G.; Tonnesen, M.G.; Henson, P.M. Stimulation of eosinophil adherence to human vascular endothelial cells in vitro by platelet-activating factor. J. Immunol. 1988, 140, 3161–3166. [Google Scholar] [CrossRef] [PubMed]
- Little, M.M.; Casale, T.B. Comparison of platelet-activating factor-induced chemotaxis of normodense and hypodense eosinophils. J. Allergy Clin. Immunol. 1991, 88, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Cossette, C.; Gravel, S.; Rokach, J.; Powell, W.S. 15R-methyl-prostaglandin D2 is a potent and selective CRTH2/DP2 receptor agonist in human eosinophils. J. Pharmacol. Exp. Ther. 2003, 304, 349–355. [Google Scholar] [CrossRef]
- Tager, A.M.; Dufour, J.H.; Goodarzi, K.; Bercury, S.D.; von Andrian, U.H.; Luster, A.D. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J. Exp. Med. 2000, 192, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Patnode, M.L.; Bando, J.K.; Krummel, M.F.; Locksley, R.M.; Rosen, S.D. Leukotriene B4 amplifies eosinophil accumulation in response to nematodes. J. Exp. Med. 2014, 211, 1281–1288. [Google Scholar] [CrossRef]
- Leonardi, A.; Secchi, A.G. Vernal keratoconjunctivitis. Int. Ophthalmol. Clin. 2003, 43, 41–58. [Google Scholar] [CrossRef]
- Fujitsu, Y.; Fukuda, K.; Kimura, K.; Seki, K.; Kumagai, N.; Nishida, T. Protection of human conjunctival fibroblasts from NO-induced apoptosis by interleukin-4 or interleukin-13. Investig. Ophthalmol. Vis. Sci. 2005, 46, 797–802. [Google Scholar] [CrossRef][Green Version]
- McKenzie, G.J.; Fallon, P.G.; Emson, C.L.; Grencis, R.K.; McKenzie, A.N. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 1999, 189, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Liu, B. Transcriptional regulation of mast cell and basophil lineage commitment. Semin. Immunopathol. 2016, 38, 539–548. [Google Scholar] [CrossRef]
- Demoulin, J.B.; Renauld, J.C. Interleukin 9 and its receptor: An overview of structure and function. Int. Rev. Immunol. 1998, 16, 345–364. [Google Scholar] [CrossRef]
- Temann, U.A.; Geba, G.P.; Rankin, J.A.; Flavell, R.A. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 1998, 188, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Gounni, A.S.; Gregory, B.; Nutku, E.; Aris, F.; Latifa, K.; Minshall, E.; North, J.; Tavernier, J.; Levit, R.; Nicolaides, N.; et al. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood 2000, 96, 2163–2171. [Google Scholar] [CrossRef]
- Solomon, A.; Shmilowich, R.; Shasha, D.; Frucht-Pery, J.; Pe’er, J.; Bonini, S.; Levi-Schaffer, F. Conjunctival fibroblasts enhance the survival and functional activity of peripheral blood eosinophils in vitro. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1038–1044. [Google Scholar]
- Peng, B.; Sun, L.; Zhang, M.; Yan, H.; Shi, G.; Xia, Z.; Dai, R.; Tang, W. Role of IL-25 on Eosinophils in the Initiation of Th2 Responses in Allergic Asthma. Front. Immunol. 2022, 13, 842500. [Google Scholar] [CrossRef]
- Isgro, M.; Bianchetti, L.; Marini, M.A.; Bellini, A.; Schmidt, M.; Mattoli, S. The C-C motif chemokine ligands CCL5, CCL11, and CCL24 induce the migration of circulating fibrocytes from patients with severe asthma. Mucosal Immunol. 2013, 6, 718–727. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, X.Y.; Jiang, P. Cytokine and Chemokine Signals of T-Cell Exclusion in Tumors. Front. Immunol. 2020, 11, 594609. [Google Scholar] [CrossRef]
- Leonardi, A. Allergy and allergic mediators in tears. Exp. Eye Res. 2013, 117, 106–117. [Google Scholar] [CrossRef]
- Bielory, L. Allergic and immunologic disorders of the eye. Part II: Ocular allergy. J. Allergy Clin. Immunol. 2000, 106, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Smith, L.; Calder, V.; Buckley, R.; Lightman, S. Clinical and immunological features of atopic keratoconjunctivitis. Int. Ophthalmol. Clin. 2003, 43, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, D.I. Minhas, B.K. Immunopathology of allergic conjunctivitis. Eur. Med. J. 2018, 3, 76–83. [Google Scholar] [CrossRef]
- Ohbayashi, M.; Manzouri, B.; Morohoshi, K.; Fukuda, K.; Ono, S.J. The Role of Histamine in Ocular Allergy. Adv. Exp. Med. Biol. 2010, 709, 43–52. [Google Scholar] [PubMed]
- Nemmer, J.M.; Kuchner, M.; Datsi, A.; Olah, P.; Julia, V.; Raap, U.; Homey, B. Interleukin-31 Signaling Bridges the Gap between Immune Cells, the Nervous System and Epithelial Tissues. Front. Med. 2021, 8, 639097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef]
- Dantas, P.; Alves, M.; Nishiwaki-Dantas, M. Topographic corneal changes in patients with vernal keratoconjunctivitis. Arq. Bras. Oftalmol. 2005, 68, 593–598. [Google Scholar] [CrossRef][Green Version]
- Totan, Y.; Hepşen, I.; Cekiç, O.; Gündüz, A.; Aydin, E. Incidence of keratoconus in subjects with vernal keratoconjunctivitis: A videokeratographic study. Ophthalmology 2001, 108, 824–827. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, F.; Qiu, J.; Yang, Y.; Zhang, C. Corneal biomechanical properties in vernal keratoconjunctivitis and its subtypes: A preliminary study. Int. Ophthalmol. 2023, 43, 2083–2090. [Google Scholar] [CrossRef]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef]
- Kugelmann, D.; Rotkopf, L.T.; Radeva, M.Y.; Garcia-Ponce, A.; Walter, E.; Waschke, J. Histamine causes endothelial barrier disruption via Ca(2+)-mediated RhoA activation and tension at adherens junctions. Sci. Rep. 2018, 8, 13229. [Google Scholar] [CrossRef]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid Res. 2009, 50, S423–S428. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.; Foudi, N.; Longrois, D.; Norel, X. The role of prostaglandin E2 in human vascular inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 55–63. [Google Scholar] [CrossRef]
- Domingo, C.; Palomares, O.; Sandham, D.A.; Erpenbeck, V.J.; Altman, P. The prostaglandin D(2) receptor 2 pathway in asthma: A key player in airway inflammation. Respir. Res. 2018, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S.H.; Kim, T.H. The Biology of Prostaglandins and Their Role as a Target for Allergic Airway Disease Therapy. Int. J. Mol. Sci. 2020, 21, 1851. [Google Scholar] [CrossRef]
- Kotowicz, K.; Callard, R.E.; Friedrich, K.; Matthews, D.J.; Klein, N. Biological activity of IL-4 and IL-13 on human endothelial cells: Functional evidence that both cytokines act through the same receptor. Int. Immunol. 1996, 8, 1915–1925. [Google Scholar] [CrossRef]
- Skaria, T.; Burgener, J.; Bachli, E.; Schoedon, G. IL-4 Causes Hyperpermeability of Vascular Endothelial Cells through Wnt5A Signaling. PLoS ONE 2016, 11, e0156002. [Google Scholar] [CrossRef] [PubMed]
- Tukler Henriksson, J.; Coursey, T.G.; Corry, D.B.; De Paiva, C.S.; Pflugfelder, S.C. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cultured Conjunctival Goblet Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4186–4197. [Google Scholar] [CrossRef]
- Pajulas, A.; Zhang, J.; Kaplan, M.H. The World according to IL-9. J. Immunol. 2023, 211, 7–14. [Google Scholar] [CrossRef]
- Galicia-Carreon, J.; Santacruz, C.; Hong, E.; Jimenez-Martinez, M.C. The ocular surface: From physiology to the ocular allergic diseases. Rev. Alerg. Mex. 2013, 60, 172–183. [Google Scholar]
- Kato, N.; Fukagawa, K.; Dogru, M.; Fujishima, H.; Tsubota, K. Mechanisms of giant papillary formation in vernal keratoconjunctivitis. Cornea 2006, 25 (Suppl. S1), S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Fujitsu, Y.; Fukuda, K.; Kumagai, N.; Nishida, T. IL-4-induced cell proliferation and production of extracellular matrix proteins in human conjunctival fibroblasts. Exp. Eye Res. 2003, 76, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Stassen, M.; Schmitt, E.; Bopp, T. From interleukin-9 to T helper 9 cells. Ann. N. Y. Acad. Sci. 2012, 1247, 56–68. [Google Scholar] [CrossRef]
- Soroosh, P.; Doherty, T.A. Th9 and allergic disease. Immunology 2009, 127, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Padmanabhan, P. Perilimbal conjunctival pigmentation in vernal keratoconjunctivitis: A new sign. Cornea 2002, 21, 432. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Meenakshi, S.; Srinivasan, B.; Baluswamy, S. Perilimbal bulbar conjunctival pigmentation in vernal conjunctivitis: Prospective evaluation of a new clinical sign in an Indian population. Cornea 2004, 23, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Luk, F.; Wong, V.; Rao, S.; Lam, D. Perilimbal conjunctival pigmentation in Chinese patients with vernal keratoconjunctivitis. Eye 2008, 22, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Dubbaka, S.; Agrawal, M.; Sati, A.; Vats, S.; Mahajan, S. An observational study on the presence of perilimbal conjunctival pigmentation in vernal keratoconjunctivitis. Indian J. Ophthalmol. 2023, 71, 1816–1821. [Google Scholar] [CrossRef]
- Leonardi, A.; Righetti, G.; Giovannini, G.; De Marchi, V.; Occhiuto, M. Diagnostic criteria of chronic conjunctivitis: Atopic keratoconjunctivitis and vernal keratoconjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 390–396. [Google Scholar] [CrossRef]
- Solomon, A. Corneal complications of vernal keratoconjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 489–494. [Google Scholar] [CrossRef]
- Wong, A.H.; Barg, S.S.; Leung, A.K. Seasonal and perennial allergic conjunctivitis. Recent Pat. Inflamm. Allergy Drug Discov. 2014, 8, 139–153. [Google Scholar] [CrossRef]
- Chigbu, D.I. The management of allergic eye diseases in primary eye care. Contact Lens Anterior Eye 2009, 32, 260–272. [Google Scholar] [CrossRef]
- Abbas, A.; Lichtman, A.; Pillai, S. Allergy. In Cellular and Molecular Immunology; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 239–264. [Google Scholar]
- Trocme, S.D.; Hallberg, C.K.; Gill, K.S.; Gleich, G.J.; Tyring, S.K.; Brysk, M.M. Effects of eosinophil granule proteins on human corneal epithelial cell viability and morphology. Investig. Ophthalmol. Vis. Sci. 1997, 38, 593–599. [Google Scholar]
- Reddy, J.C.; Basu, S.; Saboo, U.S.; Murthy, S.I.; Vaddavalli, P.K.; Sangwan, V.S. Management, clinical outcomes, and complications of shield ulcers in vernal keratoconjunctivitis. Am. J. Ophthalmol. 2013, 155, 550–559.e1. [Google Scholar] [CrossRef] [PubMed]
- Doan, S.; Papadopoulos, N.G.; Lee, J.K.; Leonardi, S.; Manti, S.; Lau, S.; Rondon, C.; Sharma, V.; Pleyer, U.; Jaumont, X.; et al. Vernal keratoconjunctivitis: Current immunological and clinical evidence and the potential role of omalizumab. World Allergy Organ. J. 2023, 16, 100788. [Google Scholar] [CrossRef]
- Ali, A.; Bielory, L.; Dotchin, S.; Hamel, P.; Strube, Y.N.J.; Koo, E.B. Management of vernal keratoconjunctivitis: Navigating a changing treatment landscape. Surv. Ophthalmol. 2023, in press.
- Leonardi, A. Management of vernal keratoconjunctivitis. Ophthalmol. Ther. 2013, 2, 73–88. [Google Scholar] [CrossRef]
- Gokhale, N.S. Systematic approach to managing vernal keratoconjunctivitis in clinical practice: Severity grading system and a treatment algorithm. Indian J. Ophthalmol. 2016, 64, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.S.; Chen, W.L.; Cheng, A.C.K.; Cung, L.X.; Dualan, I.J.; Kekunnaya, R.; Khaliddin, N.; Kim, T.I.; Lam, D.K.; Leo, S.W.; et al. Diagnosis, Management, and Treatment of Vernal Keratoconjunctivitis in Asia: Recommendations from the Management of Vernal Keratoconjunctivitis in Asia Expert Working Group. Front. Med. 2022, 9, 882240. [Google Scholar] [CrossRef]
- Andoh, T.; Kuraishi, Y. Lipid Mediators and Itch. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Dahlmann-Noor, A.; Bonini, S.; Bremond-Gignac, D.; Heegaard, S.; Leonardi, A.; Montero, J.; Silva, E.D.; Group, E.-V. Novel Insights in the Management of Vernal Keratoconjunctivitis (VKC): European Expert Consensus Using a Modified Nominal Group Technique. Ophthalmol. Ther. 2023, 12, 1207–1222. [Google Scholar] [CrossRef]
- Maharana, P.K.; Singhal, D.; Raj, N.; Sharma, N.; Titiyal, J.S. Role of combined immunomodulator therapy in severe steroid intolerant vernal keratoconjunctivitis. Eye 2021, 35, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Bandyopadhyay, S.; Kumar Bandyopadhyay, S. Efficacy, Safety and Steroid-sparing Effect of Topical Cyclosporine A 0.05% for Vernal Keratoconjunctivitis in Indian Children. J. Ophthalmic Vis. Res. 2019, 14, 412–418. [Google Scholar] [CrossRef]
- Roy, J.; Cyert, M.S. Identifying New Substrates and Functions for an Old Enzyme: Calcineurin. Cold Spring Harb. Perspect. Biol. 2020, 12, a035436. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Licthman, A.H.; Pillai, S. Immune Receptors and Signal Transduction. In Cellular and Molecular Immunology; Elsevier Saunders: Philadelphia, PA, USA, 2017; pp. 145–178. [Google Scholar]
- Murphy, K.; Weaver, C. Manipulation of the Immune Response. In Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2017; pp. 701–748. [Google Scholar]
- Gutfreund, K.; Bienias, W.; Szewczyk, A.; Kaszuba, A. Topical calcineurin inhibitors in dermatology. Part I: Properties, method and effectiveness of drug use. Postepy Dermatol. Alergol. 2013, 30, 165–169. [Google Scholar] [CrossRef]
- Murphy, K.P.; Weaver, C. Allergy and Allergic Diseases. In Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2017; pp. 601–641. [Google Scholar]
- Oray, M.; Toker, E. Tear cytokine levels in vernal keratoconjunctivitis: The effect of topical 0.05% cyclosporine a therapy. Cornea 2013, 32, 1149–1154. [Google Scholar] [CrossRef]
- Subedi, K.; Sharma, B.; Shrestha, S. Efficacy of Topical Cyclosporine 0.05% the Treatment of Vernal Keratoconjunctivitis. Nepal. J. Ophthalmol. 2020, 12, 39–47. [Google Scholar] [CrossRef]
- Bourcier, T.; Dory, A.; Dormegny, L.; Alcazar, J.; Gaucher, D.; Sauer, A. Efficacy and Safety of 0.1% Cyclosporine versus 2% Cyclosporine in the Treatment of Severe Vernal Keratoconjunctivitis in Children. Clin. Ophthalmol. 2022, 16, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Rossi, C.; Borselli, M.; Bonzano, C.; Carnovale Scalzo, G.; Nicolo, M.; Scorcia, V.; Traverso, C.E.; Vagge, A. Clinical Outcomes of Topical 0.1% Ciclosporin Cationic Emulsion Used on Label in Children with Vernal Keratoconjunctivitis. Ophthalmol. Ther. 2023, 12, 1787–1793. [Google Scholar] [CrossRef]
- Gupta, V.; Sahu, P. Topical cyclosporin A in the management of vernal keratoconjunctivitis. Eye 2001, 15 Pt 1, 39–41. [Google Scholar] [CrossRef][Green Version]
- Leonardi, A.; Doan, S.; Amrane, M.; Ismail, D.; Montero, J.; Nemeth, J.; Aragona, P.; Bremond-Gignac, D.; Group, V.S. A Randomized, Controlled Trial of Cyclosporine A Cationic Emulsion in Pediatric Vernal Keratoconjunctivitis: The VEKTIS Study. Ophthalmology 2019, 126, 671–681. [Google Scholar] [CrossRef]
- Bremond-Gignac, D.; Doan, S.; Amrane, M.; Ismail, D.; Montero, J.; Nemeth, J.; Aragona, P.; Leonardi, A.; Group, V.S. Twelve-Month Results of Cyclosporine A Cationic Emulsion in a Randomized Study in Patients With Pediatric Vernal Keratoconjunctivitis. Am. J. Ophthalmol. 2020, 212, 116–126. [Google Scholar] [CrossRef]
- Leonardi, A.; Doan, S.; Aragona, P.; Amrane, M.; Ismail, D.; Montero, J.; Nemeth, J.; Bremond-Gignac, D. Topical cyclosporine A cationic ophthalmic emulsion in paediatric vernal keratoconjunctivitis: Pooled analysis of randomised NOVATIVE and VEKTIS trials. Eye 2023, 37, 2320–2326. [Google Scholar] [CrossRef]
- Leonardi, A.; Pisella, P.J.; Benitez-Del-Castillo, J.M.; Amrane, M.; Ismail, D.; Doan, S.; Bremond-Gignac, D. NOVATIVE: A Phase II/III, Multicenter, Double-masked, Randomized Study of Cyclosporine A 0.05% and 0.1% Ophthalmic Cationic Emulsion Versus Vehicle in Patients with Vernal Keratoconjunctivitis. Clin. Ther. 2023, 45, 1284–1288. [Google Scholar] [CrossRef]
- Ozkaya, D.; Usta, G.; Karaca, U. A Case of Shield Ulcer Due to Vernal Keratoconjunctivitis. Iran. J. Allergy Asthma Immunol. 2021, 20, 505–508. [Google Scholar]
- Westland, T.; Patryn, E.K.; Nieuwendaal, C.P.; van der Meulen, I.J.E.; Mourits, M.P.; Lapid-Gortzak, R. Vernal shield ulcers treated with frequently installed topical cyclosporine 0.05% eyedrops. Int. Ophthalmol. 2018, 38, 363–368. [Google Scholar] [CrossRef]
- Hirota, A.; Shoji, J.; Inada, N.; Shiraki, Y.; Yamagami, S. Evaluation of Clinical Efficacy and Safety of Prolonged Treatment of Vernal and Atopic Keratoconjunctivitis Using Topical Tacrolimus. Cornea 2022, 41, 23–30. [Google Scholar] [CrossRef]
- Eltagoury, M.; Abou Samra, W.; Ghoneim, E. Safety and efficacy of topical tacrolimus 0.03% in the management of vernal keratoconjunctivitis: A non-randomized controlled clinical trial. Med. Hypothesis Discov. Innov. Ophthalmol. 2022, 11, 52–63. [Google Scholar] [CrossRef]
- Armstrong, V.W.; Oellerich, M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine. Clin. Biochem. 2001, 34, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pucci, N.; Caputo, R.; di Grande, L.; de Libero, C.; Mori, F.; Barni, S.; di Simone, L.; Calvani, A.; Rusconi, F.; Novembre, E. Tacrolimus vs. cyclosporine eyedrops in severe cyclosporine-resistant vernal keratoconjunctivitis: A randomized, comparative, double-blind, crossover study. Pediatr. Allergy Immunol. 2015, 26, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Kumari, R.; Ambasta, A. Comparision of efficacy and safety of 0.03% and 0.1% tacrolimus ointment in children with vernal keratoconjunctivitis. Ther. Adv. Ophthalmol. 2023, 15, 25158414231173532. [Google Scholar] [CrossRef] [PubMed]
- Caputo, R.; Marziali, E.; de Libero, C.; Di Grande, L.; Danti, G.; Virgili, G.; Villani, E.; Mori, F.; Bacci, G.M.; Lucenteforte, E.; et al. Long-Term Safety and Efficacy of Tacrolimus 0.1% in Severe Pediatric Vernal Keratoconjunctivitis. Cornea 2021, 40, 1395–1401. [Google Scholar] [CrossRef]
- Fiorentini, S.F.; Khurram, D. Therapeutic effects of topical 0.03% Tacrolimus ointment in children with refractory vernal keratoconjunctivitis in Middle East. Saudi J. Ophthalmol. 2019, 33, 117–120. [Google Scholar] [CrossRef]
- Barot, R.K.; Shitole, S.C.; Bhagat, N.; Patil, D.; Sawant, P.; Patil, K. Therapeutic effect of 0.1% Tacrolimus Eye Ointment in Allergic Ocular Diseases. J. Clin. Diagn Res. 2016, 10, NC05-9. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Zavareh, M.K.; Farzbod, F.; Mahbod, M.; Behrouz, M.J. Topical 0.005% tacrolimus eye drop for refractory vernal keratoconjunctivitis. Eye 2011, 25, 872–880. [Google Scholar] [CrossRef]
- Fukushima, A.; Ohashi, Y.; Ebihara, N.; Uchio, E.; Okamoto, S.; Kumagai, N.; Shoji, J.; Takamura, E.; Nakagawa, Y.; Namba, K.; et al. Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br. J. Ophthalmol. 2014, 98, 1023–1027. [Google Scholar] [CrossRef]
- Bardoloi, P.; Vanathi, M.; Velpandian, T.; Laxmi, M.; Gupta, N.; Lomi, N.; Tandon, R. Tear Tacrolimus Levels and Clinical Response after Adjunct Therapy with Cutaneous Application of Tacrolimus 0.1% over Upper Eyelid Skin in Chronic Vernal Keratoconjunctivitis. Cornea, 2023; in press. [Google Scholar]
- Heikal, M.A.; Soliman, T.T.; Abousaif, W.S.; Shebl, A.A. A comparative study between ciclosporine A eye drop (2%) and tacrolimus eye ointment (0.03%) in management of children with refractory vernal keratoconjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 353–361. [Google Scholar] [CrossRef]
- Labcharoenwongs, P.; Jirapongsananuruk, O.; Visitsunthorn, N.; Kosrirukvongs, P.; Saengin, P.; Vichyanond, P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac. J. Allergy Immunol. 2012, 30, 177–184. [Google Scholar] [PubMed]
- Arnon, R.; Rozen-Knisbacher, I.; Yahalomi, T.; Stanescu, N.; Niazov, Y.; Goldberg, D.; Sharabi-Nov, A.; Mostovoy, D. When to start tacrolimus ointment for vernal keratoconjunctivitis? A proposed treatment protocol. Int. Ophthalmol. 2022, 42, 1771–1780. [Google Scholar] [CrossRef]
- Al-Amri, A.M. Long-term follow-up of tacrolimus ointment for treatment of atopic keratoconjunctivitis. Am. J. Ophthalmol. 2014, 157, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Tzu, J.H.; Utine, C.A.; Stern, M.E.; Akpek, E.K. Topical calcineurin inhibitors in the treatment of steroid-dependent atopic keratoconjunctivitis. Cornea 2012, 31, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Takamura, E.; Uchio, E.; Ebihara, N.; Ohno, S.; Ohashi, Y.; Okamoto, S.; Kumagai, N.; Satake, Y.; Shoji, J.; Nakagawa, Y.; et al. Japanese guidelines for allergic conjunctival diseases 2017. Allergol. Int. 2017, 66, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Cox, L. Biologics and Allergy Immunotherapy in the Treatment of Allergic Diseases. Immunol. Allergy Clin. N. Am. 2020, 40, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Kishimoto, T.; Sumi, T.; Yamashiro, K.; Ebihara, N. Biologics for allergy: Therapeutic potential for ocular allergic diseases and adverse effects on the eye. Allergol. Int. 2023, 72, 234–244. [Google Scholar] [CrossRef] [PubMed]
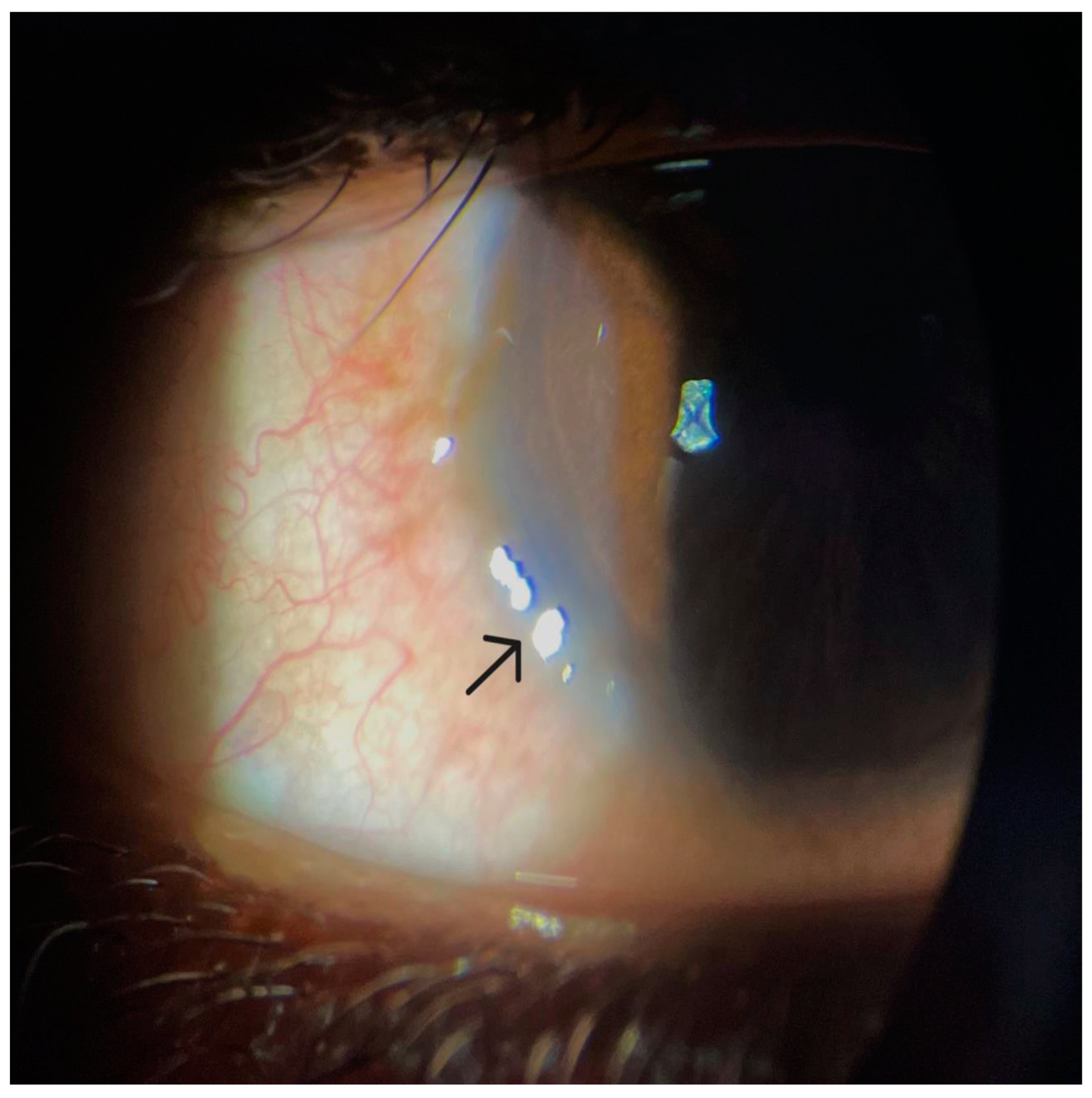

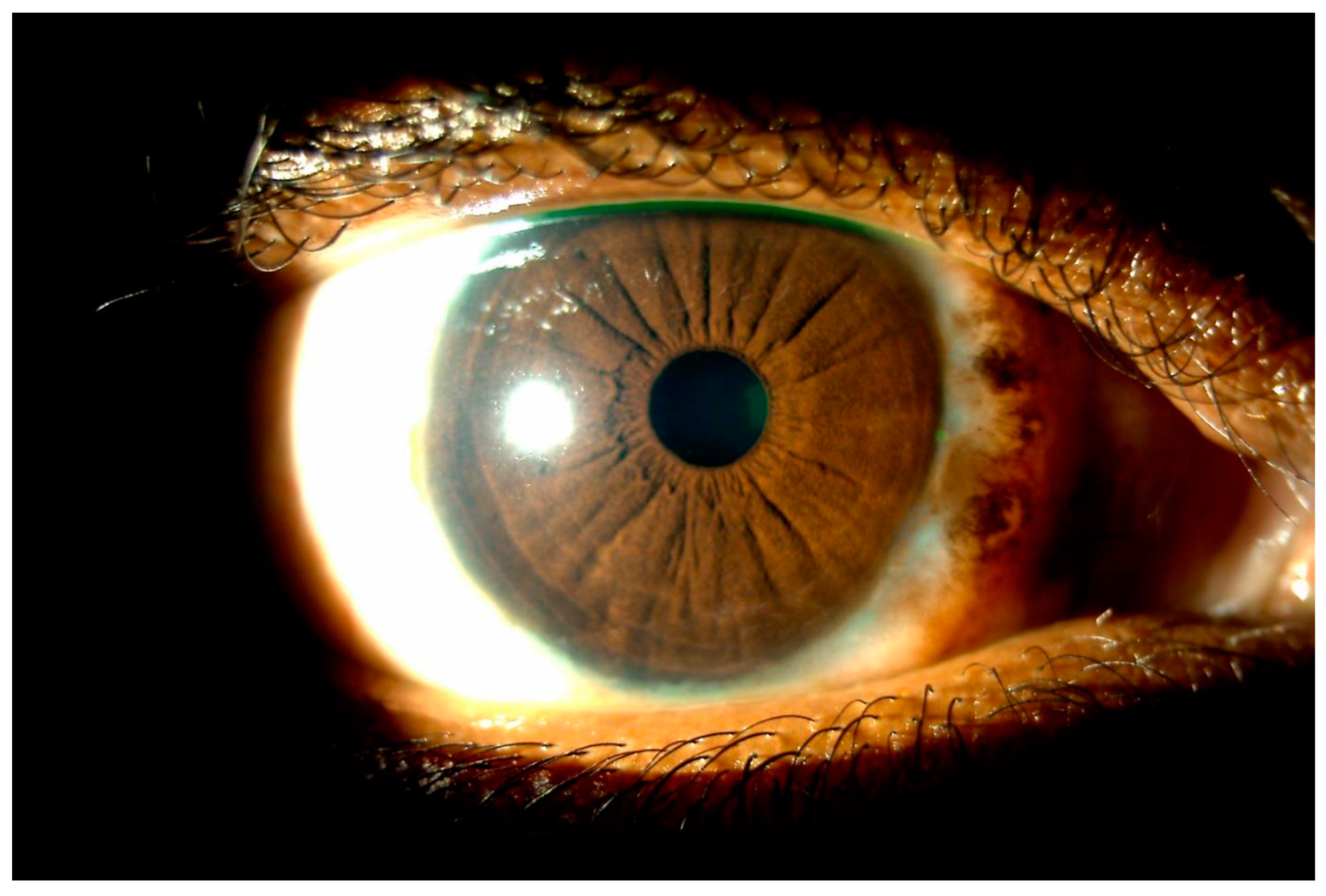
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hehar, N.K.; Chigbu, D.I. Vernal Keratoconjunctivitis: Immunopathological Insights and Therapeutic Applications of Immunomodulators. Life 2024, 14, 361. https://doi.org/10.3390/life14030361
Hehar NK, Chigbu DI. Vernal Keratoconjunctivitis: Immunopathological Insights and Therapeutic Applications of Immunomodulators. Life. 2024; 14(3):361. https://doi.org/10.3390/life14030361
Chicago/Turabian StyleHehar, Navpreet K., and DeGaulle I. Chigbu. 2024. "Vernal Keratoconjunctivitis: Immunopathological Insights and Therapeutic Applications of Immunomodulators" Life 14, no. 3: 361. https://doi.org/10.3390/life14030361
APA StyleHehar, N. K., & Chigbu, D. I. (2024). Vernal Keratoconjunctivitis: Immunopathological Insights and Therapeutic Applications of Immunomodulators. Life, 14(3), 361. https://doi.org/10.3390/life14030361





