From Satirical Poems and Invisible Poisons to Radical Surgery and Organized Cervical Cancer Screening—A Historical Outline of Cervical Carcinoma and Its Relation to HPV Infection
Abstract
1. Introduction
2. Materials and Methods
3. From Ancient Promiscuity to Microscopic Diagnosis
4. The Presence of Oncogenic Viruses: From “Invisible Poison” to Viral Genetics
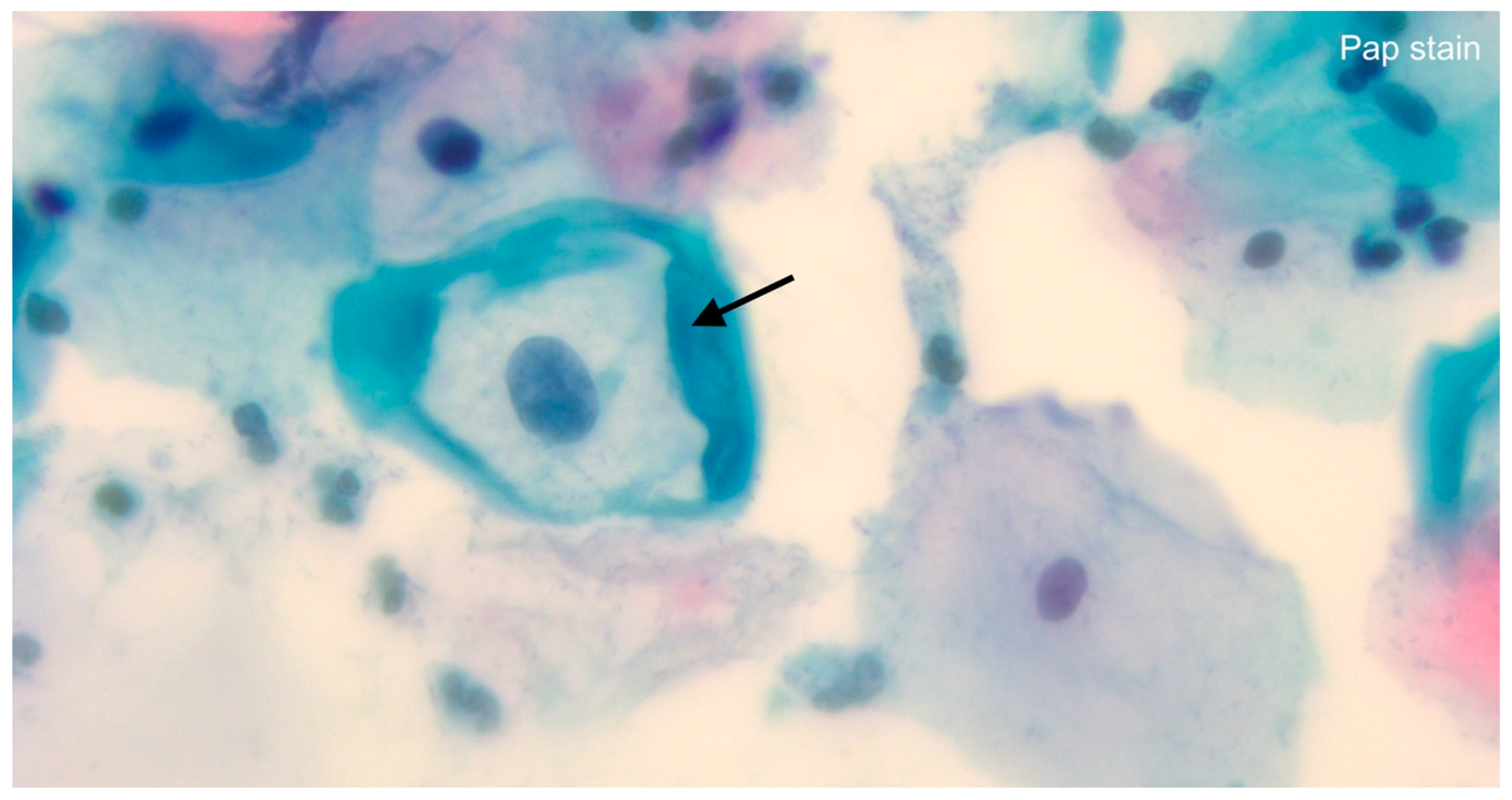
5. The HPV Test and Its Role in Cancer Screening
6. The Future of Cervical Cancer Screening—Quo Vadimus?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Daniyal, M.; Akhtar, N.; Ahmad, S.; Fatima, U.; Akram, M.; Asif, H.M. Update Knowledge on Cervical Cancer Incidence and Prevalence in Asia. Asian Pac. J. Cancer Prev. 2015, 16, 3617–3620. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 22 February 2024).
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) Prävention Des Zervixkarzinoms, Langversion 1.1. Available online: http://www.leitlinienprogramm-onkologie.de/leitlinien/zervixkarzinom-praevention/ (accessed on 17 January 2024).
- Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V.; Robert Koch-Institut. Krebs in Deutschland Für 2019/2020. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/krebs_in_deutschland_node.html (accessed on 17 January 2024).
- AWMF; Deutsche Krebshilfe; Deutsche Krebsgesellschaft. S3-Leitlinie Diagnostik, Therapie Und Nachsorge Der Patientin Mit Zervixkarzinom, Langversion, 2.2. Available online: https://register.awmf.org/de/leitlinien/detail/032-033OL (accessed on 17 January 2024).
- IARC. Cervix Cancer Screening; IARC: Lyon, France, 2005; Volume 10, ISBN 978-92-832-3010-6. [Google Scholar]
- Day, N.E. Review Article: Screening for Cancer of the Cervix. J. Epidemiol. Community Health (1979-) 1989, 43, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Lăără, E.; Day, N.; Hakama, M. Trends in mortality from cervical cancer in the nordic countries: association with organised screening programmes. Lancet 1987, 329, 1247–1249. [Google Scholar] [CrossRef]
- Sankila, R.; Démaret, E.; Hakama, M.; Lynge, E.; Schouten, L.J.; Parkin, D.M. Evaluation and Monitoring of Screening Programmes; European Commission: Luxembourg, 2001; ISBN 978-92-894-0253-8. [Google Scholar]
- Rose, P.G.; Mahdi, H. Landmark Studies of Therapeutic Vaccination in Cervical and Ovarian Cancers. Lancet Oncol. 2020, 21, 1549–1550. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical Cancer Therapies: Current Challenges and Future Perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Gottschlich, A.; van Niekerk, D.; Smith, L.W.; Gondara, L.; Melnikow, J.; Cook, D.A.; Lee, M.; Stuart, G.; Martin, R.E.; Peacock, S.; et al. Assessing 10-Year Safety of a Single Negative HPV Test for Cervical Cancer Screening: Evidence from FOCAL-DECADE Cohort. Cancer Epidemiol. Biomark. Prev. 2021, 30, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, S.I. A Note from History: Landmarks in History of Cancer, Part 1. Cancer 2011, 117, 1097–1102. [Google Scholar] [CrossRef]
- Jenkins, D. Chapter 1—A Brief History of Cervical Cancer. In Human Papillomavirus; Jenkins, D., Bosch, F.X., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–12. ISBN 978-0-12-814457-2. [Google Scholar]
- Oriel, J.D. Anal Warts and Anal Coitus. Br. J. Vener. Dis. 1971, 47, 373–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renner, C. À propos du spéculum d’étain de Récamier. Hist. Des Sci. Médicales 2006, 40, 345–350. [Google Scholar] [PubMed]
- Bliquez, L.J. Gynecology in Pompeii. In Ancient Medicine in Its Socio-Cultural Context; Brill: Leiden, The Netherlands, 1995; Volume 1, pp. 209–223. ISBN 978-90-04-41837-0. [Google Scholar]
- Osiander, F.B. Observations on the Cure of Cancer of the Womb by Excision. Edinb. Med. Surg. J. 1816, 12, 286–294. [Google Scholar] [PubMed]
- Senn, N. The early history of vaginal hysterectomy. J. Am. Med. Assoc. 1895, XXV, 476–482. [Google Scholar] [CrossRef][Green Version]
- DiMaio, D. Nuns, Warts, Viruses, and Cancer. Yale J. Biol. Med. 2015, 88, 127–129. [Google Scholar]
- Ebert, A.D.; David, M. „Was ihn vor allem charakterisierte, war seine …ungewöhnliche Klugheit“—Zum 100. Todestag von Johann Veit (1852–1917). Geburtshilfe Frauenheilkd. 2017, 77, 949–951. [Google Scholar] [CrossRef]
- Zander, J. Milestones in Gynecology and Obstetrics; Ludwig, H., Thomsen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Freund, W.A. Zu Meiner Methode der Totalen Uterus-Exstirpation; Breitkopf und Härtel: Leipzig, Germany, 1878; Volume 12, pp. 265–269. [Google Scholar]
- Toellner, R. Illustrierte Geschichte der Medizin; Andreas Verlag: Vaduz, Liechtenstein, 1992; pp. 1307–1308. ISBN 3-86070-204-1. [Google Scholar]
- Dursun, P.; Gultekin, M.; Ayhan, A. The History of Radical Hysterectomy. J. Low. Genit. Tract. Dis. 2011, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Yumpu.com Hysterektomie von der Antike bis Heute—Frauenarzt. Available online: https://www.yumpu.com/de/document/read/8470067/hysterektomie-von-der-antike-bis-heute-frauenarzt (accessed on 22 February 2024).
- Dargent, D.; Mathevet, P. 4 Schauta’s Vaginal Hysterectomy Combined with Laparoscopic Lymphadenectomy. BailliÈre’S Clin. Obstet. Gynaecol. 1995, 9, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Possover, M. Options for Laparoscopic Surgery in Cervical Carcinomas. Eur. J. Gynaecol. Oncol. 2003, 24, 471–472. [Google Scholar]
- Höckel, M.; Horn, L.-C.; Hentschel, B.; Höckel, S.; Naumann, G. Total Mesometrial Resection: High Resolution Nerve-Sparing Radical Hysterectomy Based on Developmentally Defined Surgical Anatomy. Int. J. Gynecol. Cancer 2003, 13, 791–803. [Google Scholar] [CrossRef]
- Kimmig, R. “Robotic surgery“ beim Zervixkarzinom. Gynäkologe 2012, 45, 707–713. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; de Kroon, C.; Van Driel, W.; Tidy, J.; Marth, C.; et al. An International Randomized Phase III Trial Comparing Radical Hysterectomy and Pelvic Node Dissection (RH) vs. Simple Hysterectomy and Pelvic Node Dissection (SH) in Patients with Low-Risk Early-Stage Cervical Cancer (LRESCC): A Gynecologic Cancer Intergroup Study Led by the Canadian Cancer Trials Group (CCTG CX.5-SHAPE). JCO 2023, 41, LBA5511. [Google Scholar] [CrossRef]
- Hinselmann, H. Verbesserung Der Inspektionsmöglichkeiten von Vulva, Vagina Und Portio. Münch Med. Wschr 1925, 72, 1733. [Google Scholar]
- Hübner, J. Kolposkopie ohne Menschlichkeit?! Hinselmann und die Versuche an Frauen in Auschwitz. Geburtshilfe Frauenheilkd. 2016, 76, A11. [Google Scholar] [CrossRef]
- Reich, O.; Pickel, H. 100 Years of Iodine Testing of the Cervix: A Critical Review and Implications for the Future. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 261, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, A.; Magiorkinis, E. Pioneers of Exfoliative Cytology in the 19th Century: The Predecessors of George Papanicolaou. Cytopathology 2014, 25, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Tatsumura, Y. George Papanicolaou (1883–1962): Discoverer of the Pap Smear. Singap. Med. J. 2015, 56, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Swailes, A.L.; Hossler, C.E.; Kesterson, J.P. Pathway to the Papanicolaou Smear: The Development of Cervical Cytology in Twentieth-Century America and Implications in the Present Day. Gynecol. Oncol. 2019, 154, 3–7. [Google Scholar] [CrossRef]
- Stockard, C.R.; Papanicolaou, G.N. The Existence of a Typical Oestrous Cycle in the Guinea-Pig—With a Study of Its Histological and Physiological Changes. Am. J. Anat. 1917, 22, 225–283. [Google Scholar] [CrossRef]
- Race Betterment Foundation. Race Betterment Foundation Proceedings of the Third Race Betterment Conference.; Battle Creek, MI, 1928. In Proceedings of the Third Race Betterment Conference, Battle Creek, MI, USA, 2 January 1928; Race Betterment Foundation: Battle Creek, MI, USA, 2 January 1928. [Google Scholar]
- Chantziantoniou, N.; Donnelly, A.D.; Mukherjee, M.; Boon, M.E.; Austin, R.M. Inception and Development of the Papanicolaou Stain Method. Acta Cytol. 2017, 61, 266–280. [Google Scholar] [CrossRef]
- Shaw, P.A. The History of Cervical Screening I: The Pap. Test. J. SOGC 2000, 22, 110–114. [Google Scholar] [CrossRef]
- Martin, C.E. Epidemiology of Cancer of the Cervix. II. Marital and Coital Factors in Cervical Cancer. Am. J. Public Health Nations Health 1967, 57, 803–814. [Google Scholar] [CrossRef]
- Buckley, J.D.; Doll, R.; Harris, R.W.C.; Vessey, M.P.; Williams, P.T. Case-control study of the husbands of women with dysplasia or carcinoma of the cervix uteri. Lancet 1981, 318, 1010–1015. [Google Scholar] [CrossRef]
- Elliott, R.I.K. On the prevention of carcinoma of the cervix. Lancet 1964, 283, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Beral, V. Cancer of the cervix: A sexually transmitted infection? Lancet 1974, 303, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Hochman, A.; Ratzkowski, E.; Schreiber, H. Incidence of Carcinoma of the Cervix in Jewish Women in Israel. Br. J. Cancer 1955, 9, 358–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lechevalier, H. Dmitri Iosifovich Ivanovski (1864–1920). Bacteriol. Rev. 1972, 36, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, N. On the Historical Significance of Beijerinck and His Contagium Vivum Fluidum for Modern Virology. Hist. Philos. Life Sci. 2018, 40, 41. [Google Scholar] [CrossRef]
- Ryu, W.-S. Discovery and Classification. Mol. Virol. Hum. Pathog. Viruses 2017, 3–20. [Google Scholar] [CrossRef]
- Van Epps, H.L. Peyton Rous. J. Exp. Med. 2005, 201, 320. [Google Scholar] [CrossRef]
- Rous, P.; Kidd, J.G.; Beard, J.W. Observations on the relation of the virus causing rabbit papillomas to the cancers deriving therefrom. J. Exp. Med. 1936, 64, 385–400. [Google Scholar] [CrossRef][Green Version]
- Ito, Y.; Evans, C.A. Induction of tumors in domestic rabbits with nucleic acid preparations from partially purified shope papilloma virus and from extracts of the papillomas of domestic and cottontail rabbits. J. Exp. Med. 1961, 114, 485–500. [Google Scholar] [CrossRef]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus particles in cultured lymphoblasts from burkitt’s lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Almeida, J.; Goffe, A. Antibody to wart virus in human sera demonstrated by electron microscopy and precipitin tests. Lancet 1965, 286, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Rawls, W.E.; Tompkins, W.A.; Figueroa, M.E.; Melnick, J.L. Herpesvirus Type 2: Association with Carcinoma of the Cervix. Science 1968, 161, 1255–1256. [Google Scholar] [CrossRef] [PubMed]
- Naib, Z.M.; Nahmias, A.J.; Josey, W.E.; Kramer, J.H. Genital Herpetic Infection Association with Cervical Dysplasia and Carcinoma. Cancer 1969, 23, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Hausen, H.Z.; Meinhof, W.; Scheiber, W.; Bornkamm, G.W. Attempts to Detect Virus-Specific DNA in Human Tumors. I. Nucleic Acid Hybridizations with Complementary RNA of Human Wart Virus. Int. J. Cancer 1974, 13, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Vonka, V.; Kaňka, J.; Jelínek, J.; Šubrt, I.; Suchánek, A.; Havránková, A.; Váchal, M.; Hirsch, I.; Domorázková, E.; Závadová, H.; et al. Prospective Study on the Relationship between Cervical Neoplasia and Herpes Simplex Type-2 Virus. I. Epidemiological Characteristics. Int. J. Cancer 1984, 33, 49–60. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Condylomata Acuminata and Human Genital Cancer. Cancer Res. 1976, 36, 794. [Google Scholar] [PubMed]
- zur Hausen, H. Human Papillomaviruses and Their Possible Role in Squamous Cell Carcinomas. In Current Topics in Microbiology and Immunology; Arber, W., Henle, W., Hofschneider, P.H., Humphrey, J.H., Klein, J., Koldovský, P., Koprowski, H., Maaløe, O., Melchers, F., Rott, R., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 1–30. ISBN 978-3-642-66800-5. [Google Scholar]
- Dürst, M.; Gissmann, L.; Ikenberg, H.; zur Hausen, H. A Papillomavirus DNA from a Cervical Carcinoma and Its Prevalence in Cancer Biopsy Samples from Different Geographic Regions. Proc. Natl. Acad. Sci. USA 1983, 80, 3812–3815. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomaviruses—To Vaccination and Beyond. Biochem. Mosc. 2008, 73, 498–503. [Google Scholar] [CrossRef]
- Boshart, M.; Gissmann, L.; Ikenberg, H.; Kleinheinz, A.; Scheurlen, W.; zur Hausen, H. A New Type of Papillomavirus DNA, Its Presence in Genital Cancer Biopsies and in Cell Lines Derived from Cervical Cancer. EMBO J. 1984, 3, 1151–1157. [Google Scholar] [CrossRef]
- Gissmann, L.; Boshart, M.; Dürst, M.; Ikenberg, H.; Wagner, D.; Hausen, H.Z. Presence of Human Papillomavirus in Genital Tumors. J. Investig. Dermatol. 1984, 83, S26–S28. [Google Scholar] [CrossRef][Green Version]
- Werness, B.A.; Levine, A.J.; Howley, P.M. Association of Human Papillomavirus Types 16 and 18 E6 Proteins with P53. Science 1990, 248, 76–79. [Google Scholar] [CrossRef]
- Schwarz, E.; Freese, U.K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; Hausen, H. zur Structure and Transcription of Human Papillomavirus Sequences in Cervical Carcinoma Cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef]
- Hurlin, P.J.; Kaur, P.; Smith, P.P.; Perez-Reyes, N.; Blanton, R.A.; McDougall, J.K. Progression of Human Papillomavirus Type 18-Immortalized Human Keratinocytes to a Malignant Phenotype. Proc. Natl. Acad. Sci. USA 1991, 88, 570–574. [Google Scholar] [CrossRef]
- Reeves, W.C.; Rawls, W.E.; Brinton, L.A. Epidemiology of Genital Papillomaviruses and Cervical Cancer. Rev. Infect. Dis. 1989, 11, 426–439. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X. HPV and Cervical Neoplasia: Review of Case-Control and Cohort Studies. IARC Sci. Publ. 1992, 119, 251–261. [Google Scholar] [PubMed]
- IARC. Human Papillomaviruses; IARC Publications: Lyon, France, 1995; ISBN 978-92-832-1264-5. [Google Scholar]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and Cervical Cancer: A Review of Epidemiology and Screening Uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Schiller, J.T. Human Papillomavirus Vaccines. J. Infect. Dis. 2021, 224, S367–S378. [Google Scholar] [CrossRef]
- Jeannot, E.; Ben Abdeljelil, H.; Viviano, M. HPV Vaccination for Cervical Cancer Prevention in Switzerland. Encyclopedia 2023, 3, 512–519. [Google Scholar] [CrossRef]
- de Villiers, E.M. Heterogeneity of the Human Papillomavirus Group. J. Virol. 1989, 63, 4898–4903. [Google Scholar] [CrossRef]
- The Nobel Prize in Physiology or Medicine. 2008. Available online: https://www.nobelprize.org/prizes/medicine/2008/hausen/biographical/ (accessed on 11 February 2024).
- Research, C. for B.E. and Approved Products—June 8, 2006 Approval Letter—Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant. Available online: http://wayback.archive-it.org/7993/20170722145339/https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm111283.htm (accessed on 20 January 2024).
- Cervarix|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cervarix#authorisation-details-section (accessed on 20 January 2024).
- Research, C. for B.E. and Approved Products–December 10, 2014 Approval Letter-GARDASIL 9. Available online: https://wayback.archive-it.org/7993/20190425005410/https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426520.htm (accessed on 20 January 2024).
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 Major Capsid Protein Self-Assembles into Virus-like Particles That Are Highly Immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef]
- Erman, M.; Leo, L. Merck Raises 2023 Sales Forecast as Top Drugs Beat Street Estimates. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/merck-posts-narrower-than-expected-loss-sales-top-drugs-beat-street-estimates-2023-08-01/ (accessed on 20 January 2024).
- Akhatova, A.; Azizan, A.; Atageldiyeva, K.; Ashimkhanova, A.; Marat, A.; Iztleuov, Y.; Suleimenova, A.; Shamkeeva, S.; Aimagambetova, G. Prophylactic Human Papillomavirus Vaccination: From the Origin to the Current State. Vaccines 2022, 10, 1912. [Google Scholar] [CrossRef]
- New HPV Vaccine from Innovax Receives WHO Prequalification. Available online: https://www.path.org/our-impact/media-center/new-hpv-vaccine-innovax-receives-who-prequalification/ (accessed on 8 February 2024).
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global Estimates of Human Papillomavirus Vaccination Coverage by Region and Income Level: A Pooled Analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Zou, Z.; Fairley, C.K.; Ong, J.J.; Hocking, J.; Canfell, K.; Ma, X.; Chow, E.P.F.; Xu, X.; Zhang, L.; Zhuang, G. Domestic HPV Vaccine Price and Economic Returns for Cervical Cancer Prevention in China: A Cost-Effectiveness Analysis. Lancet Glob. Health 2020, 8, e1335–e1344. [Google Scholar] [CrossRef]
- WHO Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://www.who.int/publications-detail-redirect/9789240014107 (accessed on 21 January 2024).
- Hogarth, S.; Hopkins, M.; Rotolo, D. Technological Accretion in Diagnostics: HPV Testing and Cytology in Cervical Cancer Screening. In Medical Innovation: Science, Technology and Practice; Consoli, D., Mina, A., Nelson, R.R., Ramlogan, R., Eds.; Wellcome Trust–Funded Monographs and Book Chapters; Routledge: New York, NY, USA, 2015; ISBN 978-1-138-86034-6. [Google Scholar]
- Corliss, J. Utility of ViraPapRemains to Be Established. JNCI J. Natl. Cancer Inst. 1990, 82, 252–253. [Google Scholar] [CrossRef]
- Saraiya, M.; Steben, M.; Watson, M.; Markowitz, L. Evolution of Cervical Cancer Screening and Prevention in United States and Canada: Implications for Public Health Practitioners and Clinicians. Prev. Med. 2013, 57, 426–433. [Google Scholar] [CrossRef]
- Wright, J.; Thomas, C.; Cox, J.T.; Massad, L.S.; Twiggs, L.B.; Wilkinson, E.J. 2001 ASCCP-Sponsored Consensus Conference 2001 Consensus Guidelines for the Management of Women With Cervical Cytological Abnormalities. JAMA 2002, 287, 2120–2129. [Google Scholar] [CrossRef]
- Solomon, D.; Schiffman, M.; Tarone, R.; For the ALTS Group. Comparison of Three Management Strategies for Patients With Atypical Squamous Cells of Undetermined Signifi-cance: Baseline Results From a Randomized Trial. JNCI J. Natl. Cancer Inst. 2001, 93, 293–299. [Google Scholar] [CrossRef]
- Cain, J.M.; Howett, M.K. Preventing Cervical Cancer. Science 2000, 288, 1753–1755. [Google Scholar] [CrossRef]
- Herbst, A.L.; Pickett, K.E.; Follen, M.; Noller, K.L. The Management of ASCUS Cervical Cytologic Abnormalities and HPV Testing: A Cautionary Note. Obstet. Gynecol. 2001, 98, 849–851. [Google Scholar] [CrossRef]
- Premarket Approval (PMA). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P890064S009 (accessed on 20 January 2024).
- ACOG Practice Bulletin. Int. J. Gynecol. Obstet. 2003, 83, 237–247. [CrossRef]
- Saslow, D.; Runowicz, C.D.; Solomon, D.; Moscicki, A.-B.; Smith, R.A.; Eyre, H.J.; Cohen, C. American Cancer Society Guideline for the Early Detection of Cervical Neoplasia and Cancer. CA A Cancer J. Clin. 2002, 52, 342–362. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force Screening for Cervical Cancer: Recommendations and Rationale. Am. J. Nurs. 2003, 103, 101–102, 105–106, 108–109.
- Rosenwald, M.S. Digene’s Ads Take Their Case to Women; Washington Post: Washington, DC, USA, 2005. [Google Scholar]
- Luechtefeld, L. Driving Awareness. Available online: https://www.mddionline.com/medical-device-markets/driving-awareness (accessed on 20 January 2024).
- Committee on Practice Bulletins—Gynecology ACOG Practice Bulletin Number 131: Screening for Cervical Cancer. Obstet. Gynecol. 2012, 120, 1222–1238. [CrossRef]
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.M.; Garcia, F.A.R.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C.; et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. J. Low. Genit. Tract. Dis. 2012, 16, 175–204. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2012, 156, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Roche Roche’s Cobas HPV Test Receives FDA Approval for First-Line Cervical Cancer Screening Using SurePath Preservative Fluid. Available online: https://www.prnewswire.com/news-releases/roches-cobas-hpv-test-receives-fda-approval-for-first-line-cervical-cancer-screening-using-surepath-preservative-fluid-300688266.html (accessed on 20 January 2024).
- Luu, H.N.; Adler-Storthz, K.; Dillon, L.M.; Follen, M.; Scheurer, M.E. Comparing the Performance of Hybrid Capture II and Polymerase Chain Reaction (PCR) for the Identification of Cervical Dysplasia in the Screening and Diagnostic Settings. Clin. Med. Insights Oncol. 2013, 7, 247–255. [Google Scholar] [CrossRef]
- Qiagen. Qiagen Digene® HC2 Sample Conversion Kit Instructions for Use; QIAGEN Sciences Inc.: Germantown, MD, USA, 2013. [Google Scholar]
- IQWiG HPV-Test: Hinweise auf Nutzen im Primärscreening|IQWiG.de. Available online: https://www.iqwig.de/presse/pressemitteilungen/pressemitteilungen-detailseite_10827.html (accessed on 21 January 2024).
- IQWiG Einladung und Entscheidungshilfe Zervixkarzinom-Screening: IQWiG Legt Finale Fassung vor. Available online: https://www.iqwig.de/presse/pressemitteilungen/pressemitteilungen-detailseite_10228.html (accessed on 21 January 2024).
- Gemeinsamer Bundesausschuss Richtlinie Für Organisierte Krebsfrüherkennungsprogramme Und Krebsfrüherkennungs-Richtlinie: Programm zur Früherkennung von Zervixkarzinomen—Gemeinsamer Bundesausschuss. Available online: https://www.g-ba.de/beschluesse/3597/ (accessed on 21 January 2024).
- WHO. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention. Available online: https://www.who.int/publications-detail-redirect/9789240030824 (accessed on 21 January 2024).
- European Comission Proposal for a Council Recommendation (CR) on Strengthening Prevention through Early Detection: A New Approach on Cancer Screening Replacing CR 2003/878/EC—European Commission. Available online: https://health.ec.europa.eu/publications/proposal-council-recommendation-cr-strengthening-prevention-through-early-detection-new-approach_en (accessed on 21 January 2024).
- US Preventive Services Task Force Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674–686. [CrossRef]
- ACOG Updated Cervical Cancer Screening Guidelines. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines (accessed on 21 January 2024).
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.-C.T.; Walter, L.C.; et al. Cervical Cancer Screening for Individuals at Average Risk: 2020 Guideline Update from the American Cancer Society. CA A Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- Shastri, S.S.; Temin, S.; Almonte, M.; Basu, P.; Campos, N.G.; Gravitt, P.E.; Gupta, V.; Lombe, D.C.; Murillo, R.; Nakisige, C.; et al. Secondary Prevention of Cervical Cancer: ASCO Resource–Stratified Guideline Update. JCO Glob. Oncol. 2022, 8, e2200217. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, L.; Yang, R.; Meng, Y.; Qiu, L. Ki-67 and P16 Proteins in Cervical Cancer and Precancerous Lesions of Young Women and the Diagnostic Value for Cervical Cancer and Precancerous Lesions. Oncol. Lett. 2019, 18, 1351–1355. [Google Scholar] [CrossRef]
- Ogilvie, G.S.; Patrick, D.M.; Schulzer, M.; Sellors, J.W.; Petric, M.; Chambers, K.; White, R.; FitzGerald, J.M. Diagnostic Accuracy of Self Collected Vaginal Specimens for Human Papillomavirus Compared to Clinician Collected Human Papillomavirus Specimens: A Meta-Analysis. Sex. Transm. Infect. 2005, 81, 207–212. [Google Scholar] [CrossRef]
- Shaikh, R.; Daniel, A.; Lyng, F.M. Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review. Molecules 2023, 28, 2502. [Google Scholar] [CrossRef]
- Ramos, I.R.; Meade, A.D.; Ibrahim, O.; Byrne, H.J.; McMenamin, M.; McKenna, M.; Malkin, A.; Lyng, F.M. Raman Spectroscopy for Cytopathology of Exfoliated Cervical Cells. Faraday Discuss. 2016, 187, 187–198. [Google Scholar] [CrossRef]
- Zheng, C.; Qing, S.; Wang, J.; Lü, G.; Li, H.; Lü, X.; Ma, C.; Tang, J.; Yue, X. Diagnosis of Cervical Squamous Cell Carcinoma and Cervical Adenocarcinoma Based on Raman Spectroscopy and Support Vector Machine. Photodiagnosis Photodyn. Ther. 2019, 27, 156–161. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.-X.; Ma, C.-L.; Zheng, X.-X.; Lv, X.-Y.; Lv, G.-D.; Tang, J.; Wu, G.-H. Raman Spectroscopic Study of Cervical Precancerous Lesions and Cervical Cancer. Lasers Med. Sci. 2021, 36, 1855–1864. [Google Scholar] [CrossRef]
- Hou, X.; Shen, G.; Zhou, L.; Li, Y.; Wang, T.; Ma, X. Artificial Intelligence in Cervical Cancer Screening and Diagnosis. Front. Oncol. 2022, 12, 851367. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Bell, D.; Antani, S.; Xue, Z.; Yu, K.; Horning, M.P.; Gachuhi, N.; Wilson, B.; Jaiswal, M.S.; Befano, B.; et al. An Observational Study of Deep Learning and Automated Evaluation of Cervical Images for Cancer Screening. JNCI J. Natl. Cancer Inst. 2019, 111, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Ng, M.T.A.; Qiao, Y. The Challenges of Colposcopy for Cervical Cancer Screening in LMICs and Solutions by Artificial Intelligence. BMC Med. 2020, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Cardona, H.D.; Rodriguez-Lopez, M.; Arrivillaga, M.; Vergara-Sanchez, C.; García-Cifuentes, J.P.; Bermúdez, P.C.; Jaramillo-Botero, A. Artificial Intelligence for Cervical Cancer Screening: Scoping Review, 2009–2022. Int. J. Gynecol. Obstet. 2023. [Google Scholar] [CrossRef]
- Liang, P.; Sun, G.; Wei, S. Application of Deep Learning Algorithm in Cervical Cancer MRI Image Segmentation Based on Wireless Sensor. J. Med. Syst. 2019, 43, 156. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, S.; Zhang, S.; Wang, M.; Ding, Y.; Fang, J.; Wu, Q.; Qian, W.; Liu, Z.; Sun, K.; et al. Development of a Deep Learning Model to Identify Lymph Node Metastasis on Magnetic Resonance Imaging in Patients With Cervical Cancer. JAMA Netw. Open 2020, 3, e2011625. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, A.; Plesa, A.; Fudulu, A.; Iancu, I.V.; Anton, G.; Botezatu, A. Epigenetic Approaches for Cervical Neoplasia Screening (Review). Exp. Ther. Med. 2021, 22, 1481. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-González, A.; Lizano, M.; Candelaria, M.; Cetina, L.; Arce, C.; Cervera, E. Epigenetics of Cervical Cancer. An Overview and Therapeutic Perspectives. Mol. Cancer 2005, 4, 38. [Google Scholar] [CrossRef]
- Huang, Y.; Song, H.; Hu, H.; Cui, L.; You, C.; Huang, L. Trichosanthin Inhibits DNA Methyltransferase and Restores Methylation-Silenced Gene Expression in Human Cervical Cancer Cells. Mol. Med. Rep. 2012, 6, 872–878. [Google Scholar] [CrossRef]
- Cervical Cancer Immunotherapy|Immune Checkpoint Inhibitors. Available online: https://www.cancer.org/cancer/types/cervical-cancer/treating/immunotherapy.html (accessed on 22 February 2024).
- Ding, H.; Zhang, J.; Zhang, F.; Xu, Y.; Yu, Y.; Liang, W.; Li, Q. Effectiveness of Combination Therapy with ISA101 Vaccine for the Treatment of Human Papillomavirus-Induced Cervical Cancer. Front. Oncol. 2022, 12, 990877. [Google Scholar] [CrossRef]
- Ladbury, C.; Germino, E.; Novak, J.; Liu, J.; Horne, Z.; Dyer, B.; Glaser, S. Combination Radiation and Immunotherapy in Gynecologic Malignancies—A Comprehensive Review. Transl. Cancer Res. 2021, 10, 2609–2619. [Google Scholar] [CrossRef]


| HPV Types | IARC Classification (2012) |
|---|---|
| 16; 18; 31; 33; 35; 39; 45; 51; 52; 56; 58; 59 | 1 (carcinogenic; high risk) |
| 68 | 2a (probably carcinogenic) |
| 26; 30; 34; 53; 66; 67; 69; 70; 73; 82; 85; 97 | 2b (possibly carcinogenic) |
| Gamma-HPV; Beta-HPV; 6; 11 | 3 (not classifiable) |
| 40; 42; 43; 44; 54; 61; 72; 81 | low-risk types |
| HPV Test | HPV Types | Technique |
|---|---|---|
| Digene Hybrid Capture 2 High-Risk HPV DNA Test (QIAGEN, Gaithersburg, Inc.) | 16; 18; 31; 33; 35; 39; 45; 51; 52; 56; 58; 59; 68 | DNA: whole genome |
| COBAS HPV Test (Roche Diagnostics) | 16; 18; 31; 33;35; 39; 45; 51; 52; 56; 58; 59; 66; 68 | DNA: L1 |
| Cervista™ HPV HR and GenFind™ DNA Extraction Kit (Hologic) | 16; 18; 31; 33; 35; 39; 45; 51; 52; 56; 58; 59; 66; 68 | DNA E6/E7/L1 |
| APTIMA HPV Assay (Hologic) | 16; 18; 31; 33; 35; 39; 45; 51; 52; 56; 58; 59; 66; 68 | RNA E6/E7 |
| Abbot RT High-Risk HPV Test | 16; 18; 31; 33;35; 39; 45; 51; 52; 56; 58; 59; 66; 68 | DNA L1 |
| BD Onclarity HPV Test | 16; 18; 31; 45; 51; 52; 33–58; 56–59–66; 35; 39–68 | DNA E6/E7 |
| PapilloCheck® HPV Test | 16; 18; 31; 33; 35; 39; 45; 51; 52; 53; 56; 58; 59; 66; 68; 70; 73; 82; 6; 11; 40; 42; 43; 44 | DNA E1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, L.; Klamminger, G.G.; Bier, B.; Eltze, E. From Satirical Poems and Invisible Poisons to Radical Surgery and Organized Cervical Cancer Screening—A Historical Outline of Cervical Carcinoma and Its Relation to HPV Infection. Life 2024, 14, 307. https://doi.org/10.3390/life14030307
Jung L, Klamminger GG, Bier B, Eltze E. From Satirical Poems and Invisible Poisons to Radical Surgery and Organized Cervical Cancer Screening—A Historical Outline of Cervical Carcinoma and Its Relation to HPV Infection. Life. 2024; 14(3):307. https://doi.org/10.3390/life14030307
Chicago/Turabian StyleJung, Leonard, Gilbert Georg Klamminger, Bert Bier, and Elke Eltze. 2024. "From Satirical Poems and Invisible Poisons to Radical Surgery and Organized Cervical Cancer Screening—A Historical Outline of Cervical Carcinoma and Its Relation to HPV Infection" Life 14, no. 3: 307. https://doi.org/10.3390/life14030307
APA StyleJung, L., Klamminger, G. G., Bier, B., & Eltze, E. (2024). From Satirical Poems and Invisible Poisons to Radical Surgery and Organized Cervical Cancer Screening—A Historical Outline of Cervical Carcinoma and Its Relation to HPV Infection. Life, 14(3), 307. https://doi.org/10.3390/life14030307







