Second Trimester Amniotic Fluid Angiotensinogen Levels Linked to Increased Fetal Birth Weight and Shorter Gestational Age in Term Pregnancies
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obs. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Botsis, D.; Iliodromiti, Z. The fetus that is small for gestational age. Ann. N. Y. Acad. Sci. 2006, 1092, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Unterscheider, J.; O’Donoghue, K.; Malone, F.D. Guidelines on fetal growth restriction: A comparison of recent national publications. Am. J. Perinatol. 2015, 32, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Powel, J.E.; Zantow, E.W.; Bialko, M.F.; Farley, L.G.; Lawlor, M.L.; Mullan, S.J.; Vricella, L.K.; Tomlinson, T.M. Predictive index for adverse perinatal outcome in pregnancies complicated by fetal growth restriction. Ultrasound Obstet. Gynecol. 2023, 61, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Unterscheider, J.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Definition and management of fetal growth restriction: A survey of contemporary attitudes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 174, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Huppi, P.S.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Gratacós, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef] [PubMed]
- Pergialiotis, V.; Bellos, I.; Fanaki, M.; Vrachnis, N.; Doumouchtsis, S.K. Risk factors for severe perineal trauma during childbirth: An updated meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 94–100. [Google Scholar] [CrossRef]
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Botsis, D.; Vrachnis, N.; Christodoulakos, G. Doppler assessment of the intrauterine growth-restricted fetus. Ann. N. Y. Acad. Sci. 2006, 1092, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Pringle, K.G.; Tadros, M.A.; Callister, R.J.; Lumbers, E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: Roles in trophoblast invasion and angiogenesis? Placenta 2011, 32, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Powers, R.W.; Rajakumar, A.; von Versen-Höynck, F.; Gallaher, M.J.; Lykins, D.L.; Roberts, J.M.; Hubel, C.A. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1009–E1016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vrachnis, N.; Loukas, N.; Vrachnis, D.; Antonakopoulos, N.; Christodoulaki, C.; Tsonis, O.; George, M.; Iliodromiti, Z. Phthalates and fetal growth velocity: Tracking down the suspected links. J. Matern. Fetal Neonatal Med. 2021, 35, 4985–4993. [Google Scholar] [CrossRef]
- Loukas, N.; Vrachnis, D.; Antonakopoulos, N.; Pergialiotis, V.; Mina, A.; Papoutsis, I.; Iavazzo, C.; Fotiou, A.; Stavros, S.; Valsamakis, G.; et al. Prenatal Exposure to Bisphenol A: Is There an Association between Bisphenol A in Second Trimester Amniotic Fluid and Fetal Growth? Medicina 2023, 59, 882. [Google Scholar] [CrossRef]
- Valias, G.R.; Gomes, P.R.L.; Amaral, F.G.; Alnuaimi, S.; Monteiro, D.; O’Sullivan, S.; Zangaro, R.; Cipolla-Neto, J.; Acuna, J.; Baltatu, O.C.; et al. Urinary Angiotensinogen-Melatonin Ratio in Gestational Diabetes and Preeclampsia. Front. Mol. Biosci. 2022, 9, 800638. [Google Scholar] [CrossRef]
- Colόn, N.S.; Pantho, A.F.; Afroze, S.H.; Ashraf, A.; Akter, R.; Kuehl, T.J.; Uddin, M.N. Reduced urinary angiotensinogen excretion in preeclampsia. Pregnancy Hypertens. 2022, 27, 1–5. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Yildirim, T.; Yilmaz, R.; Aybal-Kutlugun, A.; Altun, B.; Kucukozkan, T.; Erdem, Y. Association between urinary angiotensinogen, hypertension and proteinuria in pregnant women with preeclampsia. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 514–520. [Google Scholar] [CrossRef]
- Tamanna, S.; Morosin, S.K.; Delforce, S.J.; van Helden, D.F.; Lumbers, E.R.; Pringle, K.G. Renin-angiotensin system (RAS) enzymes and placental trophoblast syncytialisation. Mol. Cell. Endocrinol. 2022, 547, 111609. [Google Scholar] [CrossRef]
- Narita, T.; Ichihara, A.; Matsuoka, K.; Takai, Y.; Bokuda, K.; Morimoto, S.; Itoh, H.; Seki, H. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta 2016, 37, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Itakura, A.; Mizutani, S. Involvement of placental peptidases associated with renin-angiotensin systems in preeclampsia. Biochim. Biophys. Acta 2005, 1751, 68–72. [Google Scholar] [CrossRef]
- He, D.; Peng, X.; Xie, H.; Peng, R.; Li, Q.; Guo, Y.; Wang, W.; He, H.; Chen, Y. Genetic Variations in Angiotensinogen Gene and Risk of Preeclampsia: A Pilot Study. J. Clin. Med. 2023, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Bilardo, C.M.; Chalouhi, G.E.; Ghi, T.; Kagan, K.O.; Lau, T.K.; Papageorghiou, A.T.; Raine-Fenning, N.J.; Stirnemann, J.; et al. ISUOG practice guidelines: Performance of first-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2013, 41, 102–113. [Google Scholar] [CrossRef]
- IBM Corp. Released 2012. IBM SPSS Statistics for Windows; Version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Vrachnis, D.; Antonakopoulos, N.; Fotiou, A.; Pergialiotis, V.; Loukas, N.; Valsamakis, G.; Iavazzo, C.; Stavros, S.; Maroudias, G.; Panagopoulos, P.; et al. Is There a Correlation between Apelin and Insulin Concentrations in Early Second Trimester Amniotic Fluid with Fetal Growth Disorders? J. Clin. Med. 2023, 12, 3166. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Zygouris, D.; Vrachnis, D.; Antonakopoulos, N.; Fotiou, A.; Panagopoulos, P.; Kolialexi, A.; Pappa, K.; Mastorakos, G.; Iliodromiti, Z. Effects of Hormone Therapy and Flavonoids Capable on Reversal of Menopausal Immune Senescence. Nutrients 2021, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Merrill, D.C.; Neves, L.A.; Gruver, C.; Moorefield, C.; Brosnihan, K.B. Angiotensin II and angiotensin-(1-7) decrease sFlt1 release in normal but not preeclamptic chorionic villi: An in vitro study. Reprod. Biol. Endocrinol. 2010, 8, 135. [Google Scholar] [CrossRef]
- Anton, L.; Brosnihan, K.B. Systemic and uteroplacental renin--angiotensin system in normal and pre-eclamptic pregnancies. Ther. Adv. Cardiovasc. Dis. 2008, 2, 349–362. [Google Scholar] [CrossRef]
- Wu, W.B.; Xu, Y.Y.; Cheng, W.W.; Yuan, B.; Zhao, J.R.; Wang, Y.L.; Zhang, H.J. Decreased PGF may contribute to trophoblast dysfunction in fetal growth restriction. Reproduction 2017, 154, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Lumbers, E.R.; Delforce, S.J.; Arthurs, A.L.; Pringle, K.G. Causes and Consequences of the Dysregulated Maternal Renin-Angiotensin System in Preeclampsia. Front. Endocrinol. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lumbers, E.R.; Arthurs, A.L.; Corbisier de Meaultsart, C.; Mathe, A.; Avery-Kiejda, K.A.; Roberts, C.T.; Pipkin, F.B.; Marques, F.Z.; Morris, B.J.; et al. Regulation of the human placental (pro)renin receptor-prorenin-angiotensin system by microRNAs. Mol. Hum. Reprod. 2018, 24, 453–464. [Google Scholar] [CrossRef]
- Thombs, B.D.; Rice, D.B. Sample sizes and precision of estimates of sensitivity and specificity from primary studies on the diagnostic accuracy of depression screening tools: A survey of recently published studies. Int. J. Methods Psychiatr. Res. 2016, 25, 145–152. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Reitsma, J.B.; Di Nisio, M.; Smidt, N.; van Rijn, J.C.; Bossuyt, P.M. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 2006, 174, 469–476. [Google Scholar] [CrossRef]
- Cooper, R.; Forrester, T.; Ogunbiyi, O.; Muffinda, J. Angiotensinogen levels and obesity in four black populations. ICSHIB Investigators. J. Hypertens. 1998, 16, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Böhnke, J.; Gorzelniak, K.; Janke, J.; Schling, P.; Bader, M.; Luft, F.C.; Sharma, A.M. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 2005, 45, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Delforce, S.J.; Lumbers, E.R.; Ellery, S.J.; Murthi, P.; Pringle, K.G. Dysregulation of the placental renin-angiotensin system in human fetal growth restriction. Reproduction 2019, 158, 237–245. [Google Scholar] [CrossRef]
- Pfab, T.; Stirnberg, B.; Sohn, A.; Krause, K.; Slowinski, T.; Godes, M.; Guthmann, F.; Wauer, R.; Halle, H.; Hocher, B. Impact of maternal angiotensinogen M235T polymorphism and angiotensin-converting enzyme insertion/deletion polymorphism on blood pressure, protein excretion and fetal outcome in pregnancy. J. Hypertens. 2007, 25, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, M.H.; Park, H.S.; Lee, K.S.; Ha, E.H.; Pang, M.G. Associations of polymorphisms of the angiotensinogen M235 polymorphism and angiotensin-converting-enzyme intron 16 insertion/deletion polymorphism with preeclampsia in Korean women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 116, 48–53. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Varner, M.; Dizon-Townson, D.; Song, F.; Ward, K. A molecular variant of angiotensinogen is associated with idiopathic intrauterine growth restriction. Obstet. Gynecol. 2003, 101, 237–242. [Google Scholar] [CrossRef]
- Rotimi, C.; Cooper, R.; Ogunbiyi, O.; Morrison, L.; Ladipo, M.; Tewksbury, D.; Ward, R. Hypertension, serum angiotensinogen, and molecular variants of the angiotensinogen gene among Nigerians. Circulation 1997, 95, 2348–2350. [Google Scholar] [CrossRef]
- Schlemm, L.; Haumann, H.M.; Ziegner, M.; Stirnberg, B.; Sohn, A.; Alter, M.; Pfab, T.; Kalache, K.D.; Guthmann, F.; Hocher, B. New evidence for the fetal insulin hypothesis: Fetal angiotensinogen M235T polymorphism is associated with birth weight and elevated fetal total glycated hemoglobin at birth. J. Hypertens. 2010, 28, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021, 152 (Suppl. S1), 3–57. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, A.; Bonilla-Félix, M. Effects of Prematurity and Growth Restriction on Adult Blood Pressure and Kidney Volume. Adv. Chronic Kidney Dis. 2022, 29, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, E.; Farkas, B.; Bodis, J. Pathomechanisms of Prenatally Programmed Adult Diseases. Antioxidants 2023, 12, 1354. [Google Scholar] [CrossRef]
- Liefke, J.; Heijl, C.; Steding-Ehrenborg, K.; Morsing, E.; Arheden, H.; Ley, D.; Hedström, E. Fetal growth restriction followed by very preterm birth is associated with smaller kidneys but preserved kidney function in adolescence. Pediatr. Nephrol. 2023, 38, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, V.; Moritz, K.M.; Bubb, K.J.; Cock, M.L.; Wreford, N.; Harding, R.; Black, M.J. Nephrogenesis and the renal renin-angiotensin system in fetal sheep: Effects of intrauterine growth restriction during late gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1267–R1273. [Google Scholar] [CrossRef]
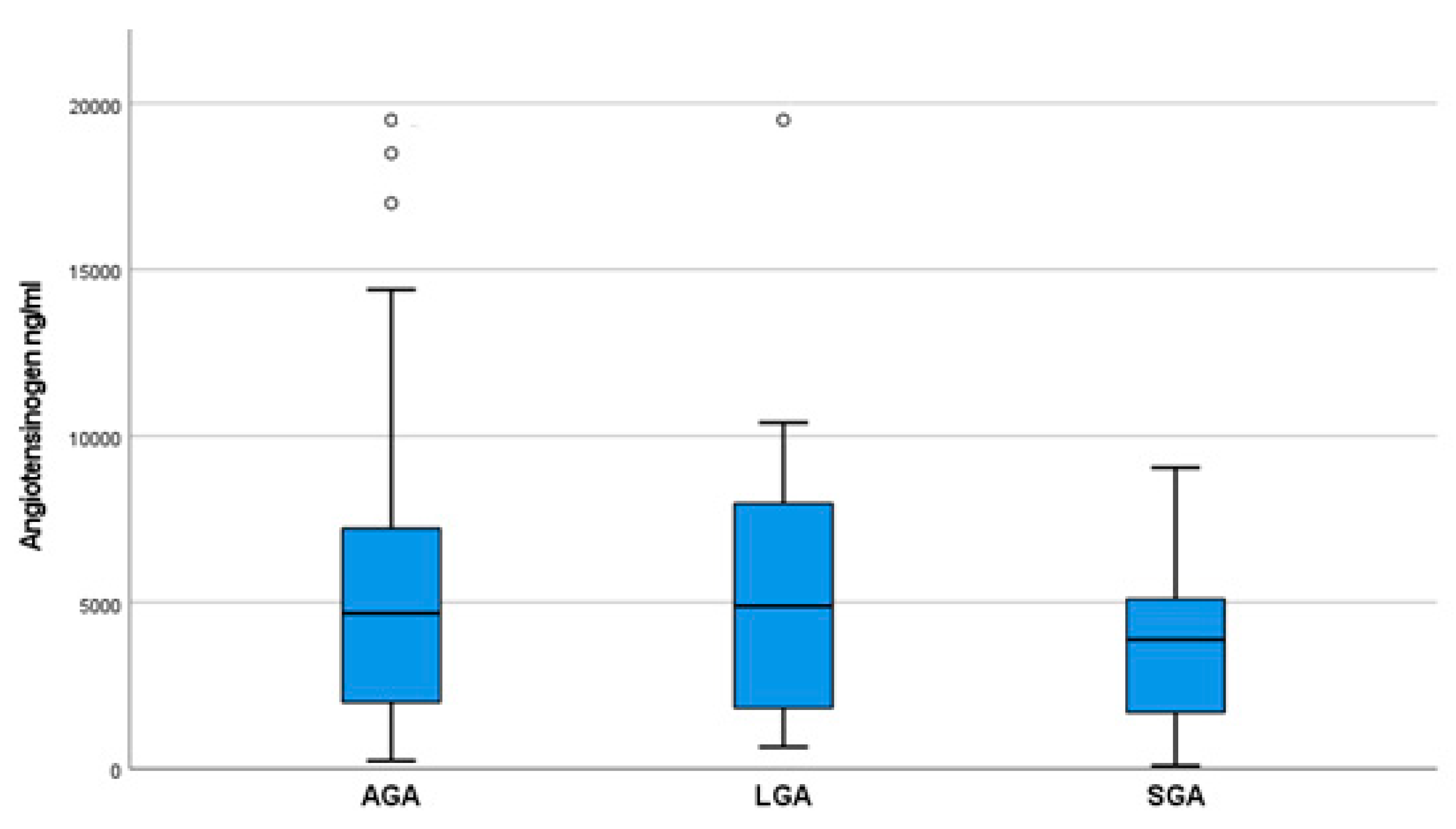
| AGA [Mean (Upper and Lower Extreme)] | SGA [Mean (Upper and Lower Extreme)] | LGA [Mean (Upper and Lower Extreme)] | p-Value | |
|---|---|---|---|---|
| Maternal age (years) | 37 (28–43) | 36 (26–41) | 35 (29–43) | 0.060 |
| Maternal weight (Kgr) | 60 (48–93) | 62 (47–100) | 59 (49–105) | 0.650 |
| Maternal height (cm) | 165 (156–174) | 167 (150–174) | 166 (160–174) | 0.070 |
| Gestational age (days) | 275 (261–285) | 267 (261–283) | 274 (268–282) | 0.011 |
| Neonatal birth weight (gr) | 3290 (2860–3750) | 2630 (1750–2860) | 3800 (3550–4330) | <0.001 |
| Neonatal sex (female/all) | 19/33 | 3/18 | 10/19 | <0.001 |
| Mean Values Compared between Groups | Significance (Two-Sided Test) |
|---|---|
| SGA–AGA | 0.441 |
| LGA–AGA | 0.889 |
| SGA–LGA | 0.429 |
| Angiotensinogen | Age | Weight | Gestational Age | Birth Weight | Percentile | |
|---|---|---|---|---|---|---|
| Angiotensinogen | 1 | 0.208 | 0.139 | −0.420 * | 0.950 ** | 0.148 |
| Age | 1 | 0.035 | 0.76 | 0.148 | 0.088 | |
| Weight | 1 | −0.152 | 0.118 | 0.139 | ||
| Gestational age | 1 | −0.157 | −0.420 * | |||
| Birth weight | 1 | 0.950 | ||||
| Percentile | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrachnis, D.; Fotiou, A.; Mantzou, A.; Pergialiotis, V.; Antsaklis, P.; Valsamakis, G.; Stavros, S.; Machairiotis, N.; Iavazzo, C.; Kanaka-Gantenbein, C.; et al. Second Trimester Amniotic Fluid Angiotensinogen Levels Linked to Increased Fetal Birth Weight and Shorter Gestational Age in Term Pregnancies. Life 2024, 14, 206. https://doi.org/10.3390/life14020206
Vrachnis D, Fotiou A, Mantzou A, Pergialiotis V, Antsaklis P, Valsamakis G, Stavros S, Machairiotis N, Iavazzo C, Kanaka-Gantenbein C, et al. Second Trimester Amniotic Fluid Angiotensinogen Levels Linked to Increased Fetal Birth Weight and Shorter Gestational Age in Term Pregnancies. Life. 2024; 14(2):206. https://doi.org/10.3390/life14020206
Chicago/Turabian StyleVrachnis, Dionysios, Alexandros Fotiou, Aimilia Mantzou, Vasilios Pergialiotis, Panagiotis Antsaklis, George Valsamakis, Sofoklis Stavros, Nikolaos Machairiotis, Christos Iavazzo, Christina Kanaka-Gantenbein, and et al. 2024. "Second Trimester Amniotic Fluid Angiotensinogen Levels Linked to Increased Fetal Birth Weight and Shorter Gestational Age in Term Pregnancies" Life 14, no. 2: 206. https://doi.org/10.3390/life14020206
APA StyleVrachnis, D., Fotiou, A., Mantzou, A., Pergialiotis, V., Antsaklis, P., Valsamakis, G., Stavros, S., Machairiotis, N., Iavazzo, C., Kanaka-Gantenbein, C., Mastorakos, G., Drakakis, P., Vrachnis, N., & Antonakopoulos, N. (2024). Second Trimester Amniotic Fluid Angiotensinogen Levels Linked to Increased Fetal Birth Weight and Shorter Gestational Age in Term Pregnancies. Life, 14(2), 206. https://doi.org/10.3390/life14020206









