First Cytogenetic Analysis of Hemidactylus mercatorius Gray, 1842 Provides Insights on Interspecific Chromosomal Diversification in the Genus Hemidactylus (Squamata: Gekkonidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Molecular Analysis
2.3. Cytogenetic Analysis
3. Results
3.1. Molecular Analysis
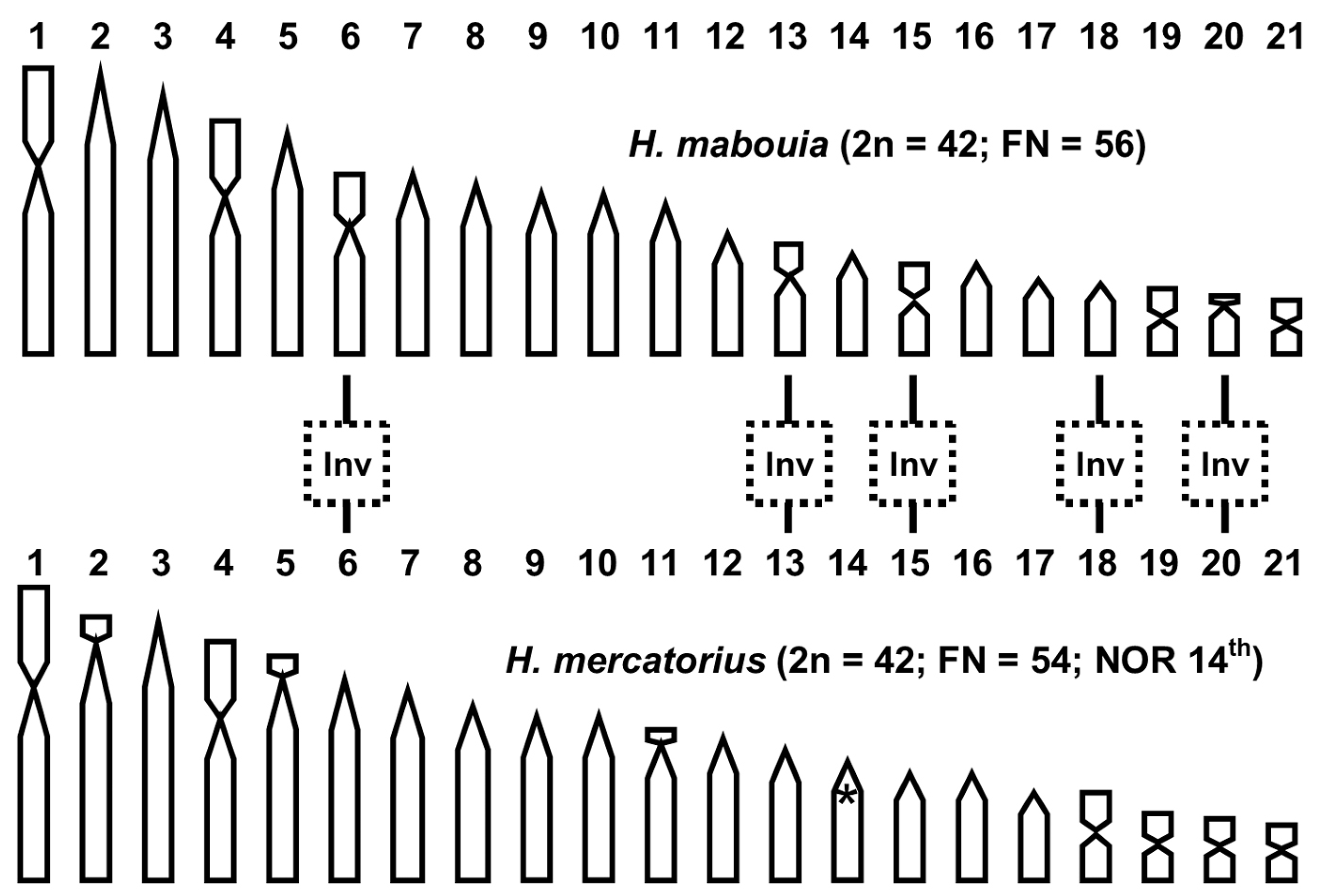
3.2. Cytogenetic Analysis
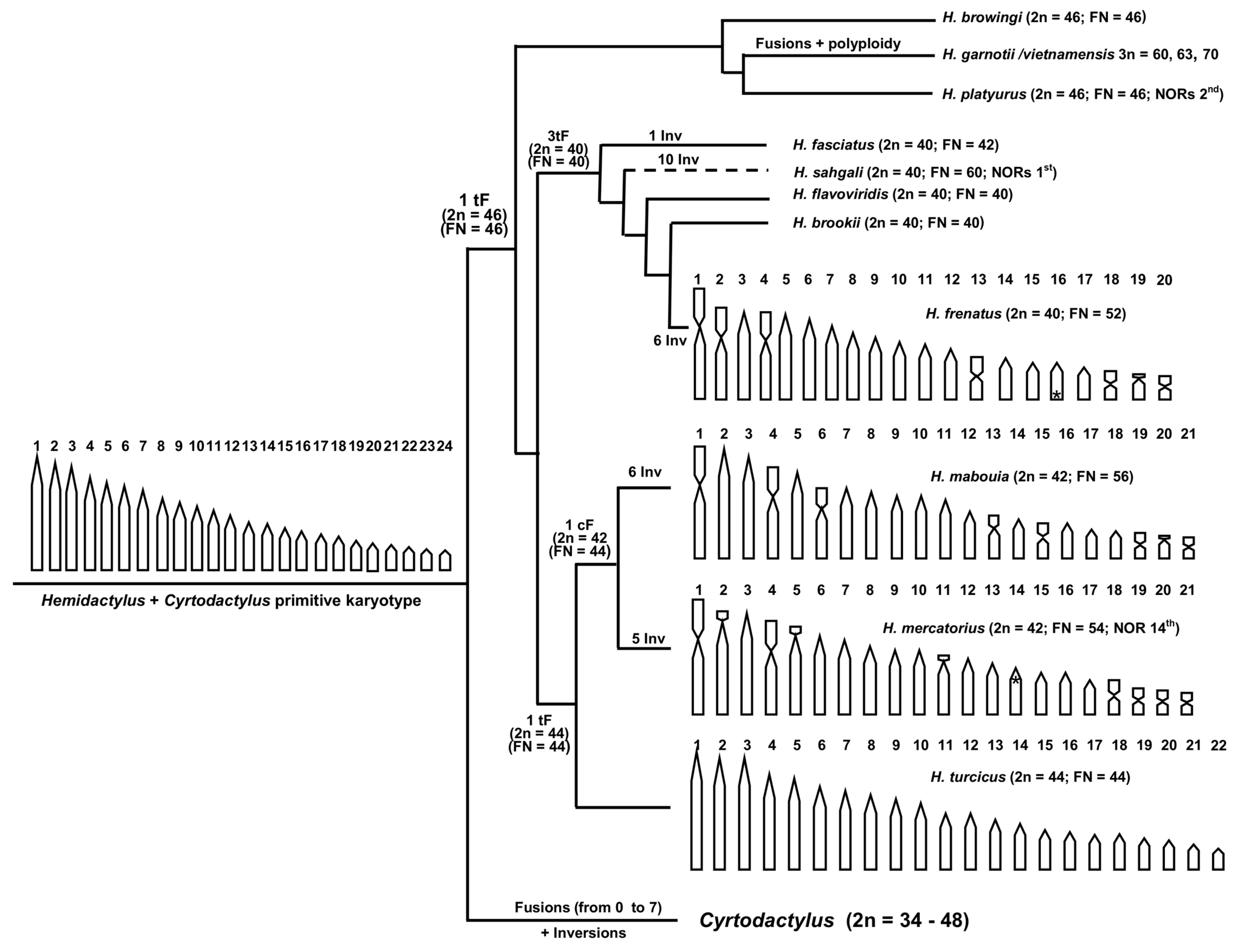
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoder, A.D.; Nowak, M.D. Has vicariance or dispersal been the predominant biogeographic force in Madagascar? Only time will tell. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 405–431. [Google Scholar] [CrossRef]
- Vences, M.; Wollenberg, K.C.; Vieites, D.R.; Lees, D.C. Madagascar as a model region of species diversification. Trends Ecol. Evol. 2009, 24, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Sillero, N.; Glaw, F.; Bora, P.; Vieites, D.R.; Vences, M. Spatial Biodiversity Patterns of Madagascar’s Amphibians and Reptiles. PLoS ONE 2016, 11, e0144076. [Google Scholar] [CrossRef] [PubMed]
- The Reptile Database 2023. Available online: http://www.reptile-database.org (accessed on 7 November 2023).
- Agarwal, I.; Ceríaco, L.M.P.; Metallinou, M.; Jackman, T.R.; Bauer, A.M. How the African house gecko (Hemidactylus mabouia) conquered the world. R. Soc. Open. Sci. 2021, 8, 210749. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. 2022. Available online: https://www.iucnredlist.org (accessed on 30 November 2023).
- Pinho, C.J.; Cardoso, L.; Rocha, S.; Vasconcelos, R. Aliens on Boats? The Eastern and Western Expansion of the African House Gecko. Genes 2023, 14, 381. [Google Scholar] [CrossRef]
- Vences, M.; Wanke, S.; Vieites, D.R.; Branch, W.R.; Glaw, F.; Meyer, A. Natural colonization or introduction? Phylogeographical relationships and morphological differentiation of house geckos (Hemidactylus) from Madagascar. Biol. J. Linn. Soc. 2004, 83, 115–130. [Google Scholar] [CrossRef]
- Arnold, E.N.; Vasconcelos, R.; Harris, D.J.; Mateo, J.A.; Carranza, S. Systematics, biogeography and evolution of the endemic Hemidactylus geckos (Reptilia, Squamata, Gekkonidae) of the Cape Verde Islands: Based on morphology and mitochondrial and nuclear DNA sequences. Zool. Scr. 2008, 37, 619–636. [Google Scholar] [CrossRef]
- Javed, S.M.M.; Srinivasulu, C.; Rao, K.L.; Raseswari, T.; Tampal, F. A divergent population of Hemidactylus frenatus Duméril & Bibron, 1836 (Reptilia: Gekkonidae) from the northern Eastern Ghats, India. J. Threat. Taxa 2010, 2, 1205–1213. [Google Scholar]
- Kluge, A.G. The evolution and geographical origin of the New World Hemidactylus mabouia-brooki complex (Gekkonidae, Sauria). Misc. Publ. Mus. Zool. Univ. Mich. 1969, 138, 1–78. [Google Scholar]
- Kluge, A.G. Gekkotan Lizard Taxonomy; Centre for Herpetology, Madras Crocodile Bank Trust: Tamil Nadu, India, 2001; Volume 26, pp. 1–209. [Google Scholar]
- Rocha, S.; Carretero, M.A.; Harris, D.J. On the diversity, colonization patterns and status of Hemidactylus spp. (Reptilia: Gekkonidae) from the Western Indian Ocean islands. Herpetol. J. 2010, 20, 83–89. [Google Scholar]
- Rato, C.; Martins, B.; Rocha, R.; Silva-Rocha, I. Uncovered genetic diversity in Hemidactylus mabouia (Reptilia: Gekkonidae) from Madeira Island reveals uncertain sources of introduction. Amphib-Reptilia 2021, 42, 369–375. [Google Scholar] [CrossRef]
- King, M. A new chromosome form of Hemidactylus frenatus (Dumeril & Bribon). Herpetologica 1978, 34, 216–218. [Google Scholar]
- Kupriyanova, L.A.; Darevsky, I.S.; Ota, H. Karyotypic uniformity in the East Asian populations of Hemidactylus frenatus (Sauria: Gekkonidae). J. Herpetol. 1989, 23, 294–296. [Google Scholar] [CrossRef]
- Castiglia, R.; Annesi, F.; Bezerra, A.M.R.; García, A.; Flores-Villela, O. Cytotaxonomy and DNA taxonomy of lizards (Squamata, Sauria) from a tropical dry forest in the Chamela-Cuixmala Biosphere Reserve on the coast of Jalisco, Mexico. Zootaxa 2010, 2508, 1–29. [Google Scholar] [CrossRef]
- Trifonov, V.A.; Giovannotti, M.; O’Brien, C.M.P.; Wallduck, M.; Lovell, F.; Rens, W.; Parise-Maltempi, P.P.; Caputo, V.; Ferguson-Smith, M.A. Chromosomal evolution in Gekkonidae. I. Chromosome painting between Gekko and Hemidactylus species reveals phylogenetic relationships within the group. Chromosome Res. 2011, 19, 843–855. [Google Scholar] [CrossRef]
- Patawang, I.; Tanomtong, A. Karyological Analysis of Asian House Gecko (Hemidactylus frenatus) and Frilly House Gecko (H. platyurus) from Northeastern Thailand. In Proceedings of the 19th National Genetics Conference 2015, Khon Kaen, Thailand, 15–17 July 2015. [Google Scholar]
- Kirkpatrick, M.; Barton, N. Chromosome inversions, local adaptation and speciation. Genetics 2006, 173, 419–434. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Andreone, F.; Aprea, G.; Glaw, F.; Odierna, G.; Guarino, F.M. When can chromosomes drive speciation? The peculiar case of the Malagasy tomato frogs (genus Dyscophus). Zool. Anz. 2017, 268, 41–46. [Google Scholar] [CrossRef]
- Olmo, E. Trends in the evolution of reptilian chromosomes. Integr. Comp. Biol. 2008, 48, 486–493. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Guarino, F.M.; Odierna, G. Lizards as Model Organisms of Sex Chromosome Evolution: What We Really Know from a Systematic Distribution of Available Data? Genes 2021, 12, 1341. [Google Scholar] [CrossRef]
- Beçak, M.L.; Beçak, W.; Denaro, L. Chromosome Polymorphism, Geographical Variation and Karyotypes in Sauria. Caryologia 1972, 25, 313–326. [Google Scholar] [CrossRef]
- McBee, K.; Bickham, J.W.; Dixon, J.R. Male heterogamety and chromosomal variation in Caribbean geckos. J. Herpetol. 1987, 21, 68–71. [Google Scholar] [CrossRef]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef]
- Cocca, W.; Rosa, G.M.; Andreone, F.; Aprea, G.; Bergò, P.E.; Mattioli, F.; Mercurio, V.; Randrianirina, J.E.; Rosado, D.; Vences, M.; et al. The herpetofauna (Amphibia, Crocodylia, Squamata, Testudines) of the Isalo Massif, southwest Madagascar: Combining morphological, molecular and museum data. Salamandra 2018, 54, 178–200. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Lab Press: New York, NY, USA, 1989. [Google Scholar]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Paabo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Smíd, J.; Carranza, S.; Kratochvíl, L.; Gvoždík, V.; Nasher, A.K.; Moravec, J. Out of Arabia: A complex biogeographic history of multiple vicariance and dispersal events in the gecko genus Hemidactylus (Reptilia: Gekkonidae). PLoS ONE 2013, 8, e64018. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Mezzasalma, M.; Brunelli, E.; Odierna, G.; Guarino, F.M. First insights on the karyotype diversification of the Endemic Malagasy Leaf-Toed Geckos (Squamata: Gekkonidae: Uroplatus). Animals 2022, 12, 2054. [Google Scholar] [CrossRef]
- Howell, W.M.; Black, D.A. Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: A1-step method. Experientia 1980, 36, 1014–1015. [Google Scholar] [CrossRef]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Andreone, F.; Glaw, F.; Guarino, F.M.; Odierna, G.; Petraccioli, A.; Picariello, O. Changes in heterochromatin content and ancient chromosome fusion in the endemic Malagasy boid snakes Sanzinia and Acrantophis (Squamata: Serpentes). Salamandra 2019, 55, 140–144. [Google Scholar]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Rocha, S.; Carretero, M.A.; Harris, D.J. Diversity and phylogenetic relationships of Hemidactylus geckos from the Comoro Islands. Mol. Phylogenet. Evol. 2005, 35, 292–299. [Google Scholar] [CrossRef]
- Chrostek, G.; Domaradzka, A.; Yurchenko, A.; Kratochvíl, L.; Mazzoleni, S.; Rovatsos, M. Cytogenetic Analysis of Seven Species of Gekkonid and Phyllodactylid Geckos. Genes 2023, 14, 178. [Google Scholar] [CrossRef]
- Montefalcone, G.; Tempesta, S.; Rocchi, M.; Archidiacono, N. Centromere repositioning. Genome Res. 1999, 9, 1184–1188. [Google Scholar] [CrossRef]
- De Smet, W.H.O. Description of the orcein stained karyotypes of 27 lizard species (Lacertilia, Reptilia) belonging to the families Iguanidae, Agamidae, Chameleontidae and Gekkonidae (Ascalabota). Acta Zool. Pathol. Antverpiensia 1981, 76, 35–72. [Google Scholar]
- Ota, H.; Hikida, T.; Lue, K.Y. Polyclony in a triploid gecko, Hemidactylus stejnegeri, from Taiwan, with notes on its bearing on the chromosomal diversity of the H. garnotii-vietnamensis complex (Sauria: Gekkonidae). Genetica 1989, 79, 183–189. [Google Scholar] [CrossRef]
- Ejere, V.C.; Adegoke, J.A. Karyological Study of the Banded Gecko, Hemidactylus fasciatus fasciatus Gray (Gekkonidae; Reptilia). Cytologia 2001, 66, 133–137. [Google Scholar] [CrossRef][Green Version]
- Thongnetr, W.; Aiumsumang, S.; Kongkaew, R.; Tanomtong, A.; Suwannapoom, C.; Phimphan, S. Cytogenetic characterisation and chromosomal mapping of microsatellite and telomeric repeats in two gecko species (Reptilia, Gekkonidae) from Thailand. Comp. Cytogen. 2021, 15, 41–52. [Google Scholar] [CrossRef]
- Prasopsin, S.; Muanglen, N.; Ditcharoen, S.; Suwannapoom, C.; Tanomtong, A.; Thongnetr, W. First Report on Classical and Molecular Cytogenetics of Doi Inthanon Bent-toed Gecko, Cyrtodactylus inthanon Kunya et al., 2015 (Squamata: Gek-konidae) in Thailand. Caryologia 2022, 75, 109–117. [Google Scholar] [CrossRef]
- King, M. Monophyletism in the Gekkonidae: A chromosomal perspective. Aust. J. Zool. 1987, 35, 641–654. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Guarino, F.M.; Aprea, G.; Petraccioli, A.; Crottini, A.; Odierna, G. Karyological evidence for diversification of Italian slow worm populations (Squamata, Anguidae). Comp. Cytogenet. 2013, 7, 217–227. [Google Scholar] [CrossRef]
- Srikulnath, K.; Uno, Y.; Nishida, C.; Ota, H.; Matsuda, Y. Karyotype Reorganization in the Hokou Gecko (Gekko hokouensis, Gekkonidae): The Process of Microchromosome Disappearance in Gekkota. PLoS ONE 2015, 10, e0134829. [Google Scholar] [CrossRef]
- King, M.; Rofe, R. Karyotypic variation in the Australian gekko Phyllodactylus marmoratus (Gray) (Gekkonidae: Reptilia). Chromosoma 1976, 54, 75–87. [Google Scholar] [CrossRef]
- Aprea, G.; Andreone, F.; Fulgione, D.; Petraccioli, A.; Odierna, G. Chromosomal rearrangements occurred repeatedly and independently during species diversification in Malagasy geckos, genus Paroedura. Afr. Zool. 2013, 48, 96–108. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Brunelli, E.; Odierna, G.; Guarino, F.M. Chromosome Diversity and Evolution of the Endemic Malagasy Velvet Geckos of the Genus Blaesodactylus (Reptilia, Gekkonidae). Animals 2023, 13, 2068. [Google Scholar] [CrossRef]
- Naveira, H.; Rojo, V.; Gómez-Seoane, I.; Ferguson-Smith, A.; Pereira, J.C.; Martínez-Lage, A. Chromosome evolution in Iberolacerta, a genus that deviates from the standard karyotype formula of Lacertidae. Genetica 2023, 151, 267–279. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Di Febbraro, M.; Guarino, F.M.; Odierna, G.; Russo, D. Cold-blooded in the Ice Age: “refugia within refugia”, inter-and intraspecific biogeographic diversification of European whipsnakes (Squamata, Colubridae, Hierophis). Zoology 2018, 127, 84–94. [Google Scholar] [CrossRef]
- Petraccioli, A.; Guarino, F.M.; Kupriyanova, L.; Mezzasalma, M.; Odierna, G.; Picariello, O.; Capriglione, T. Isolation and Characterization of Interspersed Repeated Sequences in the Common Lizard, Zootoca vivipara, and Their Conservation in Squamata. Cytogenet. Genome Res. 2019, 157, 65–76. [Google Scholar] [CrossRef]
- Keating, S.E.; Blumer, M.; Grismer, L.L.; Lin, A.; Nielsen, S.V.; Thura, M.K.; Wood, P.L., Jr.; Quah, E.S.H.; Gamble, T. Sex Chromosome Turnover in Bent-Toed Geckos (Cyrtodactylus). Genes 2021, 12, 116. [Google Scholar] [CrossRef]
- Ezaz, T.; Sarre, S.; O’Meally, D.; Graves, J.M.; Georges, A. Sex chromosome evolution in lizards: Independent origins and rapid transitions. Cytogenet. Genome Res. 2009, 127, 249–260. [Google Scholar] [CrossRef]
- Gamble, T. A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex Dev. 2010, 4, 88–103. [Google Scholar] [CrossRef] [PubMed]

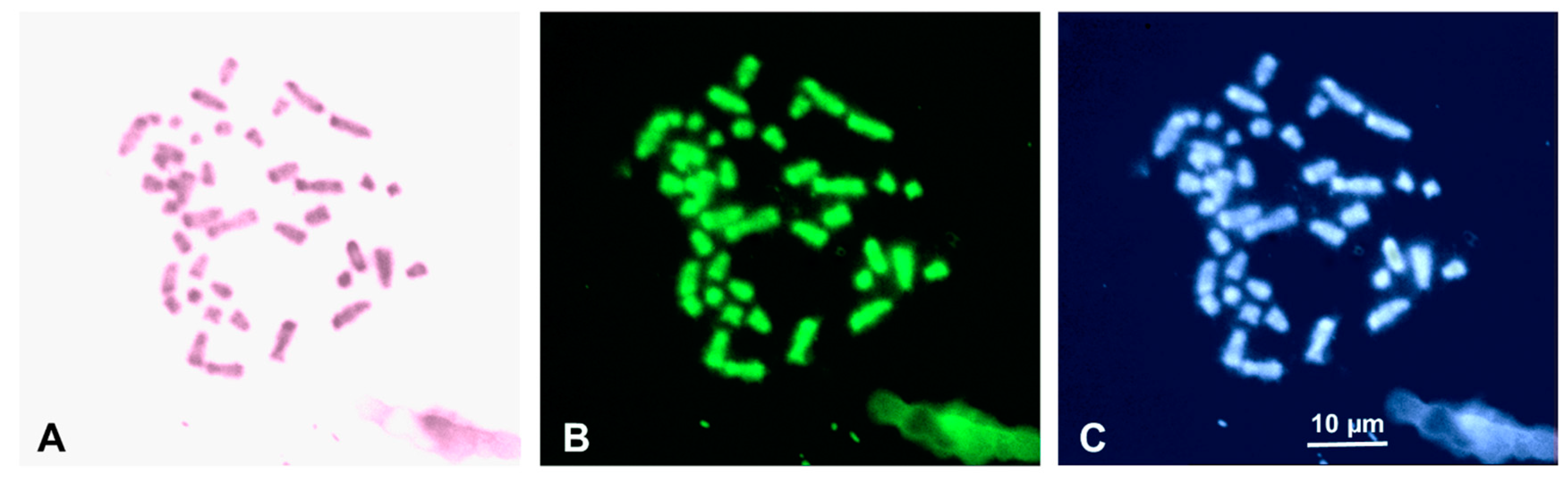
| Specimen | Locality | Sex |
|---|---|---|
| GA 507 | Mandrivazo | male |
| GA 508 | Mandrivazo | female |
| GA 509 | Mandrivazo | male |
| GA 510 | Mandrivazo | male |
| FAZC 11897 * | Analalava forest | juvenile |
| FAZC 11898 * | Analalava forest | male |
| Cp | RL | CI |
|---|---|---|
| 1 | 9.0 ± 1.1 | 43.5 ± 3.7 (M) |
| 2 | 8.9 ± 1.0 | 10.6 ± 1.7 (T) |
| 3 | 8.3 ± 0.9 | 4.2 ± 2.0 (T) |
| 4 | 7.6 ± 0.8 | 34.1 ± 2.5 (sM) |
| 5 | 7.0 ± 0.7 | 15.6 ± 3.0 (sT) |
| 6 | 6.1 ± 0.7 | 3.3 ± 1.1 (T) |
| 7 | 6.0 ± 0.4 | 5.2 ± 2.1 (T) |
| 8 | 6.0 ± 0.5 | 4.8 ± 3.0 (T) |
| 9 | 5.5 ± 0.6 | 6.3 ± 2.9 (T) |
| 10 | 4.9 ± 0.6 | 2.8 ± 1.2 (T) |
| 11 | 4.1 ± 0.5 | 15.8 ± 3.5 (sT) |
| 12 | 3.9 ± 0.4 | 4.8 ± 2.8 (T) |
| 13 | 3.4 ± 0.4 | 9.3 ± 3.7 (T) |
| 14 | 3.3 ± 0.5 | 7.4 ± 4.2 (T) |
| 15 | 2.7 ± 0.3 | 6.5 ± 3.0 (T) |
| 16 | 2.6 ± 0.7 | 10.2 ± 1.9 (T) |
| 17 | 2.6 ± 0.6 | 3.2 ± 2.1 (T) |
| 18 | 2.5 ± 0.4 | 42.8 ± 4.2 (M) |
| 19 | 2.1 ± 0.5 | 44.4 ± 2.7 (M) |
| 20 | 1.9 ± 0.5 | 42.9 ± 3.8 (M) |
| 21 | 1.8 ± 0.4 | 45.3 ± 4.0 (M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzasalma, M. First Cytogenetic Analysis of Hemidactylus mercatorius Gray, 1842 Provides Insights on Interspecific Chromosomal Diversification in the Genus Hemidactylus (Squamata: Gekkonidae). Life 2024, 14, 181. https://doi.org/10.3390/life14020181
Mezzasalma M. First Cytogenetic Analysis of Hemidactylus mercatorius Gray, 1842 Provides Insights on Interspecific Chromosomal Diversification in the Genus Hemidactylus (Squamata: Gekkonidae). Life. 2024; 14(2):181. https://doi.org/10.3390/life14020181
Chicago/Turabian StyleMezzasalma, Marcello. 2024. "First Cytogenetic Analysis of Hemidactylus mercatorius Gray, 1842 Provides Insights on Interspecific Chromosomal Diversification in the Genus Hemidactylus (Squamata: Gekkonidae)" Life 14, no. 2: 181. https://doi.org/10.3390/life14020181
APA StyleMezzasalma, M. (2024). First Cytogenetic Analysis of Hemidactylus mercatorius Gray, 1842 Provides Insights on Interspecific Chromosomal Diversification in the Genus Hemidactylus (Squamata: Gekkonidae). Life, 14(2), 181. https://doi.org/10.3390/life14020181






