The Internal Anatomy and Water Current System of Cambrian Archaeocyaths of South China
Abstract
1. Introduction
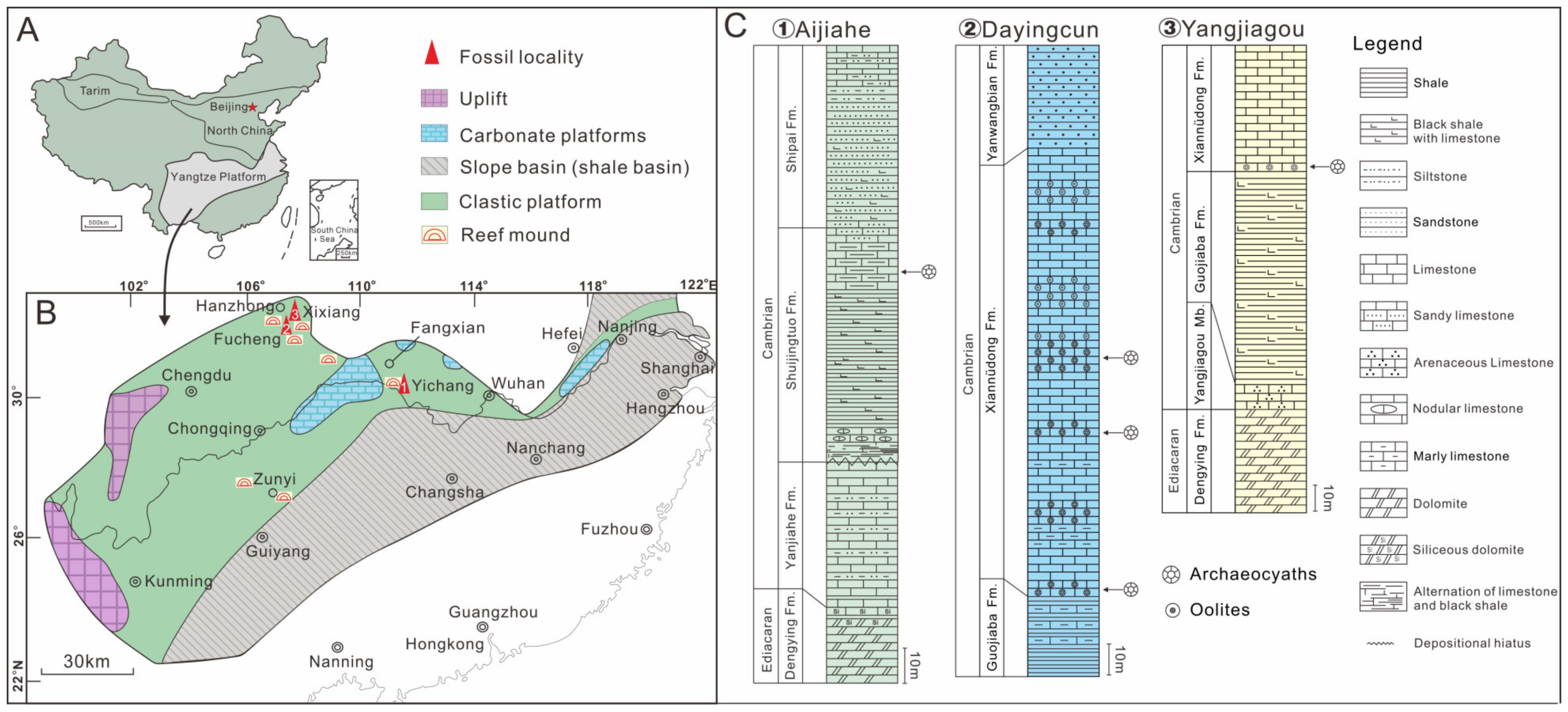
2. Stratigraphy, Material, and Methods
3. Results
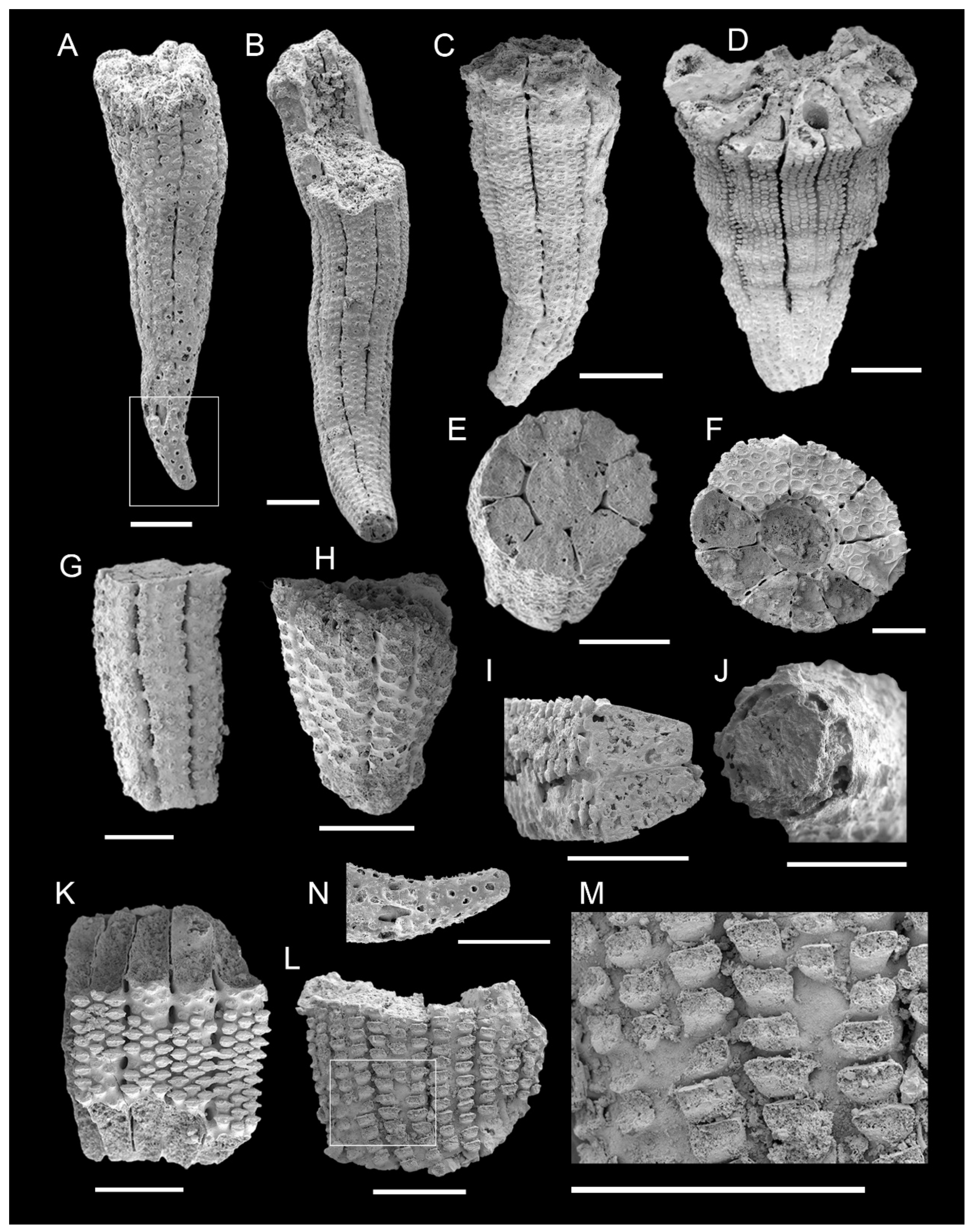
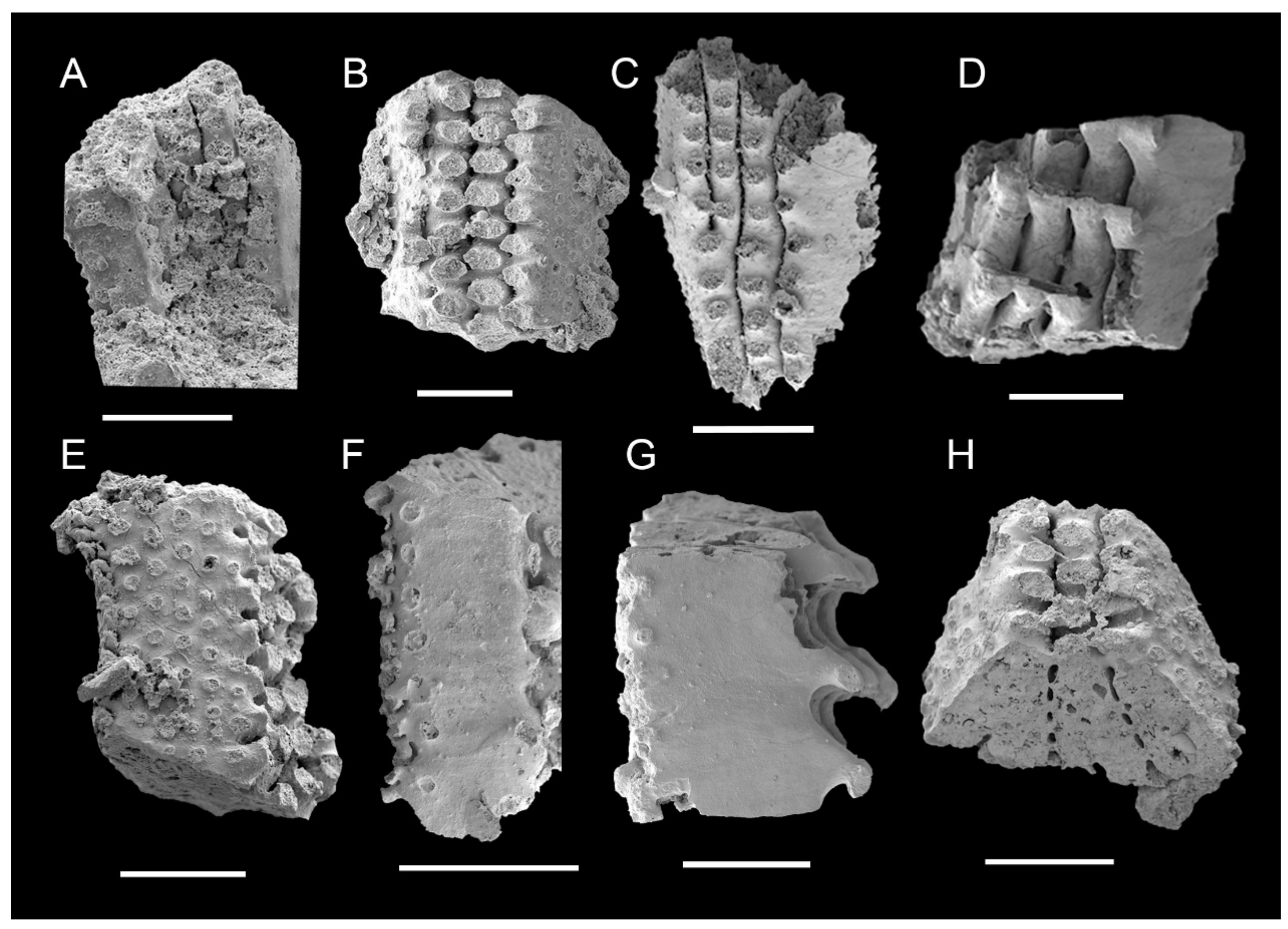
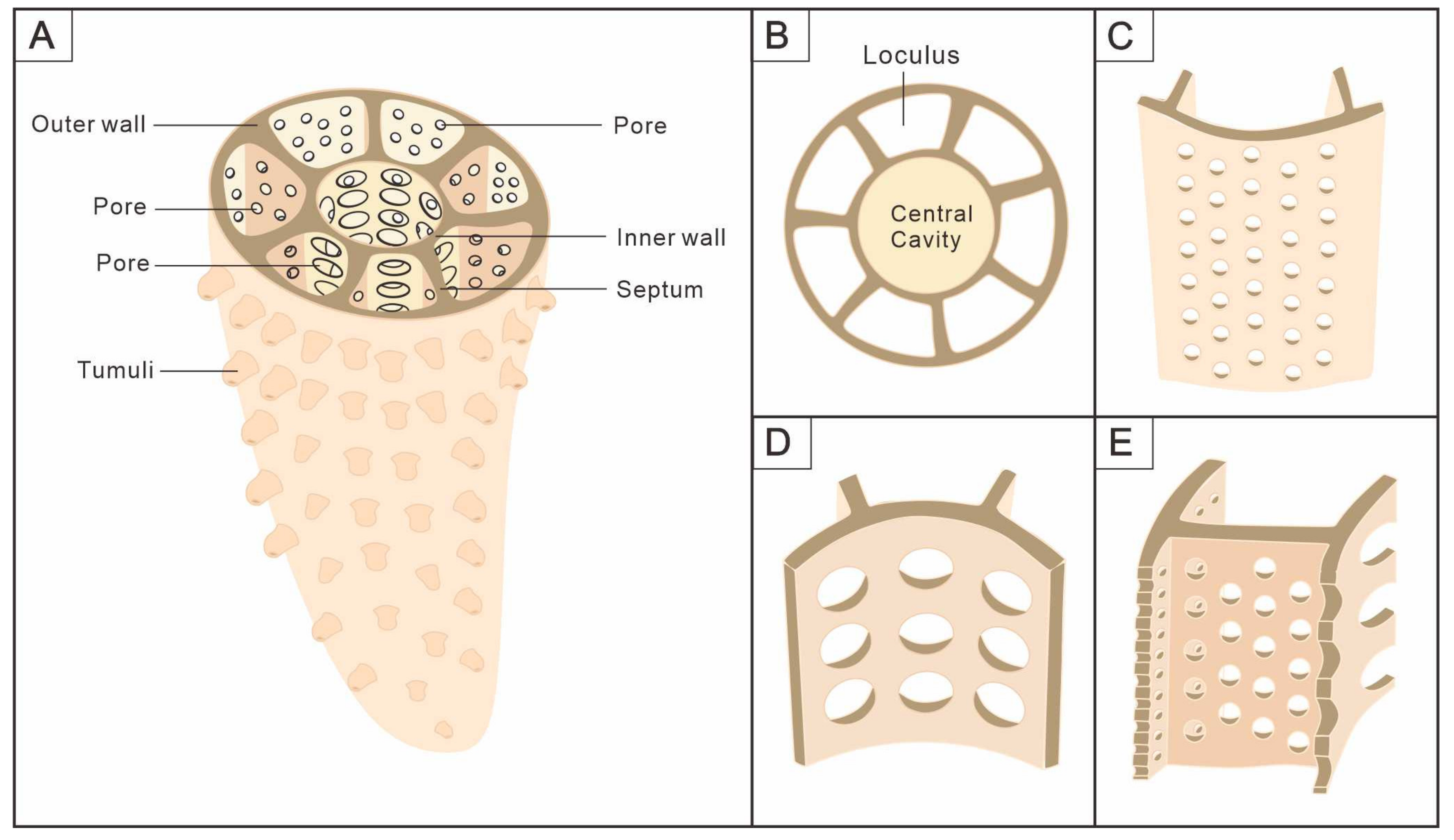
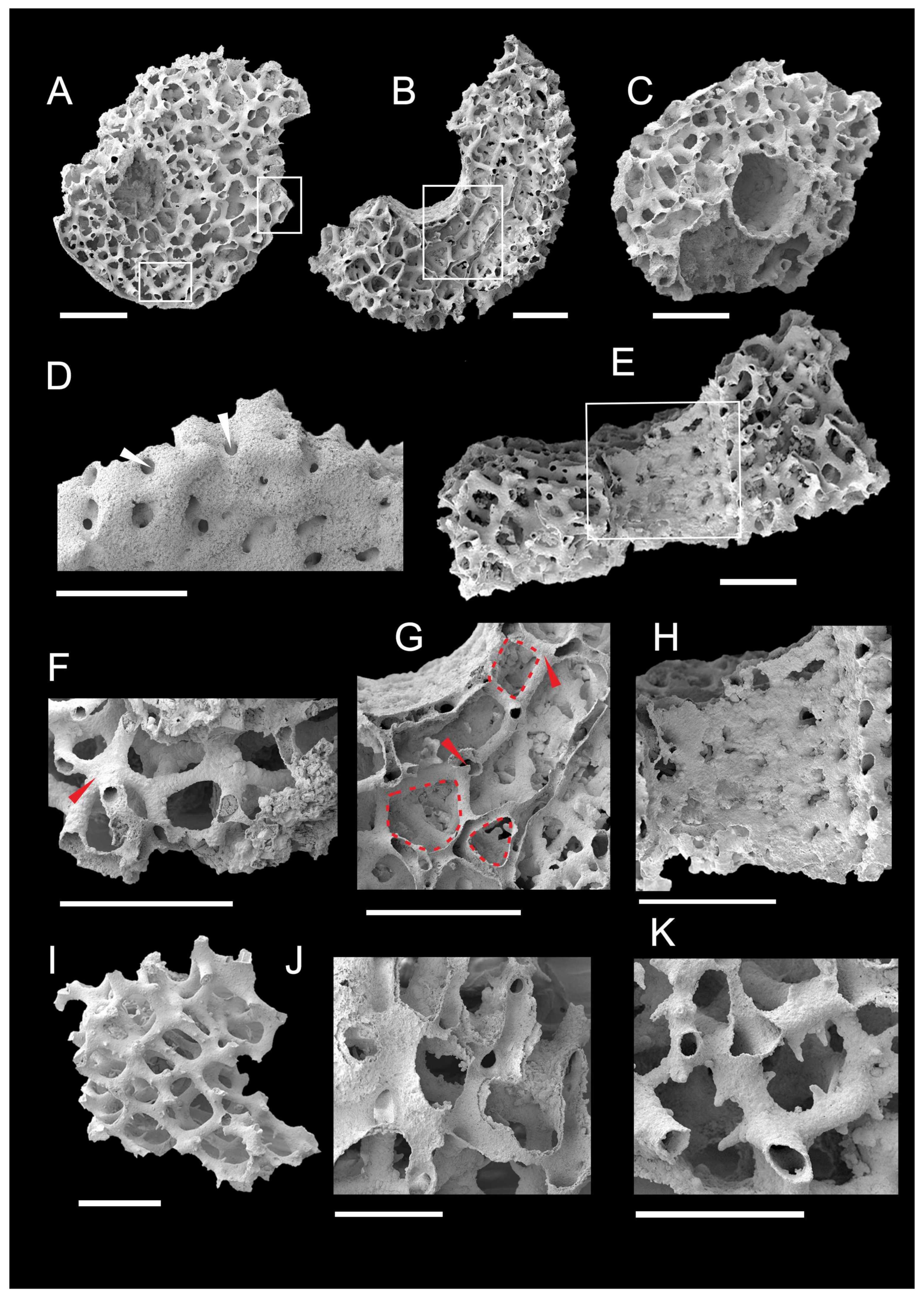
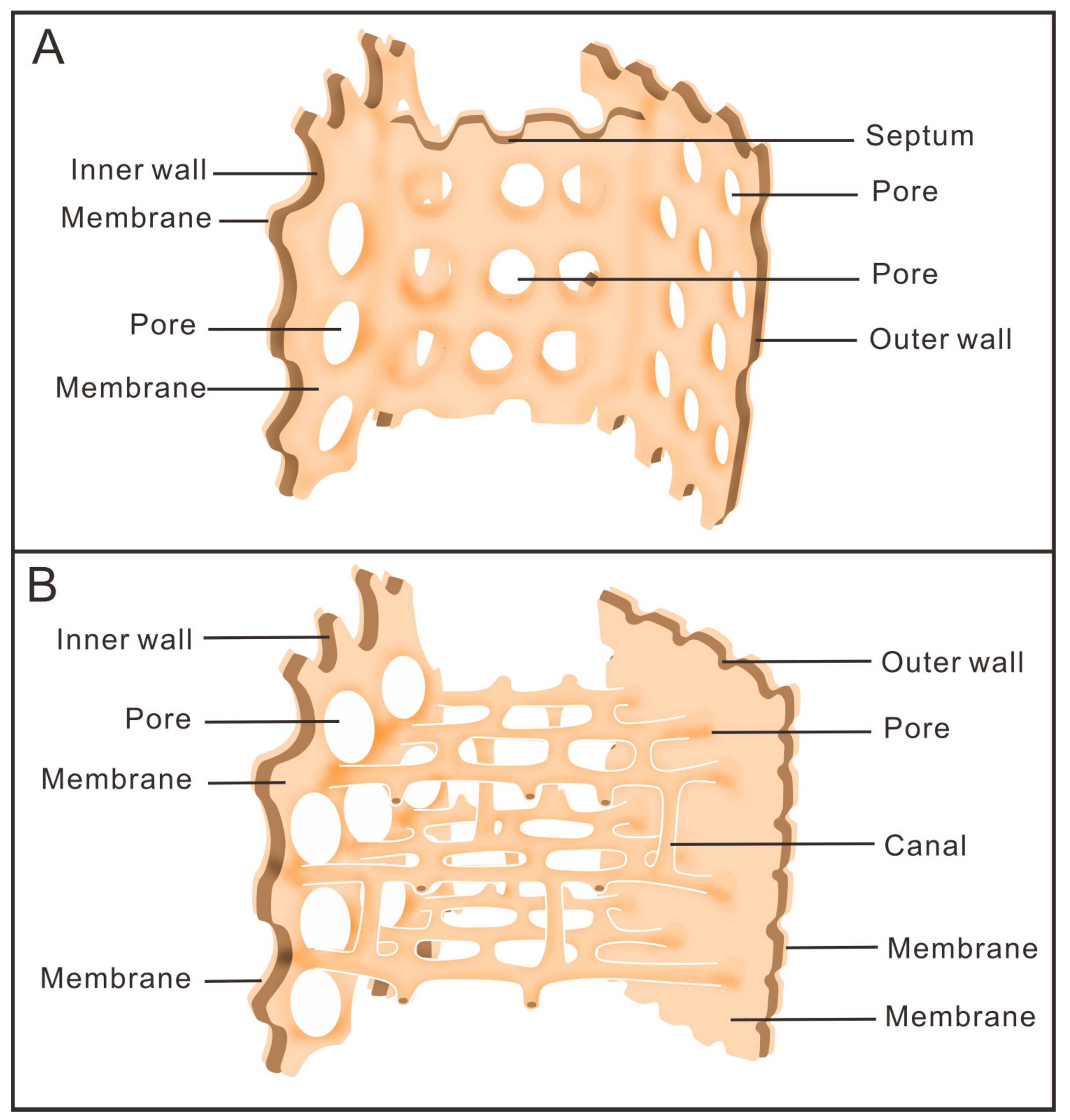
3.1. Morphology of Archaeocyaths with Septa
3.2. Morphology of Archaeocyaths with Canals
4. Discussion
4.1. Comparison of the Two Archaeocyaths
4.2. Comparison with Coral
4.3. Comparison with Sponge
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sepkoski, J.J. A kinetic model of Phanerozoic taxonomic diversity II: Early Phanerozoic families and multiple equilibria. Paleobiology 1979, 5, 222–251. [Google Scholar] [CrossRef]
- James, N.P.; Debrenne, F. Lower Cambrian bioherms: Pioneer reefs of the Phanerozoic. Acta Palaeontol. Pol. 1980, 25, 655–668. [Google Scholar]
- Zhang, X.L.; Shu, D.G.; Han, J.; Zhang, Z.F.; Liu, J.N.; Fu, D.J. Triggers for the Cambrian explosion: Hypotheses and problems. Gondwana Res. 2014, 25, 896–909. [Google Scholar] [CrossRef]
- Debrenne, F.; Zhuravlev, A.Y.; Kruse, P.D. General features of Archaeocyatha. In Treatise on Invertebrate Paleontology; Stearn, C.W., Ed.; Part E: Porifera, Revised, Hypercalcified Porifera; University of Kansas Paleontological Institute: Lawrence, KS, USA, 2015; Volume 5, pp. 845–922. [Google Scholar]
- Shu, D.G.; Han, J. The core value of Chengjiang fauna: The formation of animal kingdom and the birth of basic human organs. Earth Sci. Front. 2020, 27, 382–412, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Hanfield, R.C. Archaeocyatha from the Mackenzie and Cassiar Mountains, Northwest Territories, Yukon Territory and British Columbia. Geol. Surv. Can. Bull. 1971, 201, 1–119. [Google Scholar]
- Gravestock, D.I. Archaeocyatha from lower parts of the Lower Cambrian carbonate sequence in South Australia. Mem. Assoc. Australas. Paleontol. 1984, 2, 1–139. [Google Scholar]
- Debrenne, F.; Kruse, P.D. Shackleton limestone archaeocyaths. Alcheringa 1986, 10, 235–278. [Google Scholar] [CrossRef]
- Zhang, J.M.; Yuan, K.X. Archaeocyath reefs from Lower Cambrian Tianheban Formation at Wangjiaping, Yichang, Hubei and their diagenesis. Chin. J. Geol. 1994, 29, 236–245, (In Chinese with English abstract). [Google Scholar]
- Kruse, P.D.; Zhuravlev, A.Y.; James, N.P. Primordial metazoan-calcimicrobial reefs: Tommotian (Early Cambrian) of the Siberian platform. Palaios 1995, 10, 291–321. [Google Scholar] [CrossRef]
- Riding, R.; Zhuravlev, A.Y. Structure and diversity of oldest sponge-microbe reefs: Lower Cambrian, Aldan River, Siberia. Geology 1995, 23, 649–652. [Google Scholar] [CrossRef]
- Gandin, A.; Debrenne, F. Distribution of the archaeocyath-calcimicrobial bioconstructions on the Early Cambrian shelves. Palaeoworld 2010, 19, 222–241. [Google Scholar] [CrossRef]
- Menéndez, S.; Rodríguez-Martínez, M.; Moreno-Eris, E.; Perejón, A.; Reitner, J. Palaeoenvironmental and geochemical approach of Archaeocyath-rich facies from Lower Cambrian of Western Gondwana margin at Central Iberian Zone (Urda, Toledo Mountains, Spain). Geophys. Res. Abstr. 2010, 12, 9359. [Google Scholar]
- Stone, P.; Thomson, M.R.A.; Rushton, W.A. An Early Cambrian archaeocyath–trilobite fauna in limestone erratics from the Upper Carboniferous Fitzroy Tillite Formation, Falkland Islands. Earth Environ. Sci. Trans. R. Soc. Edinb. 2011, 102, 201–225. [Google Scholar] [CrossRef]
- Yang, A.H.; Zhu, M.Y.; Zhuravlev, A.Y.; Yuan, K.X.; Zhang, J.M.; Chen, Y.Q. Archaeocyathan zonation of the Yangtze Platform: Implications for regional and global correlation of lower Cambrian stages. Geol. Mag. 2016, 153, 388–409. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, M.; Buggisch, W.; Menendez, S.; Moreno-Eiris, E.; Perejón, A. Reconstruction of a Ross lost Cambrian Series 2 mixed siliciclastic–carbonate platform from carbonate clasts of the Shackleton Range, Antarctica. Earth Environ. Sci. Trans. R. Soc. Edinb. 2022, 113, 175–226. [Google Scholar] [CrossRef]
- Yuan, K.X.; Zhang, S.G. Archaeocyatha. In Stratigraphy and Palaeontology of Sinian to Permian in the Eastern Part of the Yangtze Gorge; Three Yangtze Gorges Geological Research Unit, Geological Bureau of Hubei Province, Ed.; Geological Publishing House: Beijing, China, 1978; pp. 138–141. (In Chinese) [Google Scholar]
- Yuan, K.X.; Zhang, S.G. Lower Cambrian Archaeocyatha of central and southwestern China. Acta Palaeontol. Pol. 1980, 19, 380–391, (In Chinese with English abstract). [Google Scholar]
- Yuan, K.X.; Zhang, S.G. Lower Cambrian archaeocyathid assemblages of central and southwestern China. Spec. Pap. Geol. Soc. Am. 1981, 187, 39–54. [Google Scholar] [CrossRef]
- Yuan, K.X.; Zhang, S.G. Biogeographical provinces of Early Cambrian archaeocyathids in China. Nanjing Inst. Geol. Palaeontol. Acad. Sin. Bull. 1983, 6, 101–116, (In Chinese with English Abstract). [Google Scholar]
- Zhang, S.G.; Yuan, K.X. Lower Cambrian archaeocyathids in Weiganping from Fuquan, China. Acta Palaeontol. Sin. 1984, 23, 543–553, (In Chinese with English abstract). [Google Scholar]
- Yuan, K.X.; Zhu, M.Y.; Zhang, J.M.; Vaniten, H. Biostratigraphy of archaeocyathan horizons in the lower Cambrian Fucheng section, South Shaanxi Province: Implications for regional correlations and archaeocyathan evolution. Acta Palaeontol. Sin. 2001, 40 (Suppl. S1), 115–129. [Google Scholar]
- Yang, A.H.; Yuan, K.X. New archaeocyaths from the early Cambrian of Shaanxi and Guizhou provinces, South China. Geobios 2012, 45, 591–601. [Google Scholar] [CrossRef]
- Luo, M.; Liu, F.; Liang, Y.; Strotz, L.C.; Wang, J.; Hu, Y.; Song, B.; Holmer, L.E.; Zhang, Z. First Report of Small Skeletal Fossils from the Upper Guojiaba Formation (Series 2, Cambrian), Southern Shaanxi, South China. Biology 2023, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Rozanov, A.Y.; Missarzhevskiy, V.V.; Volkova, N.A.; Voronova, L.G.; Krylov, I.N.; Keller, B.M.; Korolyuk, I.K.; Lendzion, K.; Mikhnyak, R.; Pykhova, N.G.; et al. The Tommotian Stage and the Cambrian lower boundary problem. Trans. Acad. Sci. USSR Nauka 1969, 206, 1–380. (In Russian) [Google Scholar]
- Zhuravlev, A.Y. The early history of the Metazoa—A paleontologist’s viewpoint. Biol. Bull. Rev. 2015, 5, 415–461. [Google Scholar] [CrossRef]
- Bayfield. On the junction of the transition and primary rocks of Canada and Labrador. Q. J. Geol. Soc. Lond. 1845, 1, 450–459. [Google Scholar] [CrossRef]
- Billings, E. New Species of Lower Silurian Fossils; Geological Survey of Canada: Montreal, QC, Canada, 1861. [Google Scholar]
- Meek, F.B. Preliminary notice of a remarkable new genus of corals, probably typical of a new family. Am. J. Sci. 1868, 2, 62–64. [Google Scholar] [CrossRef]
- Bornemann, J.G. Die Versteinerungen Des Cambrischen Schichtensystems Der Insel Sardinien Nebst Vergleichenden Untersuchungen über Analoge Vorkommnisse Aus Andern Ländern; Engelmann: Halle, Germany, 1886. [Google Scholar]
- Hinde, G.J. On Archaeocyathus, Billings, and on other Genera, allied to or associated with it, from the Cambrian Strata of North America, Spain, Sardinia, and Scotland. J. Geol. Soc. 1889, 45, 125–128. [Google Scholar] [CrossRef]
- Walcott, C.D. The Fauna of the Lower Cambrian or Olenellus Zone; 10th Annual Report; United States Geological Survey: Washington, DC, USA, 1894; pp. 599–602.
- Toll, E. Beiträge zur Kenntniss des Sibirschen Cambrium. Imp. Acad. Sci. St. Petersburg Mem. 1899, 8, 1–57. [Google Scholar]
- Öpik, A.A. Cymbric Vale fauna of New South Wales and Early Cambrian biostratigraphy. Aust. Gov. Publ. Serv. Bur. Miner. Resour. Geol. Geophys. Bull. 1975, 159, 1–78. [Google Scholar]
- Nitecki, M.H.; Toomey, D.F. Nature and classification of receptaculitids. Cent. Rech. Explor.-Prod. Elf-Aquitaine 1979, 3, 725–732. [Google Scholar]
- Meek, F.B. Note on Ethmophyllum and Archaeocyathus. Am. J. Sci. 2d Ser. 1868, 46, 144. [Google Scholar]
- Billings, E. Paleozoic Fossils; V. 1. Dawson Brothers; Geological Survey of Canada: Montreal, QC, USA, 1865; pp. 1–426.
- Walcott, C.D. Cambrian faunas of North America. U. S. Geol. Surv. Bull. 1886, 30, 72–89. [Google Scholar]
- Rowland, S.M. Archaeocyaths—A history of phylogenetic interpretation. J. Paleontol. 2001, 75, 1065–1078. [Google Scholar] [CrossRef]
- Balsam, W.L.; Vogel, S. Water movement in archaeocyathids: Evidence and implications of passive flow in models. J. Paleontol. 1973, 47, 979–984. [Google Scholar]
- Savarese, M. Functional analysis of archaeocyathan skeletal morphology and its paleobiological implications. Paleobiology 1992, 18, 464–480. [Google Scholar] [CrossRef]
- Zhuravlev, A.Y. A functional morphological approach to the biology of the Archaeocyatha. Neues Jahrb. Geol. Paläontol. Abh. 1993, 190, 315–327. [Google Scholar]
- Rigby, J.K.; Gangloff, R.A. Phylum Archaeocyatha. In Fossil Invertebrates; Boardman, R.S., Cheetham, A.H., Rowell, A.J., Eds.; Blackwell Scientific Publications: Palo Alto, CA, USA, 1987; pp. 107–115. [Google Scholar]
- Dzik, J. Evolution of ‘small shelly fossils’ assemblages of the Early Paleozoic. Acta Palaeontol. Pol. 1994, 39, 247–313. [Google Scholar]
- Wrona, R. Cambrian microfossils from glacial erratics of King George Island, Antarctica. Acta Palaeontol. Pol. 2004, 49, 13–56. [Google Scholar]
- Skovsted, C.B. Small Shelly fauna from the upper Lower Cambrian Bastion and Ella Island formations, north-east Greenland. J. Paleontol. 2006, 80, 1087–1112. [Google Scholar] [CrossRef]
- Smith, E.F.; Macdonald, F.A.; Petach, T.A.; Bold, U.; Schrag, D.P. Integrated stratigraphic, geochemical, and paleontological late Ediacaran to early Cambrian records from southwestern Mongolia. Geol. Soc. Am. Bull. 2016, 128, 442–468. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, Z.L.; Li, G.X.; Holmer, L.E. The Cambrian brachiopod fauna from the first-trilobite age Shuijingtuo Formation in the Three Gorges area of China. Paleoworld 2016, 25, 333–355. [Google Scholar] [CrossRef]
- Pruss, S.B.; Dwyer, C.H.; Smith, E.F.; Macdonald, F.A.; Tosca, N.J. Phosphatized early Cambrian archaeocyaths and small shelly fossils (SSFs) of southwestern Mongolia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 513, 166–177. [Google Scholar] [CrossRef]
- Wang, X.F.; Erdtmann, B.D.; Chen, X.H.; Mao, X.D. Integrated sequence-, bio- and chemostratigraphy of the terminal Proterozoic to Lowermost Cambrian. Epis. J. Int. Geosci. 1998, 21, 178–189. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Yang, A. Integrated Ediacaran (Sinian) chronostratigraphy of South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 254, 7–61. [Google Scholar] [CrossRef]
- Zhang, Z.; Robson, S.P.; Emig, C.; Shu, D. Early Cambrian radiation of brachiopods: A perspective from South China. Gondwana Res. 2008, 14, 241–254. [Google Scholar] [CrossRef]
- Wood, R.A.; Zhuravlev, A.Y.; Debrenne, F. Functional biology and ecology of Archaeocyatha. Palaios 1992, 7, 131–156. [Google Scholar] [CrossRef]
- Zhuravleva, I.T. O polozhenii arkheotsiat v filogeneticheskoy sisteme [On the position of archaeocyaths in the phylogenetic system]. Paleontol. Zhurnal 1959, 4, 30–40. [Google Scholar]
- Debrenne, F.; Zhuravlev, A.Y.; Kruse, P.D.; Selden, P.A. Systematic descriptions: Archaeocyatha. In Treatise on Invertebrate Paleontology; Stearn, C.W., Ed.; Part E, Porifera, Revised, Hypercalcified Porifera; University of Kansas Paleontological Institute: Lawrence, KS, USA, 2015; Volume 5, pp. 923–1084. [Google Scholar]
- Debrenne, F.; Zhuravlev, A.Y. Irregular Archaeocyaths: Morphology, Ontogeny, Systematics, Biostratigraphy, Palaeoecology; French National Museum Natural History: Paris, France, 1992; pp. 1–212. [Google Scholar]
- Kouchinsky, A.; Alexander, R.; Bengtson, S.; Bowyer, F.; Clausen, S.; Holmer, L.E.; Zhuravlev, A.Y. Early-middle Cambrian stratigraphy and faunas from northern Siberia. Acta Palaeontol. Pol. 2022, 67, 341–464. [Google Scholar] [CrossRef]
- Hill, D. Coelenterata. Rugosa and Tabulata. Part F. Supplement 1. In Treatise on Invertebrate Paleontology; Robison, R.A., Ed.; Geological Society of America: Boulder, CO, USA, 1981; Volume 1. [Google Scholar]
- Debrenne, F.; Reitner, J. Sponges, cnidarians, and ctenophores. In The Ecology of the Cambrian Radiation; Zhuravlev, A., Riding, R., Eds.; Columbia University Press: New York, NY, USA; Chichester, UK, 2000; pp. 301–325. [Google Scholar] [CrossRef]
- Taylor, T.G. The Archaeocyathinæ from the Cambrian of South Australia: With an account of the morphology and affinities of the whole class. Mem. Soc. S. Aust. 1910, 2, 55–188. [Google Scholar]
- Cohen, A.L.; McConnaughey, T.A. Geochemical perspectives on coral mineralization. Rev. Mineral. Geochem. 2003, 54, 151–187. [Google Scholar] [CrossRef]
- McKee, E.H. Ontogenetic stages of the archaeocyathid Ethmophyllum whitneyi Meek. J. Paleontol. 1963, 37, 287–293. [Google Scholar]
- Kruse, P.D.; Debrenne, F. Review of archaeocyath microstructure. Mem. Assoc. Australas. Palaeontol. 1989, 8, 133–141. [Google Scholar]
- Zhuravlev, A.Y. Poriferan aspects of archaeocyathan skeletal function. Mem. Assoc. Australas. Palaeontol. 1989, 8, 387–399. [Google Scholar]
- Kruse, P.D. Are archaeocyaths sponges, or are sponges archaeocyaths. Aust. Geol. Soc. Spec. Publ. 1990, 16, 310–323. [Google Scholar]
- Wood, R.A. Reefbuilding sponges. Am. Sci. 1990, 78, 224–235. [Google Scholar]
- Leys, S.P.; Hill, A. The physiology and molecular biology of sponge tissues. Adv. Mar. Biol. 2012, 62, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Vacelet, J. Une nouvelle relique du Secondaire: Un représentant actuel des éponges fossiles Sphinctozoaires. Comptes Rendus Acad. Sci. 1977, 285, 509–511. [Google Scholar]
- Wörheide, G.; Reitner, J. “Living fossil” sphinctozoan coralline sponge colonies in shallow water caves of the Osprey Reef (Coral Sea) and the Astrolabe Reefs (Fiji Islands). In Global and Regional Controls on Biogenic Sedimentation, Vol. 1. Reef Evolution; Reitner, J., Neuweiler, F., Gunkel, F., Eds.; Geologisch-Paläontologisches Institut der Georg-August-Universität: Göttingen, Germany, 1996; pp. 145–148. [Google Scholar] [CrossRef]
- Debrenne, F.; Vacelet, J. Archaeocyatha: Is the sponge model consistent with their structural organization. Palaeontogr. Am. 1984, 54, 358–369. [Google Scholar]
- Pickett, J. Vaceletia, a living archaeocyathid. N. Z. Geol. Surv. Rec. 1985, 9, 77. [Google Scholar]
- Reitner, J.; Wörheide, G.; Lange, R.; Thiel, V. Biomineralization of calcified skeletons in three Pacific coralline demosponges-an approach to the evolution of basal skeletons. Cour. Forschungsinstitut Senckenberg 1997, 201, 371–383. [Google Scholar] [CrossRef]
- Senowbari-Daryan, B.; Schäfer, P. Sphinctozoen (Kalkschwämme) aus den norischen Riffen von Sizilien. Facies 1986, 14, 235–283. [Google Scholar] [CrossRef]
- Luo, C.; Reitner, J. First report of fossil “keratose” demosponges in Phanerozoic carbonates: Preservation and 3-D reconstruction. Sci. Nat. 2014, 101, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.L. The Cell Biology of Sponges; Springer Science & Business Media: New York, NY, USA, 1984. [Google Scholar] [CrossRef]
- Zhuravleva, I.T. Arkheotsiaty Sibiri: Odnostennye arkheotsiaty: Otriady Monocyathida i Rhizacyathida; Izd-vo Akademii Nauk SSSR: Moscow, Russia, 1963. [Google Scholar]
- Zhuravleva, I.T. Biologiya arkheotsiat [Biology of archaeocyaths]. Tr. Inst. Geol. Geofiz. Sib. Otd. 1974, 276, 107–124. [Google Scholar]
- Zhuravleva, I.T.; Elkina, V.N. Arkheotsiaty Sibiri: Etmophilloidnye Arkheotsiaty [Arqueociatos de Siberia: Arqueociatos ethmofiloides]. Tr. Inst. Geol. Geofiz. Sib. Otd. Akad. Nauk SSSR 1974, 230, 1–167. [Google Scholar]
- Zhuravleva, I.T.; EI, M. Comparaison entre les Archaeata et les Porifera. Colloq. Int. CNRS 1979, 291, 521–526. [Google Scholar]
- Boyajian, G.E.; LaBarbera, M. Biomechanical analysis of passive flow of stromatoporoids—Morphologic, paleoecologic, and systematic implications. Lethaia 1987, 20, 223–229. [Google Scholar] [CrossRef]
- Hartman, W.D. Modern and ancient sclerospongiae. Ser. Geol. Notes Short Course 1983, 7, 116–129. [Google Scholar] [CrossRef]
- Stearn, C.W. The stromatoporoid animal. Lethaia 1975, 8, 89–100. [Google Scholar] [CrossRef]
- Briggs, D.E.; Siveter, D.J.; Siveter, D.J.; Sutton, M.D.; Rahman, I.A. An edrioasteroid from the Silurian Herefordshire Lagerstätte of England reveals the nature of the water vascular system in an extinct echinoderm. Proc. R. Soc. B. 2017, 284, 20171189. [Google Scholar] [CrossRef]
- Peel, J.S.; Streng, M.; Geyer, G.; Kouchinsky, A.; Skovsted, C.B. ‘Ovatoryctocara granulate’ assemblage (Cambrian series 2-series 3 boundary) of Londal, North Greenland. Australas. Palaeontol. Mem. 2016, 49, 241–282. [Google Scholar]
- McMenamin, M.A.S. Dynamic Paleontology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- McMenamin, M.A.S. The Cambrian Explosion: Macroevolution and biomineralization. Acad. Biol. 2023, 1. [Google Scholar] [CrossRef]

| H | D(C) | Int | IS | N | RK | IK | IC | D (OWP) | D (IWP) | D (SPP) | D (TAP) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | 27 | 52 | 54 | 61 | 54 | 21 | 23 | 54 | 103 | 48 | 65 | 4 |
| Min | 1.04 | 0.59 | 0.1 | 0.1 | 6 | 8.38 | 0.19 | 0.29 | 0.01 | 0.05 | 0.03 | 0.06 |
| Max | 4.58 | 1.88 | 0.57 | 0.48 | 12 | 17.19 | 0.32 | 1.4 | 0.11 | 0.16 | 0.1 | 0.14 |
| Mean | 2.04 | 1.06 | 0.31 | 0.24 | 9 | 11.74 | 0.28 | 0.81 | 0.05 | 0.1 | 0.07 | 0.09 |
| SD | 0.6 | 0.34 | 0.11 | 0.07 | 1 | 2.95 | 0.026 | 0.21 | 0.03 | 0.03 | 0.018 | 0.015 |
| D(C) | Int | IK | D (CA) | T (CA) | D (OWP) | D (IWP) | D (SPI) | L (SPI) | |
|---|---|---|---|---|---|---|---|---|---|
| Count | 3 | 2 | 2 | 26 | 20 | 5 | 3 | 3 | 3 |
| Min | 1.47 | 0.46 | 0.23 | 0.02 | 0.003 | 0.029 | 0.05 | 0.015 | 0.02 |
| Max | 2.01 | 0.95 | 0.58 | 0.137 | 0.018 | 0.112 | 0.11 | 0.035 | 0.04 |
| Mean | 1.82 | 0.72 | 0.38 | 0.06 | 0.008 | 0.056 | 0.08 | 0.026 | 0.03 |
| SD | 0.16 | 0.18 | 0.13 | 0.015 | 0.003 | 0.022 | 0.018 | 0.005 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Song, B.; Liang, Y.; Liang, K.; Zhang, Z. The Internal Anatomy and Water Current System of Cambrian Archaeocyaths of South China. Life 2024, 14, 167. https://doi.org/10.3390/life14020167
Wang J, Song B, Liang Y, Liang K, Zhang Z. The Internal Anatomy and Water Current System of Cambrian Archaeocyaths of South China. Life. 2024; 14(2):167. https://doi.org/10.3390/life14020167
Chicago/Turabian StyleWang, Jiayue, Baopeng Song, Yue Liang, Kun Liang, and Zhifei Zhang. 2024. "The Internal Anatomy and Water Current System of Cambrian Archaeocyaths of South China" Life 14, no. 2: 167. https://doi.org/10.3390/life14020167
APA StyleWang, J., Song, B., Liang, Y., Liang, K., & Zhang, Z. (2024). The Internal Anatomy and Water Current System of Cambrian Archaeocyaths of South China. Life, 14(2), 167. https://doi.org/10.3390/life14020167







