Immunomodulatory Properties of Multi-Strain Postbiotics on Human CD14+ Monocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Postbiotic Preparation
2.2. Primary PBMC Sourcing and Culture
2.3. CD14+ Monocyte Isolation
2.4. Exposure of CD14+ Monocytes to Postbiotics
2.5. RNA Extraction
2.6. TaqMan Probe Design
2.7. RT-qPCR
2.8. Luminex Analysis
2.9. Statistical Analysis
3. Results
3.1. Immune Response Timing
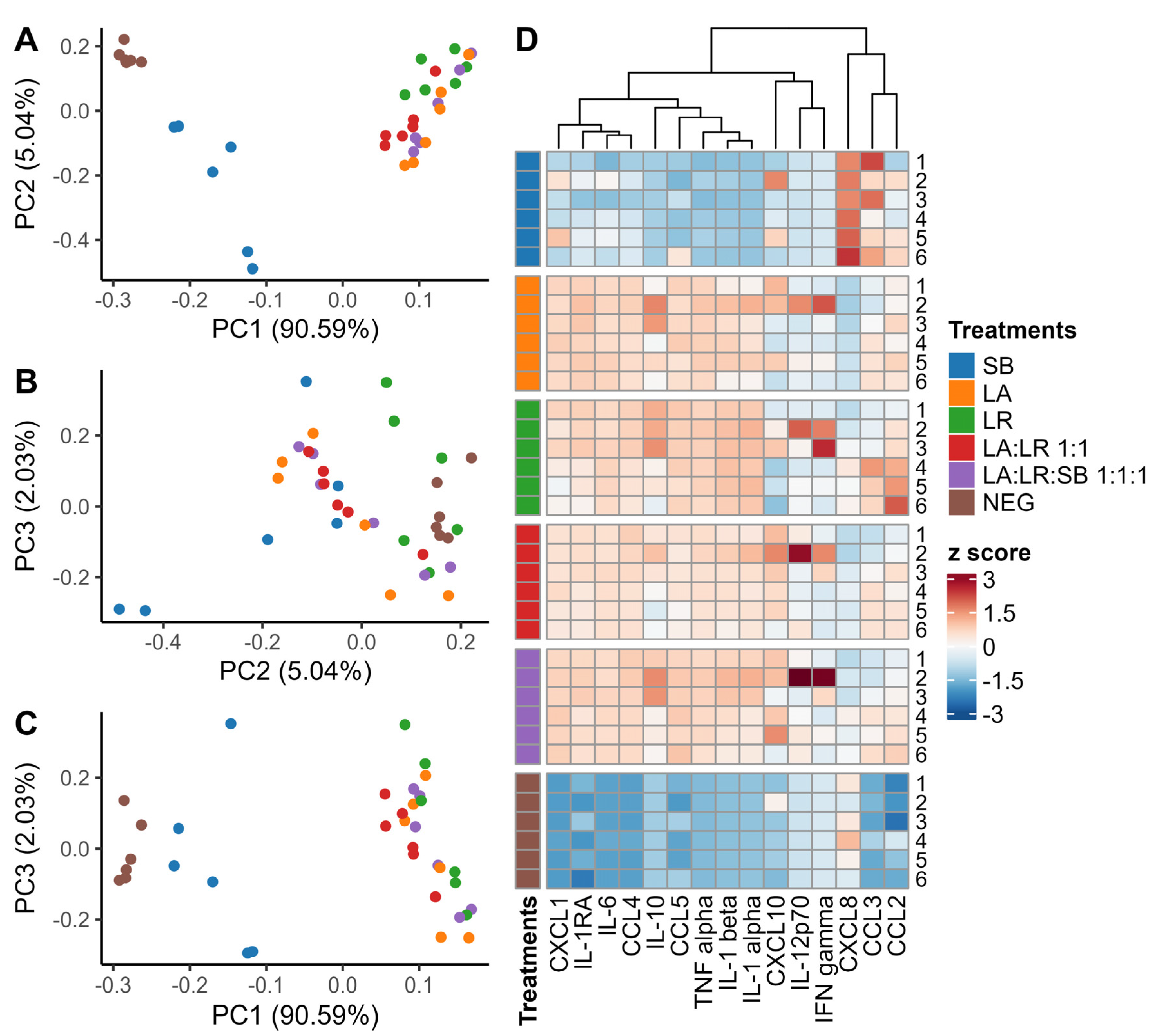
3.2. Characterization of Cytokines Following Co-Culture with Postbiotics
3.3. Chemokine Production
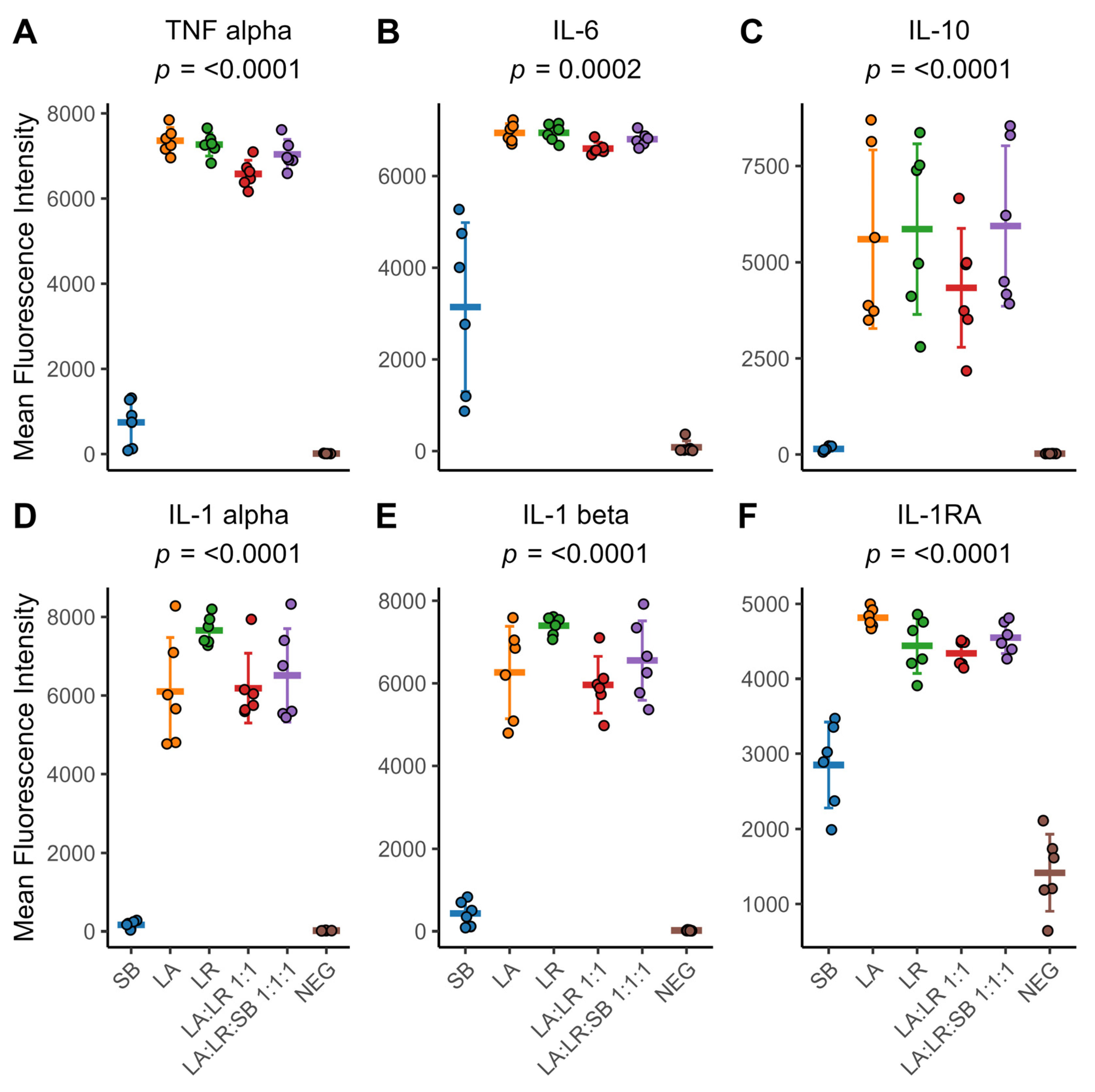
3.4. Cytokine Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef]
- Retuerto, M.; La-Monica, M.B.; Ziegenfuss, T.N.; Raub, B.; McCormick, T.S.; Ghannoum, M.A. Probiotic supplementation of pea-derived protein alters the gut microbiome balance in favor of increased protein degradation, reflected in increased levels of essential amino acid in human plasma. Gastroenterol. Hepatol. Open Access 2023, 4, 93–103. [Google Scholar] [CrossRef]
- Hager, C.L.; Isham, N.; Schrom, K.P.; Chandra, J.; McCormick, T.; Miyagi, M.; Ghannoum, M.A. Effects of a Novel Probiotic Combination on Pathogenic Bacterial-Fungal Polymicrobial Biofilms. mBio 2019, 10, e00338-19. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Liu, C.; Ma, N.; Feng, Y.; Zhou, M.; Li, H.; Zhang, X.; Ma, X. From probiotics to postbiotics: Concepts and applications. Anim. Res. One Health 2023, 1, 92–114. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jia, A.; Li, Y.; Bi, Y.; Liu, G. Microbiota regulate the development and function of the immune cells. Int. Rev. Immunol. 2018, 37, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Cheraghpour, M.; Fatemi, N.; Shadnoush, M.; Talebi, G.; Tierling, S.; Bermúdez-Humarán, L.G. Immunomodulation aspects of gut microbiome-related interventional strategies in colorectal cancer. Med. Oncol. 2024, 41, 231. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, K.M.; Fletcher, D.H.; Gileau, L.; Kandolo, A. Lactic acid bacteria decrease Salmonella enterica Javiana virulence and modulate host inflammation during infection of an intestinal epithelial cell line. Pathog. Dis. 2019, 77, ftz025. [Google Scholar] [CrossRef] [PubMed]
- Coconnier, M.H.; Bernet, M.F.; Chauviere, G.; Servin, A.L. Adhering heat-killed human Lactobacillus acidophilus, strain LB, inhibits the process of pathogenicity of diarrhoeagenic bacteria in cultured human intestinal cells. J. Diarrhoeal. Dis. Res. 1993, 11, 235–242. [Google Scholar]
- Magryś, A.; Pawlik, M. Postbiotic Fractions of Probiotics Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG Show Immune-Modulating Effects. Cells 2023, 12, 2538. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Jin, Y.; Huang, K.; Zhang, Y.; Liang, Z. Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients 2023, 15, 1484. [Google Scholar] [CrossRef]
- Soler, D.C.; Kerstetter-Fogle, A.; Young, A.B.; Rayman, P.; Finke, J.H.; Debanne, S.M.; Cooper, K.D.; Barnholtz-Sloan, J.; Sloan, A.E.; McCormick, T.S. Healthy myeloid-derived suppressor cells express the surface ectoenzyme Vanin-2 (VNN2). Mol. Immunol. 2022, 142, 1–10. [Google Scholar] [CrossRef]
- Thom, R.E.; Eastaugh, L.S.; O’Brien, L.M.; Ulaeto, D.O.; Findlay, J.S.; Smither, S.J.; Phelps, A.L.; Stapleton, H.L.; Hamblin, K.A.; Weller, S.A. Evaluation of the SARS-CoV-2 Inactivation Efficacy Associated With Buffers From Three Kits Used on High-Throughput RNA Extraction Platforms. Front. Cell. Infect. Microbiol. 2021, 11, 716436. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Nikoo, H.R.; Fotouhi, F.; Khosravi, A. Development of a robust TaqMan probe-based one-step multiplex RT-qPCR for simultaneous detection of SARS-CoV-2 and Influenza A/B viruses. BMC Microbiol. 2023, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Isali, I.; McClellan, P.; Wong, T.R.; Hijaz, S.; Fletcher, D.R.; Liu, G.; Bonfield, T.L.; Anderson, J.M.; Hijaz, A.; Akkus, O. Differential effects of macrophage subtype-specific cytokines on fibroblast proliferation and endothelial cell function in co-culture system. J. Biomed. Mater. Res. A 2024. ahead of print. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Muhammad Ali, G.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Feng, D. Therapeutic effect of Saccharomyces boulardii combined with Bifidobacterium and on cellular immune function in children with acute diarrhea. Exp. Ther. Med. 2019, 18, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Pique, N.; Berlanga, M.; Minana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Jorjao, A.L.; de Oliveira, F.E.; Leao, M.V.; Carvalho, C.A.; Jorge, A.O.; de Oliveira, L.D. Live and Heat-Killed Lactobacillus rhamnosus ATCC 7469 May Induce Modulatory Cytokines Profiles on Macrophages RAW 264.7. Sci. World J. 2015, 2015, 716749. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M. Immunomodulatory mechanisms of lactobacilli. Microb. Cell Fact. 2011, 10 (Suppl. 1), S17. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Sivamaruthi, B.; Sirilun, S.; Peerajan, S.; Kesika, P.; Chaiyasut, K.; Chaiyasut, C. Extraction of β-glucan from Saccharomyces cerevisiae: Comparison of different extraction methods and in vivo assessment of immunomodulatory effect in mice. Food Sci. Technol. Camp. 2017, 37, 124–130. [Google Scholar] [CrossRef]
- Pelizon, A.C.; Kaneno, R.; Soares, A.M.; Meira, D.A.; Sartori, A. Immunomodulatory activities associated with beta-glucan derived from Saccharomyces cerevisiae. Physiol. Res. 2005, 54, 557–564. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, K.D.; Ahmed, S.; San Valentin, E.; Di Martino, L.; McCormick, T.S.; Ghannoum, M.A. Immunomodulatory Properties of Multi-Strain Postbiotics on Human CD14+ Monocytes. Life 2024, 14, 1673. https://doi.org/10.3390/life14121673
Roberts KD, Ahmed S, San Valentin E, Di Martino L, McCormick TS, Ghannoum MA. Immunomodulatory Properties of Multi-Strain Postbiotics on Human CD14+ Monocytes. Life. 2024; 14(12):1673. https://doi.org/10.3390/life14121673
Chicago/Turabian StyleRoberts, Kyle D., Sadia Ahmed, Erin San Valentin, Luca Di Martino, Thomas S. McCormick, and Mahmoud A. Ghannoum. 2024. "Immunomodulatory Properties of Multi-Strain Postbiotics on Human CD14+ Monocytes" Life 14, no. 12: 1673. https://doi.org/10.3390/life14121673
APA StyleRoberts, K. D., Ahmed, S., San Valentin, E., Di Martino, L., McCormick, T. S., & Ghannoum, M. A. (2024). Immunomodulatory Properties of Multi-Strain Postbiotics on Human CD14+ Monocytes. Life, 14(12), 1673. https://doi.org/10.3390/life14121673





