CRISPR/Cas9 Edition of the F9 Gene in Human Mesenchymal Stem Cells for Hemophilia B Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines Selection and Culture Conditions
2.2. RNA Guides Design
2.3. Vector Design and Production
2.4. Genome Cell Knockout
2.5. CRISPR/Cas9 Knockin Assay
2.6. Lentivirus Particles Productio
2.7. Overexpression FIX Models
2.8. Validation of Lentiviral Secretory FIX Models
3. Results
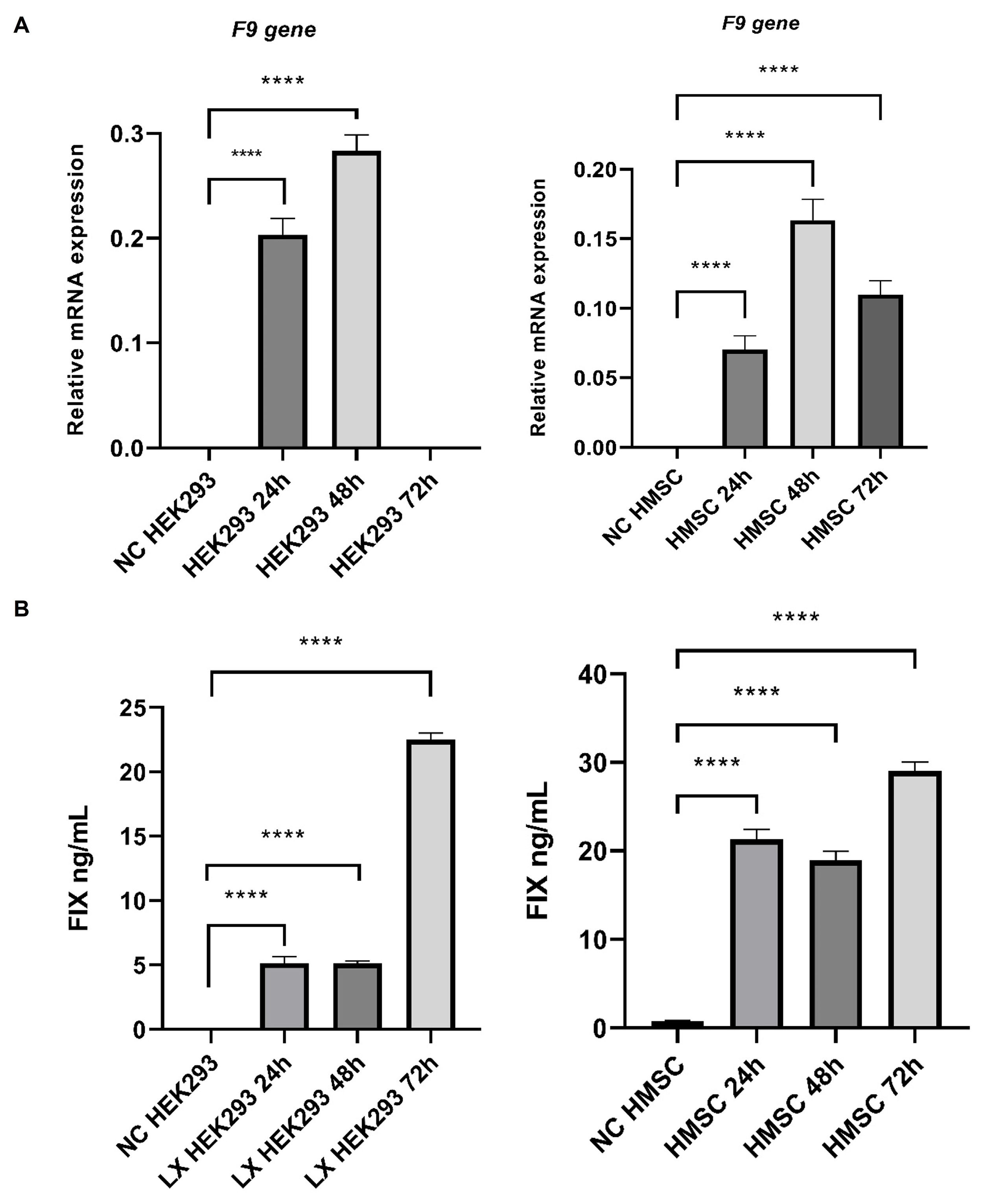
3.1. Knockout and Knockin Cellular Models with CRISPR/Cas9 System
3.2. Human F9 Gene and FIX Protein Secretion Models with Lentiviral Vectors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peyvandi, F.; Garagiola, I.; Young, G. The past and future of haemophilia: Diagnosis, treatments, and its complications. Lancet 2016, 388, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Registro Mexicano de Coagulopatías. Federación de Hemofilia de la República Mexicana, A.C. Available online: https://hemofilia.org.mx/registro-mexicano-de-coagulopatias/ (accessed on 19 September 2024).
- Nazeef, M.; Sheehan, J. New developments in the management of moderate-to-severe hemophilia B. J. Blood Med. 2016, 7, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Rallapalli, P.; Kemball-Cook, G.; Tuddenham, E.; Gomez, K.; Perkins, S. An interactive mutation database for human coagulation factor IX provides novel insights into the phenotypes and genetics of hemophilia B. J. Thromb. Haemost. 2013, 11, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M. Hemophilia therapy: The future has begun. Haematologica 2020, 105, 545–553. [Google Scholar] [CrossRef]
- Croteau, S.E.; Wang, M.; Wheeler, A.P. 2021 clinical trials update: Innovations in hemophilia therapy. Am. J. Hematol. 2021, 96, 128–144. [Google Scholar] [CrossRef]
- Lara-Navarro, I.J.; Jaloma-Cruz, A.R. Current Therapies in Hemophilia: From Plasma-Derived Factor Modalities to CRISPR/Cas Alternatives. Tohoku J. Exp. Med. 2022, 256, 197–207. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Davidoff, A.M.; Tuddenham, E.G.D. Advances in Gene Therapy for Hemophilia. Hum. Gene Ther. 2017, 28, 1004–1012. [Google Scholar] [CrossRef]
- George, L.A.; Sullivan, S.K.; Giermasz, A.; Rasko, J.E.J.; Samelson-Jones, B.J.; Ducore, J.; Cuker, A.; Sullivan, L.M.; Majumdar, S.; Teitel, J.; et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017, 377, 2215–2227. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Olmedillas López, S.; Garcia-Arranz, M.; Garcia-Olmo, D.; Liras, A. Preliminary study on non-viral transfection of F9 (factor IX) gene by nucleofection in human adipose-derived mesenchymal stem cells. PeerJ 2016, 4, e1907. [Google Scholar] [CrossRef]
- Han, A.R.; Shin, H.R.; Kwon, J.; Lee, S.B.; Lee, S.E.; Kim, E.Y.; Kweon, J.; Chang, E.J.; Kim, Y.; Kim, S.W. Highly efficient genome editing via CRISPR-Cas9 ribonucleoprotein (RNP) delivery in mesenchymal stem cells. BMB Rep. 2024, 57, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, W.; Zhao, L.; Yao, M.; Wu, C.; Su, P.; Yang, L.; Wang, G. Generation of an mESC model with a human hemophilia B nonsense mutation via CRISPR/Cas9 technology. Stem Cell Res. Ther. 2022, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for sgRNA Design, Off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; An, B.; Yu, B.; Peng, X.; Yuan, H.; Yang, Q.; Chen, X.; Yu, T.; Wang, L.; Zhang, X.; et al. CRISPR/Cas9-mediated knockin of human factor IX into swine factor IX locus effectively alleviates bleeding in hemophilia B pigs. Haematologica 2021, 106, 829–837. [Google Scholar] [CrossRef]
- Samelson-Jones, B.J.; George, L.A. Adeno-Associated Virus Gene Therapy for Hemophilia. Annu. Rev. Med. 2023, 74, 231–247. [Google Scholar] [CrossRef]
- de Sousa Bomfim, A.; Cristina Corrêa de Freitas, M.; Picanço-Castro, V.; de Abreu Soares Neto, M.; Swiech, K.; Tadeu Covas, D.; Maria de Sousa Russo, E. Human cell lines: A promising alternative for recombinant FIX production. Protein. Expr. Purif. 2016, 121, 149–156. [Google Scholar] [CrossRef]
- Dodd, M.; Marquez-Curtis, L.; Janowska-Wieczorek, A.; Hortelano, G. Sustained expression of coagulation factor IX by modified cord blood-derived mesenchymal stromal cells. J. Gene Med. 2014, 16, 131–142. [Google Scholar] [CrossRef]
- Hortelano, G.; Xu, N.; Vandenberg, A.; Solera, J.; Chang, P.L.; Ofosu, F.A. Persistent delivery of factor IX in mice: Gene therapy for hemophilia using implantable microcapsules. Hum. Gene Ther. 1999, 10, 1281–1288. [Google Scholar] [CrossRef]
- Lisjak, M.; De Caneva, A.; Marais, T.; Barbon, E.; Biferi, M.G.; Porro, F.; Barzel, A.; Zentilin, L.; Kay, M.A.; Mingozzi, F.; et al. Promoterless Gene Targeting Approach Combined to CRISPR/Cas9 Efficiently Corrects Hemophilia B Phenotype in Neonatal Mice. Front. Genome Ed. 2022, 4, 785698. [Google Scholar] [CrossRef]
- Morishige, S.; Mizuno, S.; Ozawa, H.; Nakamura, T.; Mazahery, A.; Nomura, K.; Seki, R.; Mouri, F.; Osaki, K.; Yamamura, K.; et al. CRISPR/Cas9-mediated gene correction in hemophilia B patient-derived iPSCs. Int. J. Hematol. 2020, 111, 225–233. [Google Scholar] [CrossRef]
- Soroka, A.B.; Feoktistova, S.G.; Mityaeva, O.N.; Volchkov, P.Y. Gene Therapy Approaches for the Treatment of Hemophilia B. Int. J. Mol. Sci. 2023, 24, 10766. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Yu, H.; Tian, Y.; Chen, X.; Chen, C.; Ren, Y.; Chen, Z.; Ren, Y.; Gong, X.; et al. Protective effect of Nr4a2 (Nurr1) against LPS-induced depressive-like behaviors via regulating activity of microglia and CamkII neurons in anterior cingulate cortex. Pharmacol. Res. 2023, 191, 106717. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, J.P.; Song, D.W.; Lee, G.S.; Choi, B.S.; Kim, M.; Lee, Y.; Kim, S.; Lee, H.; Yeom, S.C. In vivo genome editing for hemophilia B therapy by the combination of rebalancing and therapeutic gene knockin using a viral and non-viral vector. Mol. Ther. Nucleic Acids 2023, 32, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Boletín informativo de la Academia Mexicana de Ciencias. Academia Mexicana de Ciencias. Edición genética con la técnica CRISPR/Cas9. Boletín Inf. Acad. Mex. Cienc. 2016, 55, 5–18. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Navarro, I.J.; Jave-Suárez, L.F.; Marchal, J.A.; Jaloma-Cruz, A.R. CRISPR/Cas9 Edition of the F9 Gene in Human Mesenchymal Stem Cells for Hemophilia B Therapy. Life 2024, 14, 1640. https://doi.org/10.3390/life14121640
Lara-Navarro IJ, Jave-Suárez LF, Marchal JA, Jaloma-Cruz AR. CRISPR/Cas9 Edition of the F9 Gene in Human Mesenchymal Stem Cells for Hemophilia B Therapy. Life. 2024; 14(12):1640. https://doi.org/10.3390/life14121640
Chicago/Turabian StyleLara-Navarro, Irving Jair, Luis Felipe Jave-Suárez, Juan Antonio Marchal, and Ana Rebeca Jaloma-Cruz. 2024. "CRISPR/Cas9 Edition of the F9 Gene in Human Mesenchymal Stem Cells for Hemophilia B Therapy" Life 14, no. 12: 1640. https://doi.org/10.3390/life14121640
APA StyleLara-Navarro, I. J., Jave-Suárez, L. F., Marchal, J. A., & Jaloma-Cruz, A. R. (2024). CRISPR/Cas9 Edition of the F9 Gene in Human Mesenchymal Stem Cells for Hemophilia B Therapy. Life, 14(12), 1640. https://doi.org/10.3390/life14121640









