Reactivating Sleeping Intramedullary Nail in a 16-Year-Old Female with Polyostotic Fibrous Dysplasia: A Case Report on Complications and Potential Solutions
Abstract
1. Introduction
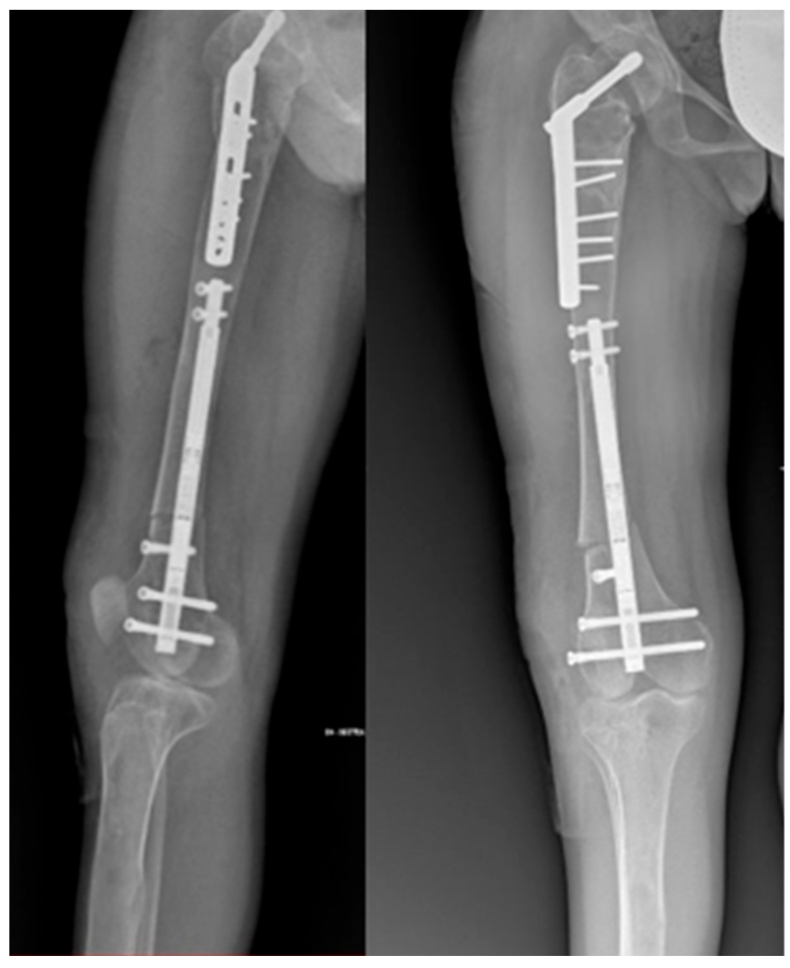
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.Y.; Shim, J.H.; Heo, C.Y. A Rare Skeletal Disorder, Fibrous Dysplasia: A Review of Its Pathogenesis and Therapeutic Prospects. Int. J. Mol. Sci. 2023, 24, 15591. [Google Scholar] [CrossRef] [PubMed]
- Hartley, I.; Zhadina, M.; Collins, M.T.; Boyce, A.M. Fibrous Dysplasia of Bone and McCune–Albright Syndrome: A Bench to Bedside Review. Calcif. Tissue Int. 2019, 104, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Vescini, F.; Falchetti, A.; Tonelli, V.; Carpentieri, M.; Cipri, C.; Cosso, R.; Kara, E.; Triggiani, V.; Grimaldi, F. Mazabraud’s Syndrome: A Case Report and Up-To-Date Literature Review. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Dicaprio, M.R. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J. Bone Jt. Surg. 2005, 87-A, 1848–1864. [Google Scholar]
- Zhai, X.; Duan, L.; Yao, Y.; Xing, B.; Deng, K.; Wang, L.; Feng, F.; Liang, Z.; You, H.; Yang, H.; et al. Clinical Characteristics and Management of Patients With McCune-Albright Syndrome With GH Excess and Precocious Puberty: A Case Series and Literature Review. Front. Endocrinol. 2021, 12, 672394. [Google Scholar] [CrossRef]
- Popkov, A.; Ducic, B.S.; Lazovi, M.; Lascombes, P.; Popkov, D. Limb lengthening and deformity correction in children with abnormal bone. Injury 2019, 50, S79–S86. [Google Scholar] [CrossRef]
- Ippolito, E.; Farsetti, P.; Caterini, R.; Gorgolini, G.; Caterini, A.; De Maio, F. Lower-limb intramedullary nailing in patients with polyostotic fibrous dysplasia who had a previous unsuccessful treatment. A report of 48 cases. J. Orthop. Traumatol. 2023, 24, 35. [Google Scholar] [CrossRef]
- Stanton, R.P.; Ippolito, E.; Springfield, D.; Lindaman, L.; Wientroub, S.; Leet, A. The surgical management of fibrous dysplasia of bone. Orphanet J. Rare Dis. 2012, 7 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Fang, X.; Liu, H.; Lang, Y.; Xiong, Y.; Duan, H. Fibrous dysplasia of bone: Surgical management options and outcomes of 22 cases. Mol. Clin. Oncol. 2018, 9, 98–103. [Google Scholar] [CrossRef]
- Hampton, M.J.; Weston-Simmons, S.; Giles, S.N.; Fernandes, J.A. Deformity correction, surgical stabilisation and limb length equalisation in patients with fibrous dysplasia: A 20-year experience. Strateg. Trauma. Limb Reconstr. 2021, 16, 41–45. [Google Scholar] [CrossRef]
- Popkov, A.; Aranovich, A.; Antonov, A.; Journeau, P.; Lascombes, P.; Popkov, D. Lower limb lengthening and deformity correction in polyostotic fibrous dysplasia using external fixation and flexible intramedullary nailing. J. Orthop. 2020, 21, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.P. Surgery for fibrous dysplasia. J. Bone Miner. Res. 2007, 22 (Suppl. 2), P105–P109. [Google Scholar] [CrossRef] [PubMed]
- Karakoyun, O.; Sokucu, S.; Erol, M.F.; Kucukkaya, M.; Kabukcuoglu, Y.S. Use of a magnetic bone nail for lengthening of the femur and tibia. J. Orthop. Surg. 2016, 24, 374–378. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Nahm, N.J.; Dahl, M.T. Re-use of Motorised Intramedullary Limb Lengthening Nails. Strateg. Trauma Limb Reconstr. 2023, 18, 106–110. [Google Scholar] [CrossRef]
- Szymczuk, V.L.; Hammouda, A.I.; Gesheff, M.G.; Standard, S.C.; Herzenberg, J.E. Lengthening With Monolateral External Fixation Versus Magnetically Motorized Intramedullary Nail in Congenital Femoral Deficiency. J. Pediatr. Orthop. 2019, 39, 458–465. [Google Scholar] [CrossRef]
- Baumgart, R. The reverse planning method for lengthening of the lower limb using a straight intramedullary nail with or without deformity correction. A new method. Oper. Orthop. Traumatol. 2009, 21, 221–233. [Google Scholar] [CrossRef]
- Li, R.; Saleh, M.; Yang, L.; Coulton, L. Radiographic classification of osteogenesis during bone distraction. J. Orthop. Res. 2006, 24, 339–347. [Google Scholar] [CrossRef]
- Iliadis, A.D.; Palloni, V.; Wright, J.; Goodier, D.; Calder, P. Pediatric Lower Limb Lengthening Using the PRECICE Nail: Our Experience with 50 Cases. J. Pediatr. Orthop. 2021, 41, e44–e49. [Google Scholar] [CrossRef]
- Alrabai, H.M. Breakage of a re-activated PRECICE® nail: A case report. Int. J. Surg. Case Rep. 2023, 106, 108182. [Google Scholar] [CrossRef]
- Couto, A.; Freitas, J.; Alegrete, N.; Coutinho, J.; Costa, G. Two consecutive limb lengthenings with the same PRECICE nail: A technical note. Strateg. Trauma Limb Reconstr. 2018, 13, 199–204. [Google Scholar] [CrossRef]
- Eltayeby, H.H.; Alrabai, H.M.; Jauregui, J.J.; Shabtai, L.Y.; Herzenberg, J.E. Post-retrieval functionality testing of PRECICE lengthening nails: The “Sleeper” nail concept. J. Clin. Orthop. Trauma 2020, 14, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Hvid, I.; Huhnstock, S.; Breen, A.B.; Steen, H. Limb lengthening and deformity correction with externally controlled motorized intramedullary nails: Evaluation of 50 consecutive lengthenings. Acta Orthop. 2019, 90, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Safi, İ.K.A.; Samadov, F.; Kanar, M.; Tüter, İ.; Özdemir, H.M. Deformity correction and limb lengthening with externally controlled motorized extendable intramedullary nails: Comparison of 2 different nails. Acta Orthop. Traumatol. Turc. 2023, 57, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.R.; McKay, J.E.; Timms, A.J.; Roskrow, T.; Fugazzotto, S.; Edel, P.; Goodier, W.D. Femoral lengthening using the Precice intramedullary limb-lengthening system: Outcome comparison following antegrade and retrograde nails. Bone Jt. J. 2019, 101-B, 1168–1176. [Google Scholar] [CrossRef]
- Alonso-Hernández, J.; Galán-Olleros, M.; Miranda-Gorozarri, C.; Egea-Gámez, R.M.; Palazón-Quevedo, Á. Two-stage Bone Lengthening with Reuse of a Single Intramedullary Telescopic Nail in Patients with Achondroplasia. J. Pediatr. Orthop. 2022, 42, E616–E622. [Google Scholar] [CrossRef]
- Gomez-Alessandri, J.; Sanpera-Iglesias, J.; Raluy-Collado, D.; Sanpera, I. Maximizing length with precice nail: A novel technique. J. Pediatr. Orthop. B 2022, 31, E85–E89. [Google Scholar] [CrossRef]
- Kariksiz, M.; Karakoyun, O. Limb lengthening with one Precice nail over its capacity. Saudi Med. J. 2019, 40, 1058–1062. [Google Scholar] [CrossRef]
- Teulières, M.; Langlais, T.; Sales de Gauzy, J.; Rölfing, J.D.; Accadbled, F. Bone Lengthening with a Motorized Intramedullary Nail in 34 Patients with Posttraumatic Limb Length Discrepancies. J. Clin. Med. 2021, 10, 2393. [Google Scholar] [CrossRef]
- Dabash, S.; Zhang, D.T.; Rozbruch, S.R.; Fragomen, A.T. Blocking Screw-assisted Intramedullary Nailing Using the Reverse-rule-of-thumbs for Limb Lengthening and Deformity Correction. Strateg. Trauma Limb Reconstr. 2019, 14, 77–84. [Google Scholar] [CrossRef]
- Wright, S.E.; David Goodier, W.; Calder, P. Regenerate Deformity with the Precice Tibial Nail. Strateg. Trauma Limb Reconstr. 2020, 15, 98–105. [Google Scholar] [CrossRef]
- Paley, D. Problems, Obstacles, and Complications of Limb Lengthening by the Ilizarov Technique. Clin. Orthop. Relat. Res. 1990, 250, 81–104. [Google Scholar] [CrossRef]
- Lascombes, P.; Popkov, D.; Huber, H.; Haumont, T.; Journeau, P. Classification of complications after progressive long bone lengthening: Proposal for a new classification. Orthop. Traumatol. Surg. Res. 2012, 98, 629–637. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todisco, M.; Viotto, M.; Campanacci, L.; Di Gennaro, G.L.; Depaoli, A.; Rocca, G.; Trisolino, G. Reactivating Sleeping Intramedullary Nail in a 16-Year-Old Female with Polyostotic Fibrous Dysplasia: A Case Report on Complications and Potential Solutions. Life 2024, 14, 1543. https://doi.org/10.3390/life14121543
Todisco M, Viotto M, Campanacci L, Di Gennaro GL, Depaoli A, Rocca G, Trisolino G. Reactivating Sleeping Intramedullary Nail in a 16-Year-Old Female with Polyostotic Fibrous Dysplasia: A Case Report on Complications and Potential Solutions. Life. 2024; 14(12):1543. https://doi.org/10.3390/life14121543
Chicago/Turabian StyleTodisco, Marco, Marianna Viotto, Laura Campanacci, Giovanni Luigi Di Gennaro, Alessandro Depaoli, Gino Rocca, and Giovanni Trisolino. 2024. "Reactivating Sleeping Intramedullary Nail in a 16-Year-Old Female with Polyostotic Fibrous Dysplasia: A Case Report on Complications and Potential Solutions" Life 14, no. 12: 1543. https://doi.org/10.3390/life14121543
APA StyleTodisco, M., Viotto, M., Campanacci, L., Di Gennaro, G. L., Depaoli, A., Rocca, G., & Trisolino, G. (2024). Reactivating Sleeping Intramedullary Nail in a 16-Year-Old Female with Polyostotic Fibrous Dysplasia: A Case Report on Complications and Potential Solutions. Life, 14(12), 1543. https://doi.org/10.3390/life14121543







