Desmodesmus pannonicus Water Extract Inhibits Melanin Synthesis and Promotes Wound Healing
Abstract
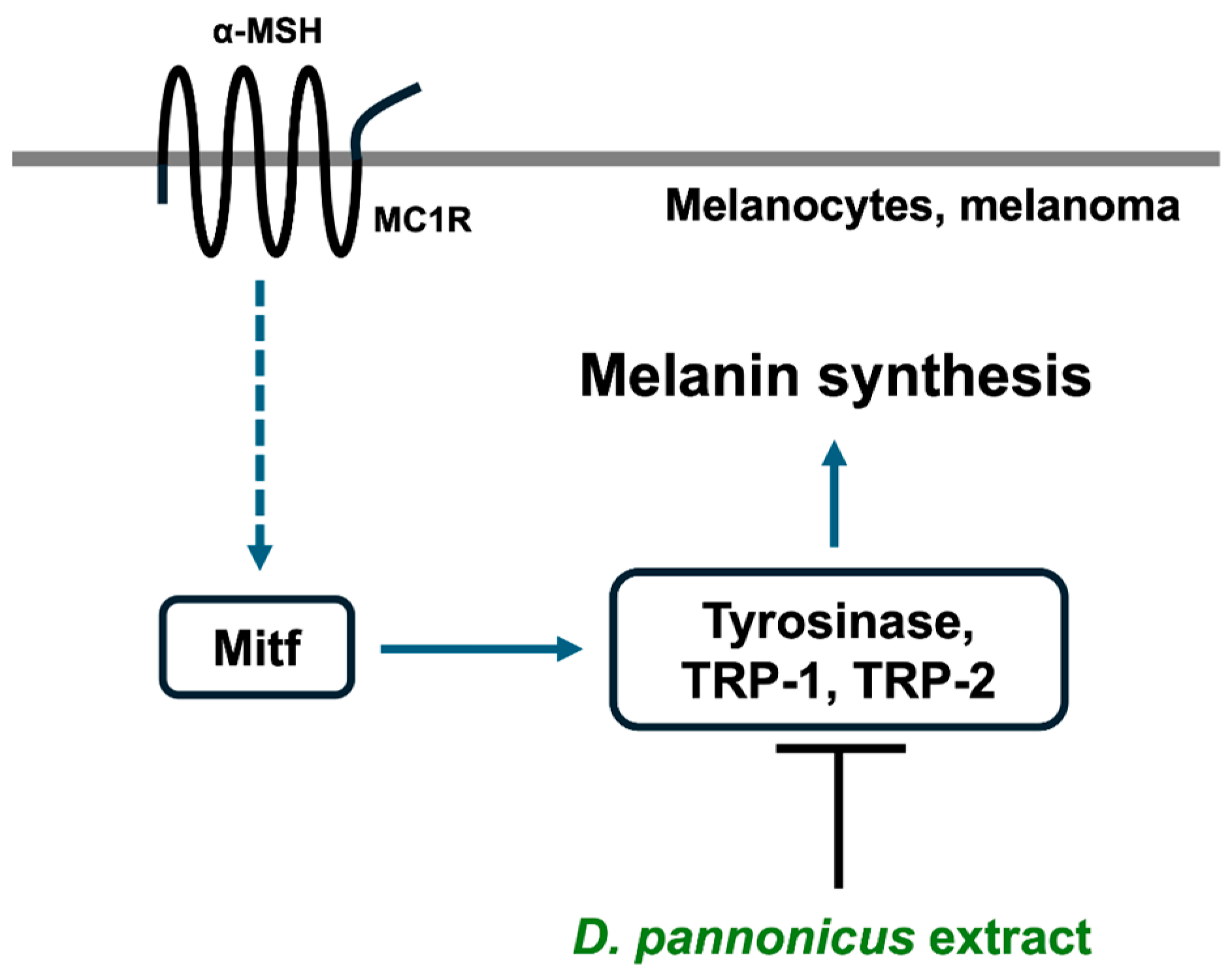
1. Introduction
2. Materials and Methods
2.1. Collection and Cultivation of Desmodesmus pannonicus
2.2. Cell Culture
2.3. Assay of 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) Radical Scavenging Activity
2.4. Cell Viability and Proliferation
2.5. Melanin Content
2.6. Activities of Mushroom Tyrosinase
2.7. Intracellular Tyrosinase Activity Analysis (DOPA Staining)
2.8. Western Blotting
2.9. Quantitative RT-PCR
2.10. Scratch Wound-Healing Assay
2.11. Statistical Analysis
3. Results
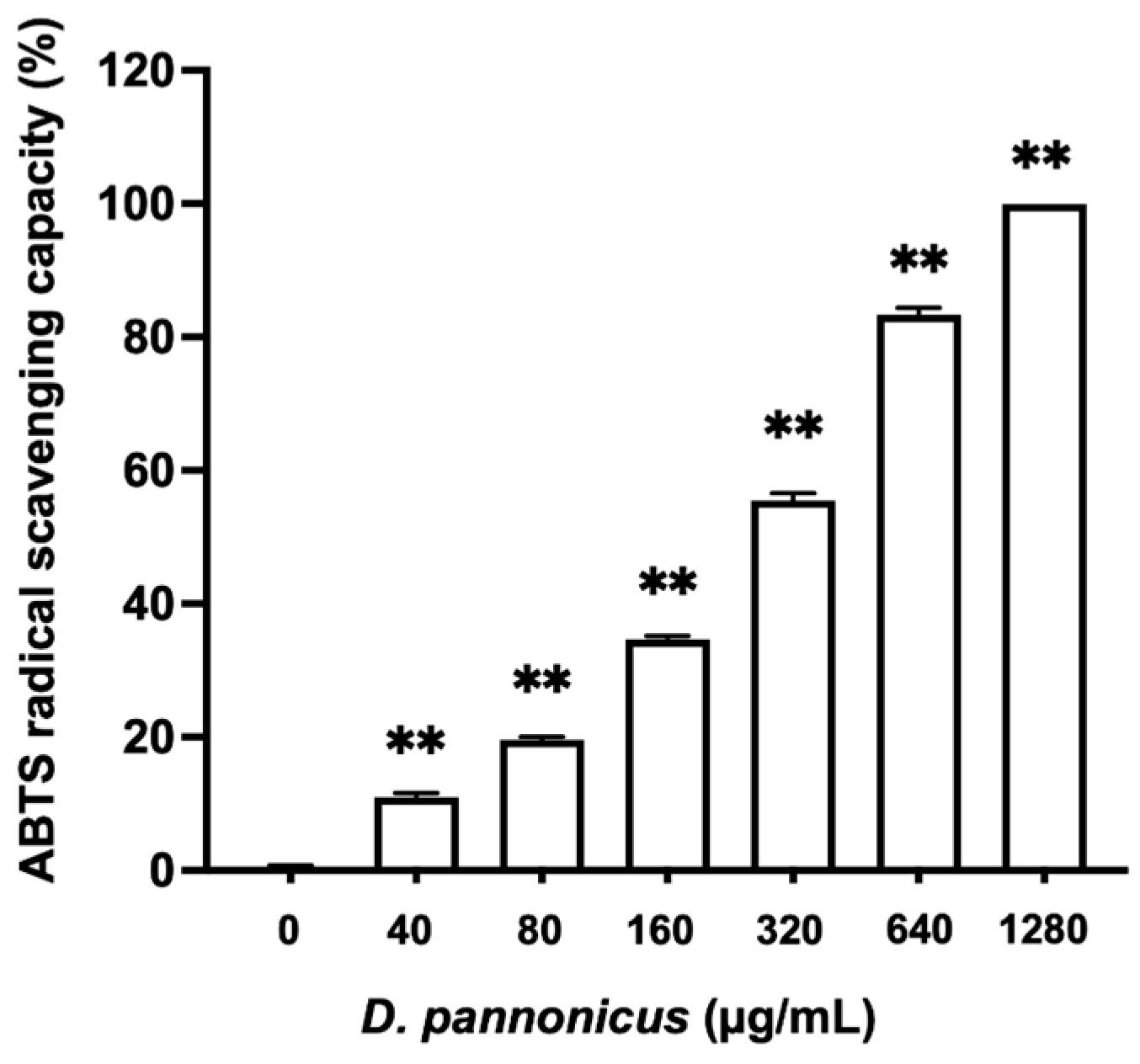
3.1. Antioxidant Activity
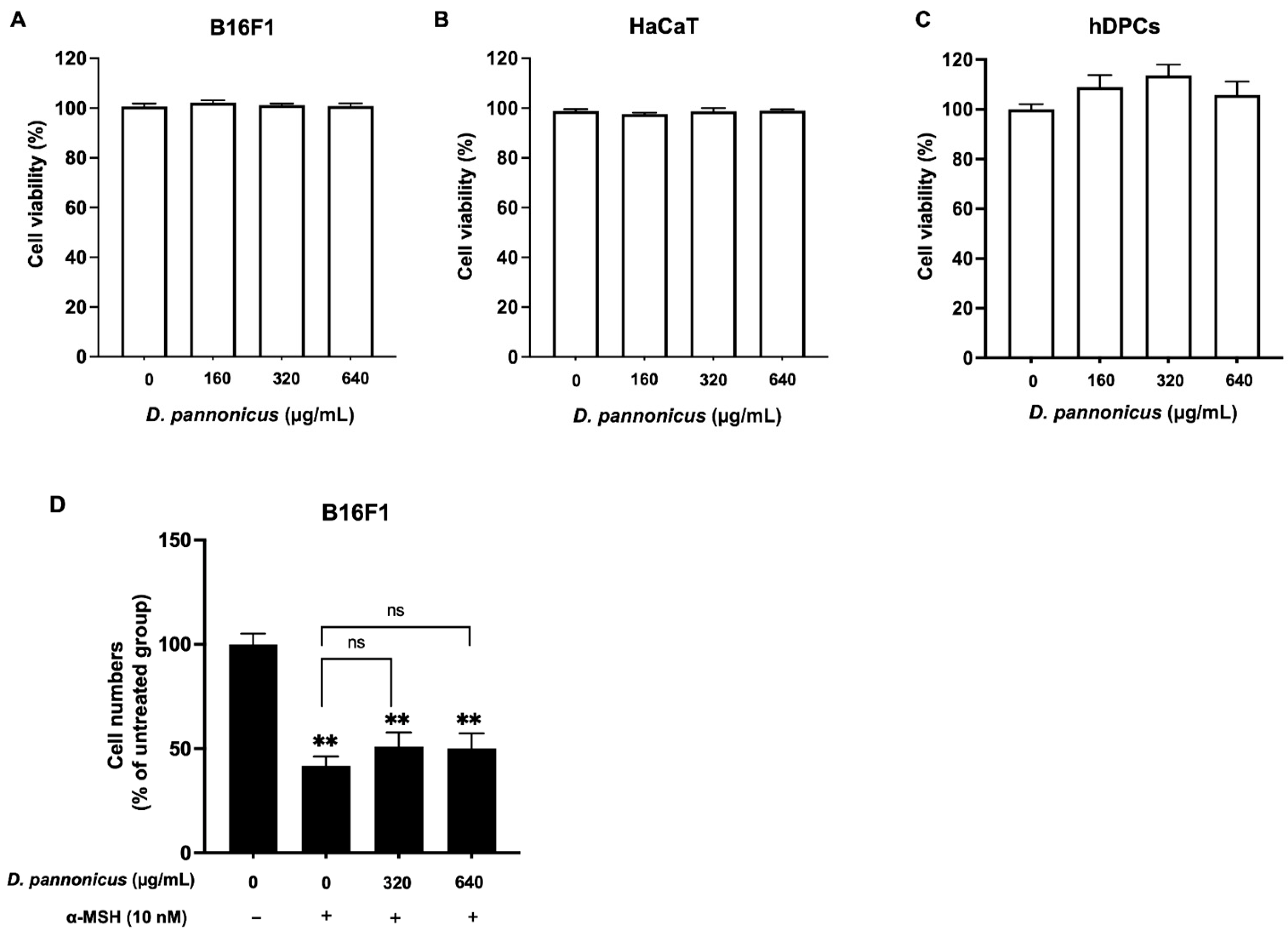
3.2. Effect of D. pannonicus on Cell Viability and Proliferation
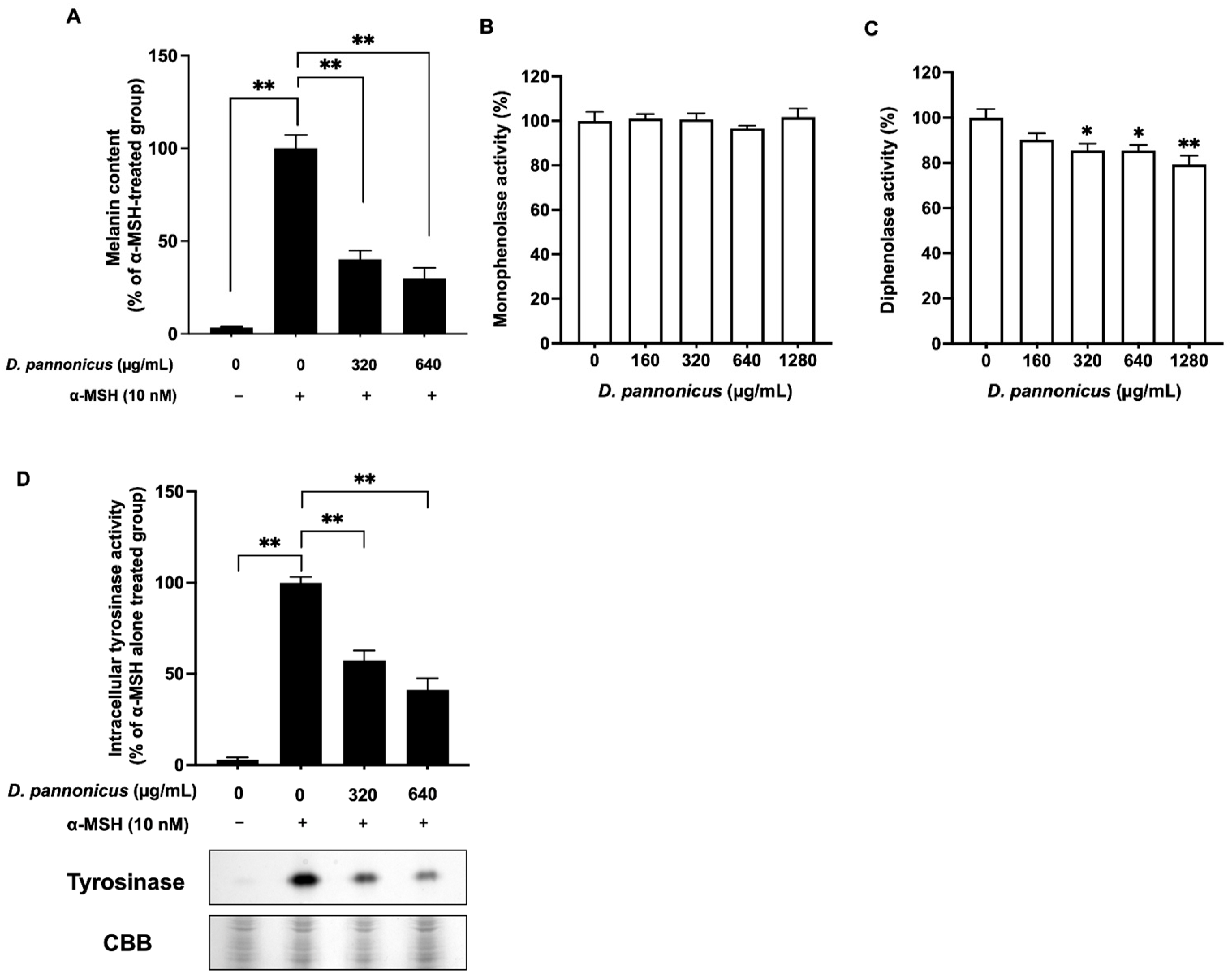
3.3. D. pannonicus Extract Inhibits Melanin Synthesis
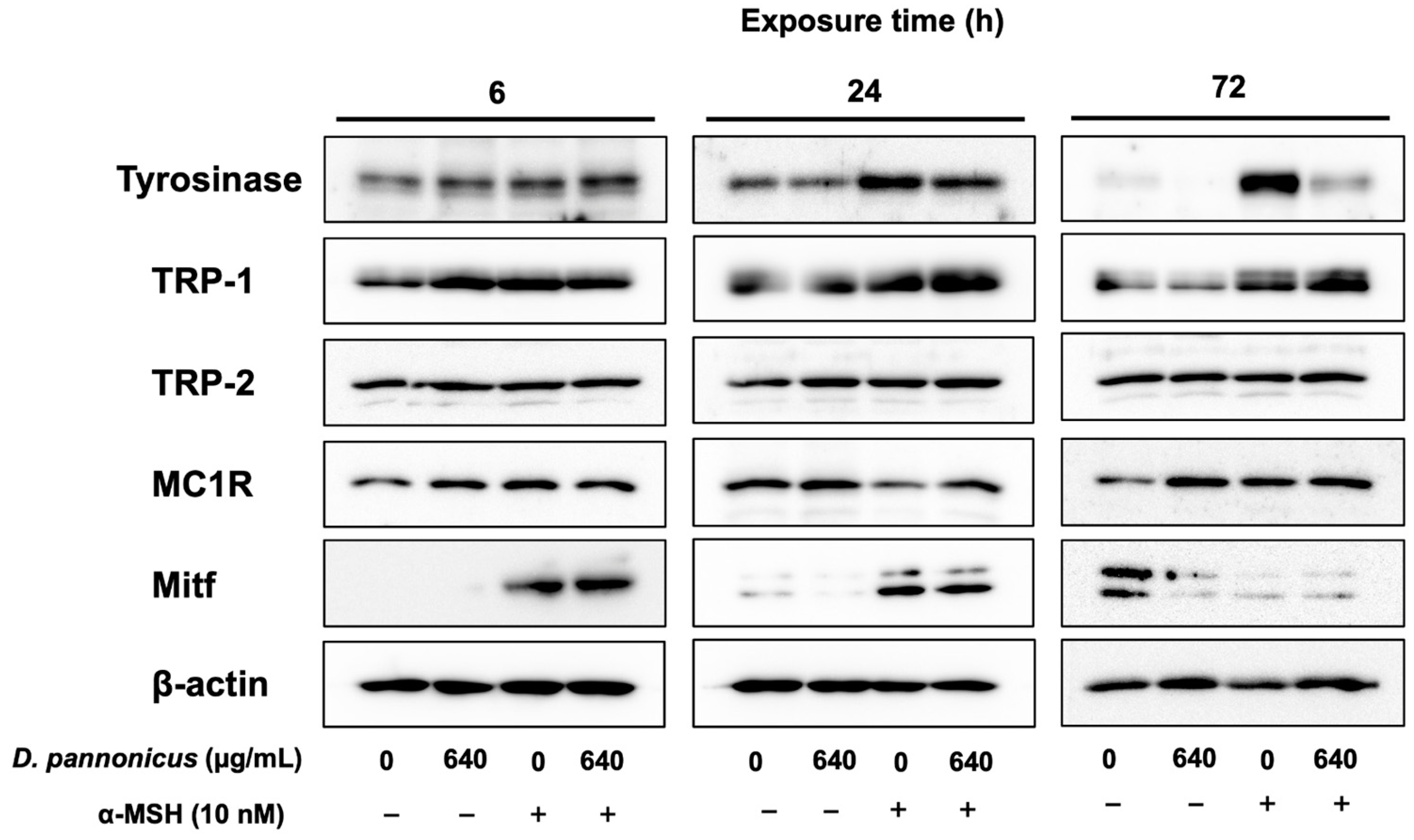
3.4. The Effects of D. pannonicus Extract on Melanogenic Protein Expression
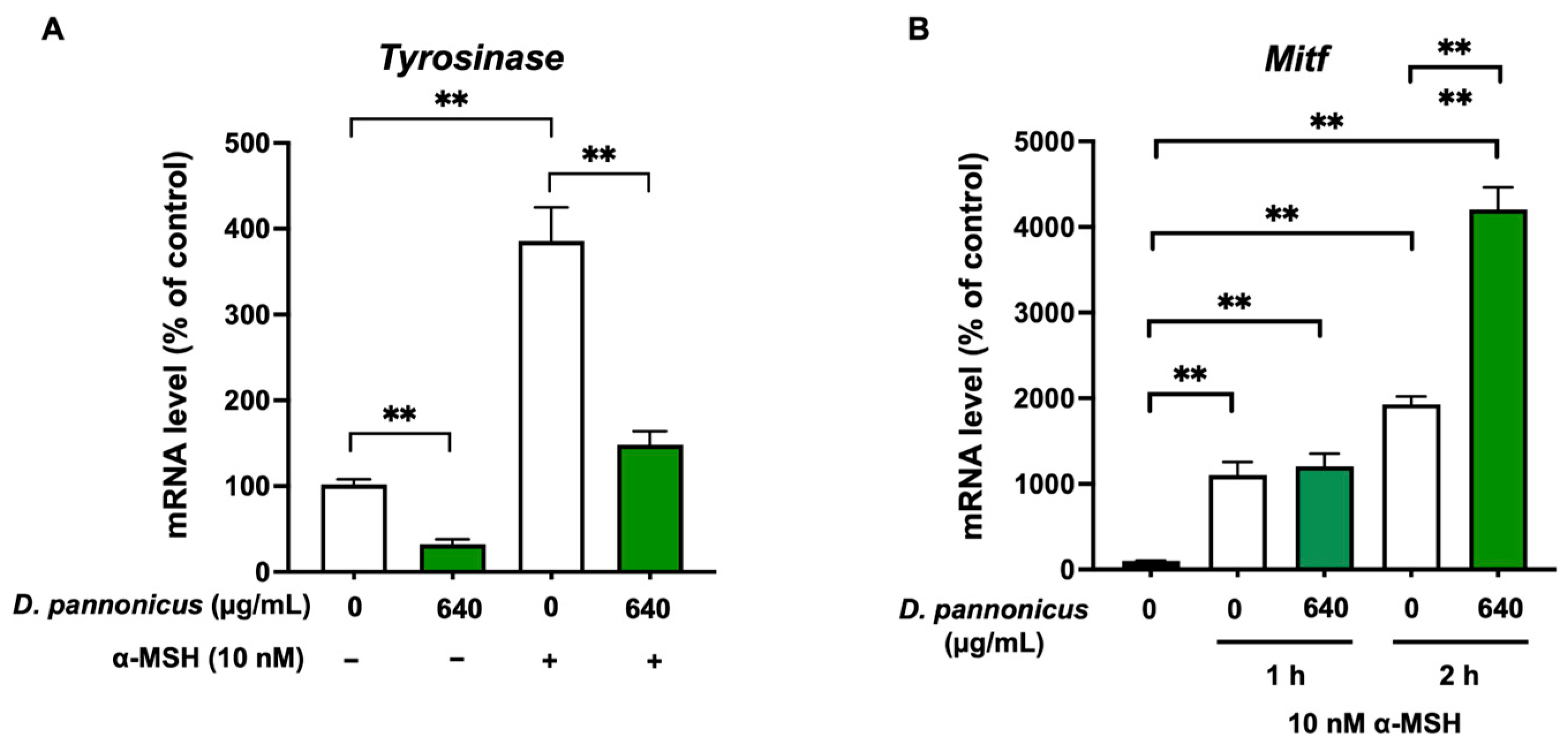
3.5. D. pannonicus Suppressed the Tyrosinase mRNA Levels
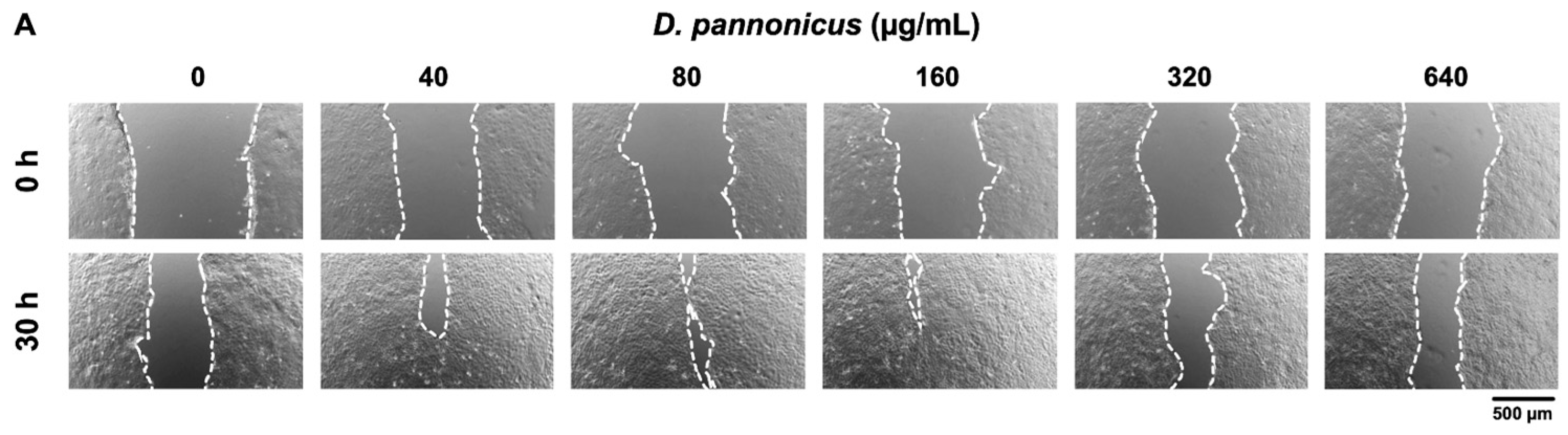
3.6. Wound Healing Effect of D. pannonicus Extract
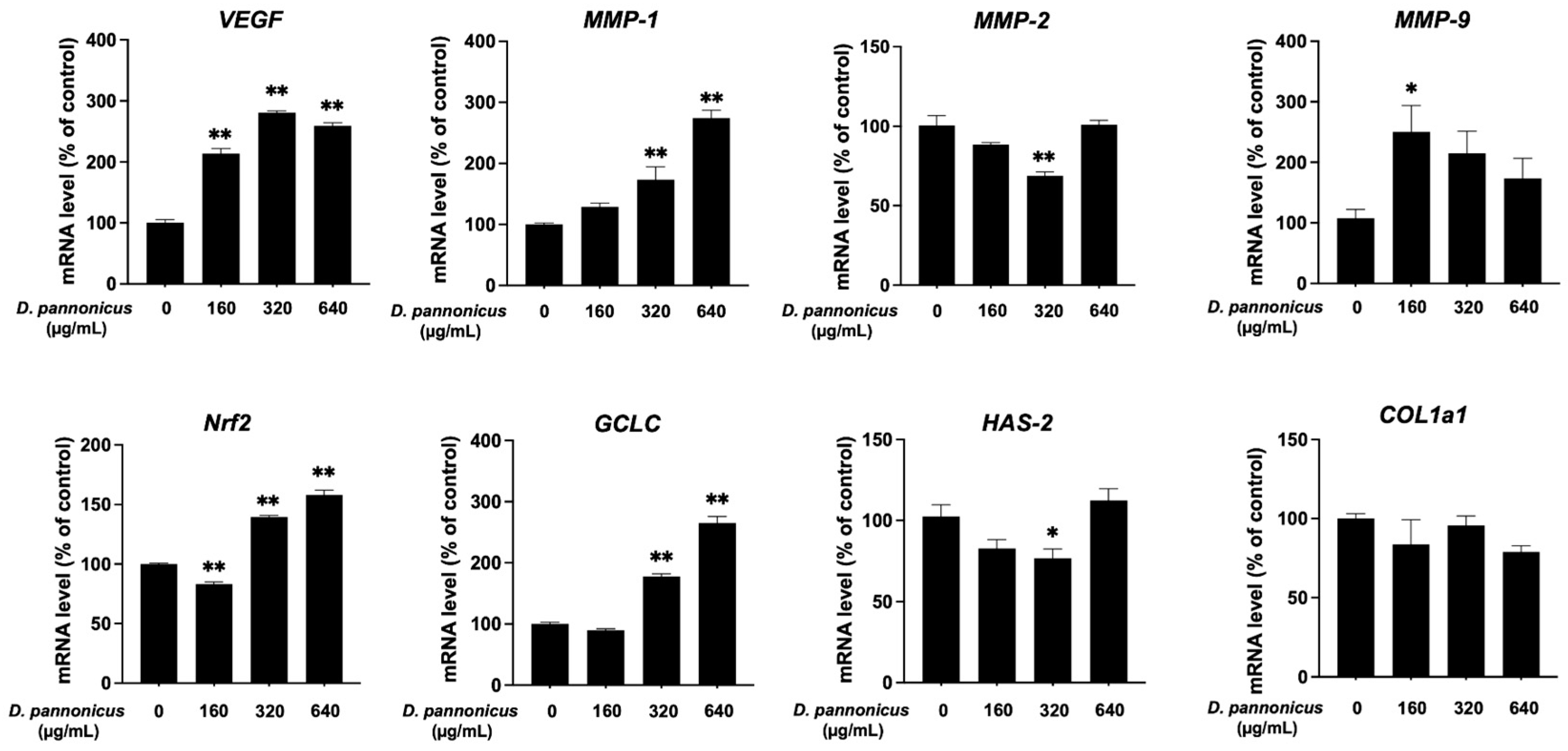
3.7. Effect of D. pannonicus Extract on Gene Expression in HaCaT Cells
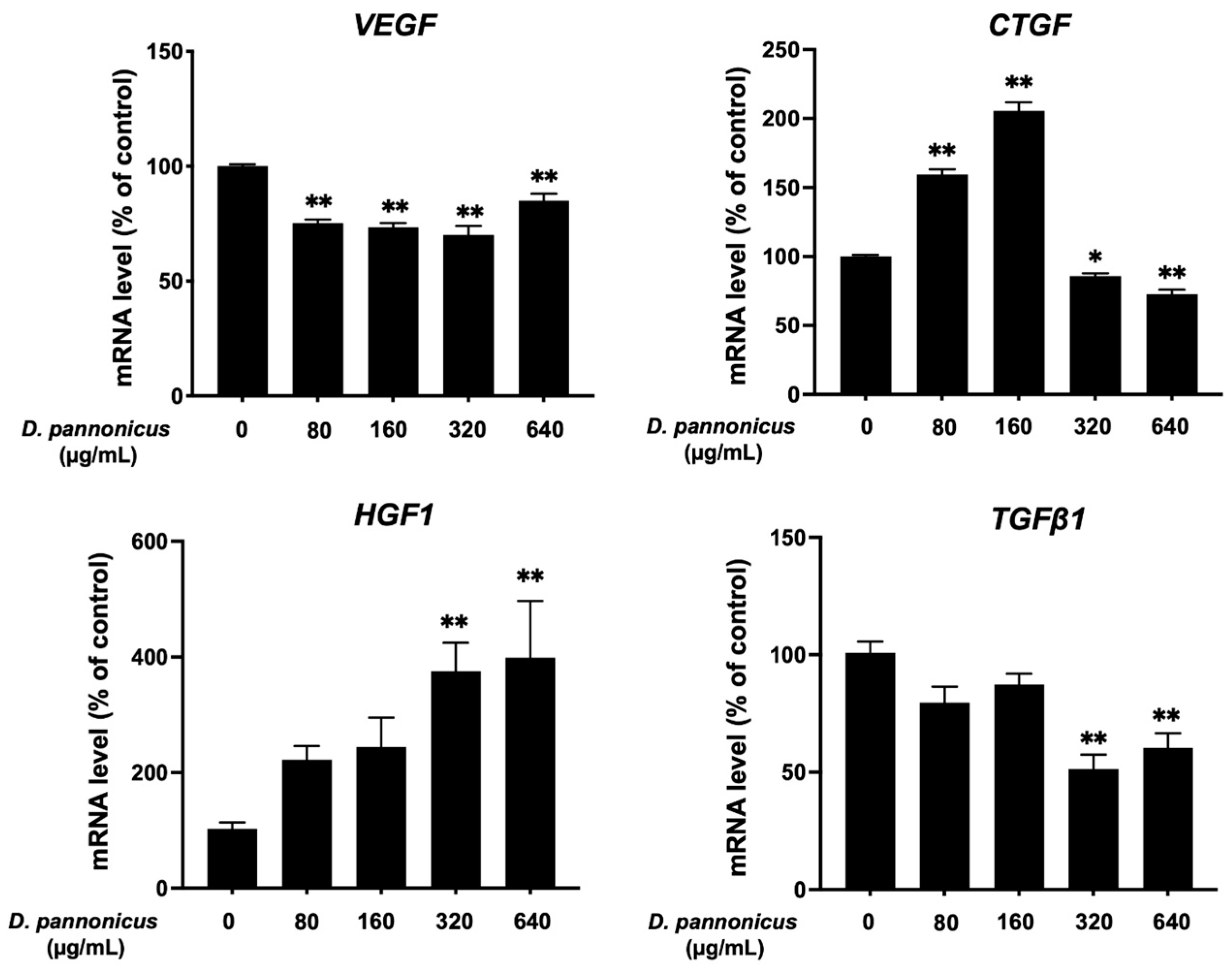
3.8. Effect of D. pannonicus Extract on Hair Growth-Related Gene Expression in HFDPCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çakirsoy, I.; Miyamoto, T.; Ohtake, N. Physiology of microalgae and their application to sustainable agriculture: A mini-review. Front. Plant Sci. 2022, 13, 1005991. [Google Scholar] [CrossRef] [PubMed]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Singh, I.; Pandey, A.; Shangdiar, S.; Rai, P.K.; Kumar, A.; Amesho, K.T.T.; Bux, F. Towards Sustainable Energy: Harnessing Microalgae Biofuels for a Greener Future. Sustainability 2023, 15, 14029. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakrim, S.; Chamkhi, I.; Taha, D.; Omari, N.E.; Mneyiy, N.E.; Hachlafi, N.E.; El-Shazly, M.; Khalid, A.; Abdalla, A.N.; et al. Bioactive substances of cyanobacteria and microalgae: Sources, metabolism, and anticancer mechanism insights. Biomed. Pharmacother. 2024, 170, 115989. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamaguchi, Y.; Sakaki, S.; Takenaka, H. Pleurochrysis carterae Hot-Water Extract Inhibits Melanogenesis in Murine Melanoma Cells. Cosmetics 2019, 6, 60. [Google Scholar] [CrossRef]
- Sato, K.; Hiraga, Y.; Yamaguchi, Y.; Sakaki, S.; Takenaka, H. Anti-Melanogenic and Anti-Oxidative Effects of Nostoc verrucosum (ashitsuki) Extracts. Cosmetics 2023, 10, 30. [Google Scholar] [CrossRef]
- Xing, X.; Dan, Y.; Xu, Z.; Xiang, L. Implications of Oxidative Stress in the Pathogenesis and Treatment of Hyperpigmentation Disorders. Oxid. Med. Cell Longev. 2022, 2022, 7881717. [Google Scholar] [CrossRef]
- Decher, H.; Schweikardt, T.; Tuczek, F. The first crystal structure of tyrosinase: All questions answered? Angew. Chem. Int. Ed. Engl. 2006, 45, 4546–4550. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Cao, M.; Wang, S.; Gao, Y.; Pan, X.; Wang, X.; Deng, R.; Liu, P. Study on physicochemical properties and antioxidant activity of polysaccharides from Desmodesmus armatus. J. Food Biochem. 2020, 44, e13243. [Google Scholar] [CrossRef]
- Ying, M.; Zeng, Z.; Li, Q.; Chen, X.; Xiong, Y.; Wu, B.; Peng, L.; Zhang, Q.; Wang, L.; Dai, Z.; et al. Water-soluble intracellular extract of Desmodesmus sp. YT enhanced the antioxidant capacity of human skin fibroblast to protect the skin from UV damage. J. Cosmet. Dermatol. 2024, 23, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nishio, T.; Usami, M.; Awaji, M.; Shinohara, S.; Sato, K. Dual effects of acetylsalicylic acid on ERK signaling and Mitf transcription lead to inhibition of melanogenesis. Mol. Cell. Biochem. 2016, 412, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Daniello, V.; Leo, V.D.; Lasalvia, M.; Hossain, M.N.; Carbone, A.; Catucci, L.; Zefferino, R.; Ingrosso, C.; Conese, M.; Gioia, S.D. Solanum lycopersicum (Tomato)-Derived Nanovesicles Accelerate Wound Healing by Eliciting the Migration of Keratinocytes and Fibroblasts. Int. J. Mol. Sci. 2024, 25, 2452. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borron, J.C.; Abdel-Malek, Z.; Jimenez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Lee, B.S.; Jo, B.-G.; Heo, S.P.; Jung, Y.S.; Kim, S.-N.; Kim, K.H.; Yang, M.H. Iridoid Glycosides and Coumarin Glycoside Derivatives from the Roots of Nymphoides peltata and Their In Vitro Wound Healing Properties. Int. J. Mol. Sci. 2024, 25, 1268. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Chen, Y.; Chen, C.; Wang, J.; Lee, Y.M.; Zheng, W.; Shang, R.; Tang, Y.; Zhang, X.; et al. P311 Facilitates the Angiogenesis and Wound Healing Function of MSCs by Increasing VEGF Production. Front. Immunol. 2022, 13, 821932. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wolter, W.R.; Anderson, B.; Schroeder, V.A.; Gao, M.; Gooyit, M.; Suckow, M.A.; Chang, M. Limitations of Knockout Mice and Other Tools in Assessment of the Involvement of Matrix Metalloproteinases in Wound Healing and the Means to Overcome Them. ACS Pharmacol. Transl. Sci. 2020, 3, 489–495. [Google Scholar] [CrossRef]
- Harder, B.; Jiang, T.; Wu, T.; Tao, S.; Rojo de la Vega, M.; Tian, W.; Chapman, E.; Zhang, D.D. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem. Soc. Trans. 2015, 43, 680–686. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genomics 2018, 50, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Song, Y.; Chen, N.; Guo, J.; Liu, W.; Guo, K.; Ling, X.; Zhang, L. 4-octyl itaconate improves the viability of D66H cells by regulating the KEAP1-NRF2-GCLC/HO-1 pathway. J. Cell. Mol. Med. 2023, 27, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Bienová, M.; Kucerová, R.; Fiurásková, M.; Hajdúch, M.; Koláŕ, Z. Androgenetic alopecia and current methods of treatment. Acta Dermatovenerol. Alp. Pannonica. Adriat. 2005, 14, 5–8. [Google Scholar] [PubMed]
- Dai, R.; Xu, Q.; Shao, Z.; Wu, X. The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles. Open Life Sci. 2023, 18, 20220723. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, M.A.; Hafez, A.M.; Elhawary, S.S.; Sedky, N.K.; Hassan, O.F.; Bakeer, R.M.; Hadi, S.A.E.; El-Desoky, A.H.; Mahgoub, S.; Mokhtar, F.A. The medicinal activity of lyophilized aqueous seed extract of Lepidium sativum L. in an androgenic alopecia model. Sci. Rep. 2023, 13, 7676. [Google Scholar] [CrossRef]
- Qi, Y.; Li, M.; Xu, L.; Chang, Z.; Shu, X.; Zhou, L. Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. PeerJ 2016, 4, e2624. [Google Scholar] [CrossRef]
- Park, S.-M.; He, Y.-C.; Gong, C.; Gao, W.; Bae, Y.-S.; Si, C.; Park, K.-H.; Choi, S.-E. Effects of taxifolin from enzymatic hydrolysis of Rhododendron mucrotulatum on hair growth promotion. Front. Bioeng. Biotechnol. 2022, 10, 995238. [Google Scholar] [CrossRef]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Tyrosinase (mouse) | TTGCCACTTCATGTCATCATAGAATATT | TTTATCAAAGGTGTGACTGCTATACAAAT |
| Mitf (mouse) | CGCCTGATCTGGTGAATCG | CCTGGCTGCAGTTCTCAAGAA |
| GAPDH (mouse) | CGTCCCGTAGACAAAATGGT | TTGATGGCAACAATCTCCAC |
| VEGF (human) | CTTCTGAGTTGCCCAGGAGA | GGATGGAGGAAGGTCAACCA |
| MMP-1 (human) | GGGAGATCATCGGGACAACTC | TGAGCATCCCCTCCAATACC |
| MMP-2 (human) | TAGCAGCGGAACAAGGAG | AAACGGGAACCAGGACAC |
| Nrf2 (human) | AACCAGTGGATCTGCCAACTACTC | CTGCGCCAAAAGCTGCAT |
| GCLC (human) | GATGCTGTCTTGCAGGGAATG | AGCGAGCTCCGTGCTGTT |
| HAS-2 (human) | TGGATGACCTACGAAGCGATTA | GCTGGATTACTGTGGCAATGAG |
| COL1a1 (human) | AGGACAAGAGGCATGTCTGGTT | TTGCAGTGGTAGGTGATGTTCTG |
| CTGF (human) | GTTTGGCCCAGACCCAACTA | GGCTCTGCTTCTCTAGCCTG |
| HGF1 (human) | AGAAATGCAGCCAGCATCAT | CACATGGTCCTGATCCAATC |
| TGFβ1 (human) | GCCCTGGACACCAACTATTG | GTCCAGGCTCCAAATGTAGG |
| GAPDH (human) | CTTTGGTATCGTGGAAGGACTC | GTAGAGGCAGGGATGATGTTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Hiraga, Y.; Yamaguchi, Y.; Sakaki, S.; Takenaka, H. Desmodesmus pannonicus Water Extract Inhibits Melanin Synthesis and Promotes Wound Healing. Life 2024, 14, 1542. https://doi.org/10.3390/life14121542
Sato K, Hiraga Y, Yamaguchi Y, Sakaki S, Takenaka H. Desmodesmus pannonicus Water Extract Inhibits Melanin Synthesis and Promotes Wound Healing. Life. 2024; 14(12):1542. https://doi.org/10.3390/life14121542
Chicago/Turabian StyleSato, Kazuomi, Yosuke Hiraga, Yuji Yamaguchi, Setsuko Sakaki, and Hiroyuki Takenaka. 2024. "Desmodesmus pannonicus Water Extract Inhibits Melanin Synthesis and Promotes Wound Healing" Life 14, no. 12: 1542. https://doi.org/10.3390/life14121542
APA StyleSato, K., Hiraga, Y., Yamaguchi, Y., Sakaki, S., & Takenaka, H. (2024). Desmodesmus pannonicus Water Extract Inhibits Melanin Synthesis and Promotes Wound Healing. Life, 14(12), 1542. https://doi.org/10.3390/life14121542






