Effect of the Complex Allele p.[Ile148Thr;Ile1023_Val1024del] in Cystic Fibrosis and Tracing of a Founder Effect in Mexican Families
Abstract
1. Introduction
2. Materials and Methods
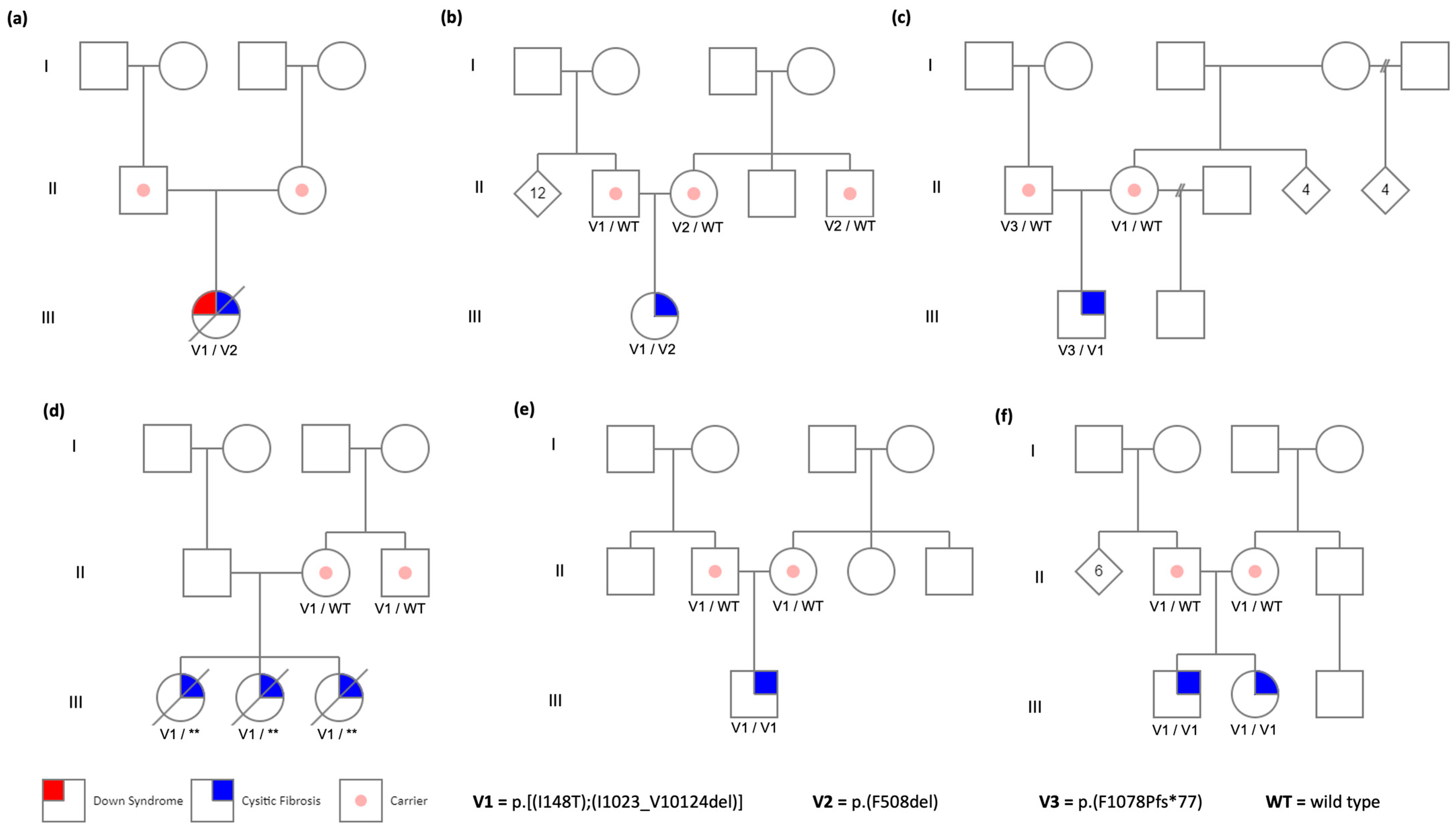
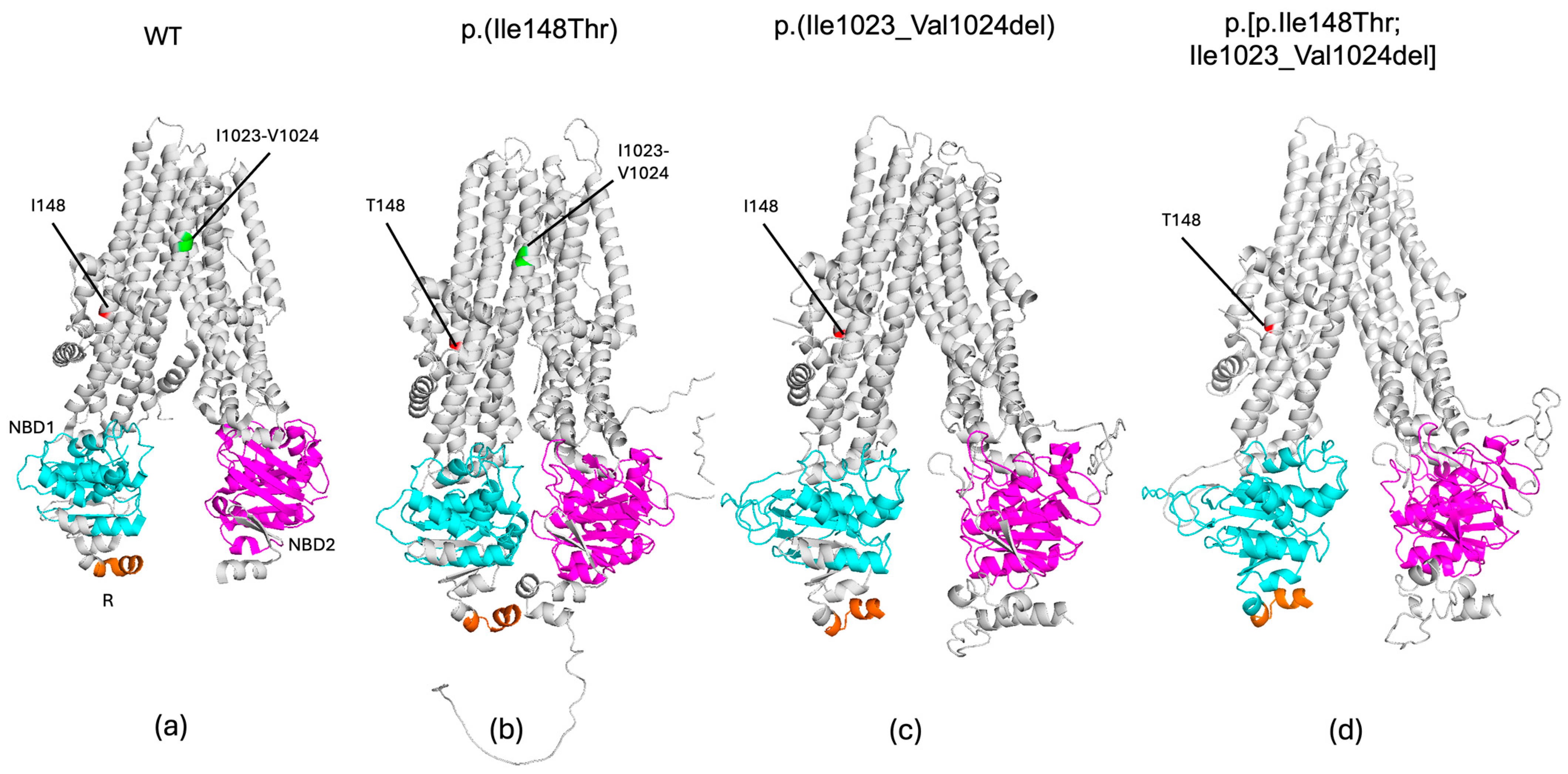
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Human Genetics Programme. The Molecular Genetic Epidemiology of Cystic Fibrosis: Report of a Joint Meeting of WHO/IECFTN/ICF(M)A/ECFS, Genoa, Italy, 19 June 2002; World Health Organization: Geneva, Switzerland, 2004; Available online: https://iris.who.int/handle/10665/68702 (accessed on 7 July 2024).
- Martínez-Hernández, A.; Larrosa, J.; Barajas-Olmos, F.; García-Ortíz, H.; Mendoza-Caamal, E.C.; Contreras-Cubas, C.; Mirzaeicheshmeh, E.; Lezana, J.L.; Orozco, L. Next-generation sequencing for identifying a novel/de novo pathogenic variant in a Mexican patient with cystic fibrosis: A case report. BMC Med. Genom. 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Kondratyeva, E.; Melyanovskaya, Y.; Efremova, A.; Krasnova, M.; Mokrousova, D.; Bulatenko, N.; Petrova, N.; Polyakov, A.; Adyan, T.; Kovalskaia, V.; et al. Clinical and Genetic Characteristics of a Patient with Cystic Fibrosis with a Complex Allele [E217G;G509D] and Functional Evaluation of the CFTR Channel. Genes 2023, 28, 1705. [Google Scholar] [CrossRef] [PubMed]
- Krasnova, M.; Efremova, A.; Bukhonin, A.; Zhekaite, E.; Bukharova, T.; Melyanovskaya, Y.; Goldshtein, D.; Kondratyeva, E. The Effect of Complex Alleles of the CFTR Gene on the Clinical Manifestations of Cystic Fibrosis and the Effectiveness of Targeted Therapy. Int. J. Mol. Sci. 2023, 25, 114. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo Ruiz, V.; de la Rosa Gildardo, F.Z.; Rafael, D.U.H. Genética Clínica, 2nd ed.; Editorial El Manual Moderno: Mexico City, Mexico, 2019; 651p. [Google Scholar]
- Martínez-Hernández, A.; Mendoza-Caamal, E.C.; Mendiola-Vidal, N.G.; Barajas-Olmos, F.; Villafan-Bernal, J.R.; Jiménez-Ruiz, J.L.; Monge-Cazares, T.; García-Ortiz, H.; Cubas-Contreras, C.; Centeno-Cruz, F.; et al. CFTR pathogenic variants spectrum in a cohort of Mexican patients with cystic fibrosis. Heliyon 2024, 10, e28984. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, V.; Farrell, P.M. Update on advances in cystic fibrosis towards a cure and implications for primary care clinicians. Curr. Probl. Pediatr. Adolesc. Health Care 2024, 54, 101637. [Google Scholar] [CrossRef]
- Bienvenu, T.; Lopez, M.; Girodon, E. Molecular Diagnosis and Genetic Counseling of Cystic Fibrosis and Related Disorders: New Challenges. Genes 2020, 11, 619. [Google Scholar] [CrossRef]
- The Clinical and Functional TRanslation of CFTR (CFTR2). Available online: http://cftr2.org (accessed on 7 July 2024).
- Chevalier, B.; Hinzpeter, A. The influence of CFTR complex alleles on precision therapy of cystic fibrosis. J. Cyst. Fibros. 2020, 19 (Suppl. 1), S15–S18. [Google Scholar] [CrossRef]
- Lucarelli, M.; Narzi, L.; Pierandrei, S.; Bruno, S.M.; Stamato, A.; d’Avanzo, M.; Strom, R.; Quattrucci, S. A new complex allele of the CFTR gene partially explains the variable phenotype of the L997F mutation. Genet. Med. 2010, 12, 548–555. [Google Scholar] [CrossRef]
- Kondratyeva, E.; Efremova, A.; Melyanovskaya, Y.; Voronkova, A.; Polyakov, A.; Bulatenko, N.; Adyan, T.; Sherman, V.; Kovalskaia, V.; Petrova, N.; et al. Evaluation of the Complex p.[Leu467Phe;Phe508del] CFTR Allele in the Intestinal Organoids Model: Implications for Therapy. Int. J. Mol. Sci. 2022, 8, 10377. [Google Scholar] [CrossRef]
- Petrova, N.V.; Kashirskaya, N.Y.; Vasilyeva, T.A.; Balinova, N.V.; Marakhonov, A.V.; Kondratyeva, E.; Zhekaite, E.K.; Voronkova, A.Y.; Kutsev, S.I.; Zinchenko, R.A. High frequency of complex CFTR alleles associated with c.1521_1523delCTT (F508del) in Russian cystic fibrosis patients. BMC Genom. 2022, 23, 252. [Google Scholar] [CrossRef]
- Progeny Genetics LLC, Aliso Viejo, CA, USA. Available online: www.progenygenetics.com (accessed on 10 June 2024).
- Claustres, M.; Thèze, C.; des Georges, M.; Baux, D.; Girodon, E.; Bienvenu, T.; Audrezet, M.P.; Dugueperoux, I.; Férec, C.; Lalau, G.; et al. CFTR-France, a national relational patient database for sharing genetic and phenotypic data associated with rare CFTR variants. Hum. Mutat. 2017, 38, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Flannick, J.; Mercader, J.M.; Fuchsberger, C.; Udler, M.S.; Mahajan, A.; Wessel, J.; Teslovich, T.M.; Caulkins, L.; Koesterer, R.; Barajas-Olmos, F.; et al. Exome sequencing of 20, 791 cases of type 2 diabetes and 24, 440 controls. Nature 2019, 570, 71–76. [Google Scholar] [CrossRef] [PubMed]
- SIGMA Type 2 Diabetes Consortium; Estrada, K.; Aukrust, I.; Bjørkhaug, L.; Burtt, N.P.; Mercader, J.M.; García-Ortiz, H.; Huerta-Chagoya, A.; Moreno-Macías, H.; Walford, G.; et al. Association of a low-frequency variant in HNF1A with type 2 diabetes in a Latino population. JAMA 2014, 31, 2305–2314. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, W.; Li, Y.; Pearce, R.; Zhang, C.; Bell, E.W.; Zhang, G.; Zhang, Y. I-TASSER-MTD: A deep-learning-based platform for multi-domain protein structure and function prediction. Nat. Protoc. 2022, 17, 2326–2353. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. Predicting deleterious amino acid substitutions. Genome Res. 2001, 11, 863–874. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Rodrigues, C.H.; Pires, D.E.; Ascher, D.B. DynaMut: Predicting the impact of mutations on protein conformation; flexibility and stability. Nucleic Acids Res. 2018, 46, W350–W355. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, Version 3.0 Schrödinger, LLC. Available online: https://www.pymol.org/ (accessed on 10 June 2024).
- Sheridan, M.B.; Aksit, M.A.; Pagel, K.; Hetrick, K.; Shultz-Lutwyche, H.; Myers, B.; Buckingham, K.J.; Pace, R.G.; Ling, H.; Pugh, E.; et al. The clinical utility of sequencing the entirety of CFTR. J. Cyst. Fibros. 2024, 23, 707–715. [Google Scholar] [CrossRef]
- Terlizzi, V.; Castaldo, G.; Salvatore, D.; Lucarelli, M.; Raia, V.; Angioni, A.; Carnovale, V.; Cirilli, N.; Casciaro, R.; Colombo, C.; et al. Genotype-phenotype correlation and functional studies in patients with cystic fibrosis bearing CFTR complex alleles. J. Med. Genet. 2017, 54, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.G.; Highsmith, W.E.; Amos, J.; Pratt, V.M.; Roa, B.; Friez, M.; Pike-Buchanan, L.L.; Buyse, I.M.; Redman, J.B.; Strom, C.M.; et al. Genotype-phenotype correlation and frequency of the 3199del6 cystic fibrosis mutation among I148T carriers: Results from a collaborative study. Genet. Med. 2004, 6, 421–425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruchon, A.F.; Ryan, S.R.; Fetni, R.; Rozen, R.; Scott, P. Frequency and phenotypic consequences of the 3199del6 CFTR mutation in French Canadians. Genet. Med. 2005, 7, 210–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terlizzi, V.; Centrone, C.; Botti, M.; Taccetti, G. G378X-I148T CFTR variant: A new complex allele in a cystic fibrosis newborn with pancreatic insufficiency. Mol. Genet. Genom. Med. 2022, 10, e2033. [Google Scholar] [CrossRef]
- Laselva, O.; Ardelean, M.C.; Bear, C.E. Phenotyping Rare CFTR Mutations Reveal Functional Expression Defects Restored by TRIKAFTATM. J. Pers. Med. 2021, 15, 301. [Google Scholar] [CrossRef]
- Secretaria de Gobernación, México. Available online: http://www.gobernacion.gob.mx/work/models/SEGOB/Resource/1353/4/images/Cobo_2012_canadienses_mpi.pdf (accessed on 5 June 2024).
- Secretaria de Gobernación, México. Available online: https://migracionesinternacionales.colef.mx/index.php/migracionesinternacionales/article/view/237/438 (accessed on 5 June 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendiola-Vidal, N.G.; Contreras-Cubas, C.; Barajas-Olmos, F.; Villafan-Bernal, J.R.; Yañez-Felix, A.L.; García-Ortiz, H.; Centeno-Cruz, F.; Mendoza-Caamal, E.; Alaez-Verson, C.; Jiménez-Ruíz, J.L.; et al. Effect of the Complex Allele p.[Ile148Thr;Ile1023_Val1024del] in Cystic Fibrosis and Tracing of a Founder Effect in Mexican Families. Life 2024, 14, 1445. https://doi.org/10.3390/life14111445
Mendiola-Vidal NG, Contreras-Cubas C, Barajas-Olmos F, Villafan-Bernal JR, Yañez-Felix AL, García-Ortiz H, Centeno-Cruz F, Mendoza-Caamal E, Alaez-Verson C, Jiménez-Ruíz JL, et al. Effect of the Complex Allele p.[Ile148Thr;Ile1023_Val1024del] in Cystic Fibrosis and Tracing of a Founder Effect in Mexican Families. Life. 2024; 14(11):1445. https://doi.org/10.3390/life14111445
Chicago/Turabian StyleMendiola-Vidal, Namibia Guadalupe, Cecilia Contreras-Cubas, Francisco Barajas-Olmos, José Rafael Villafan-Bernal, Ana Lucia Yañez-Felix, Humberto García-Ortiz, Federico Centeno-Cruz, Elvia Mendoza-Caamal, Carmen Alaez-Verson, Juan Luis Jiménez-Ruíz, and et al. 2024. "Effect of the Complex Allele p.[Ile148Thr;Ile1023_Val1024del] in Cystic Fibrosis and Tracing of a Founder Effect in Mexican Families" Life 14, no. 11: 1445. https://doi.org/10.3390/life14111445
APA StyleMendiola-Vidal, N. G., Contreras-Cubas, C., Barajas-Olmos, F., Villafan-Bernal, J. R., Yañez-Felix, A. L., García-Ortiz, H., Centeno-Cruz, F., Mendoza-Caamal, E., Alaez-Verson, C., Jiménez-Ruíz, J. L., Monge-Cázares, T., Lieberman, E., Baca, V., Lezana, J. L., Martínez-Hernández, A., & Orozco, L. (2024). Effect of the Complex Allele p.[Ile148Thr;Ile1023_Val1024del] in Cystic Fibrosis and Tracing of a Founder Effect in Mexican Families. Life, 14(11), 1445. https://doi.org/10.3390/life14111445





