Niemann–Pick C1-like 1 as a Prognostic Marker in Renal Cell Carcinoma: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Information
2.2. Analysis of Gene Expression and Gene Effect Scores Using Online Platforms
2.3. Statistical Analysis
3. Results
3.1. RCC Patient Characteristics
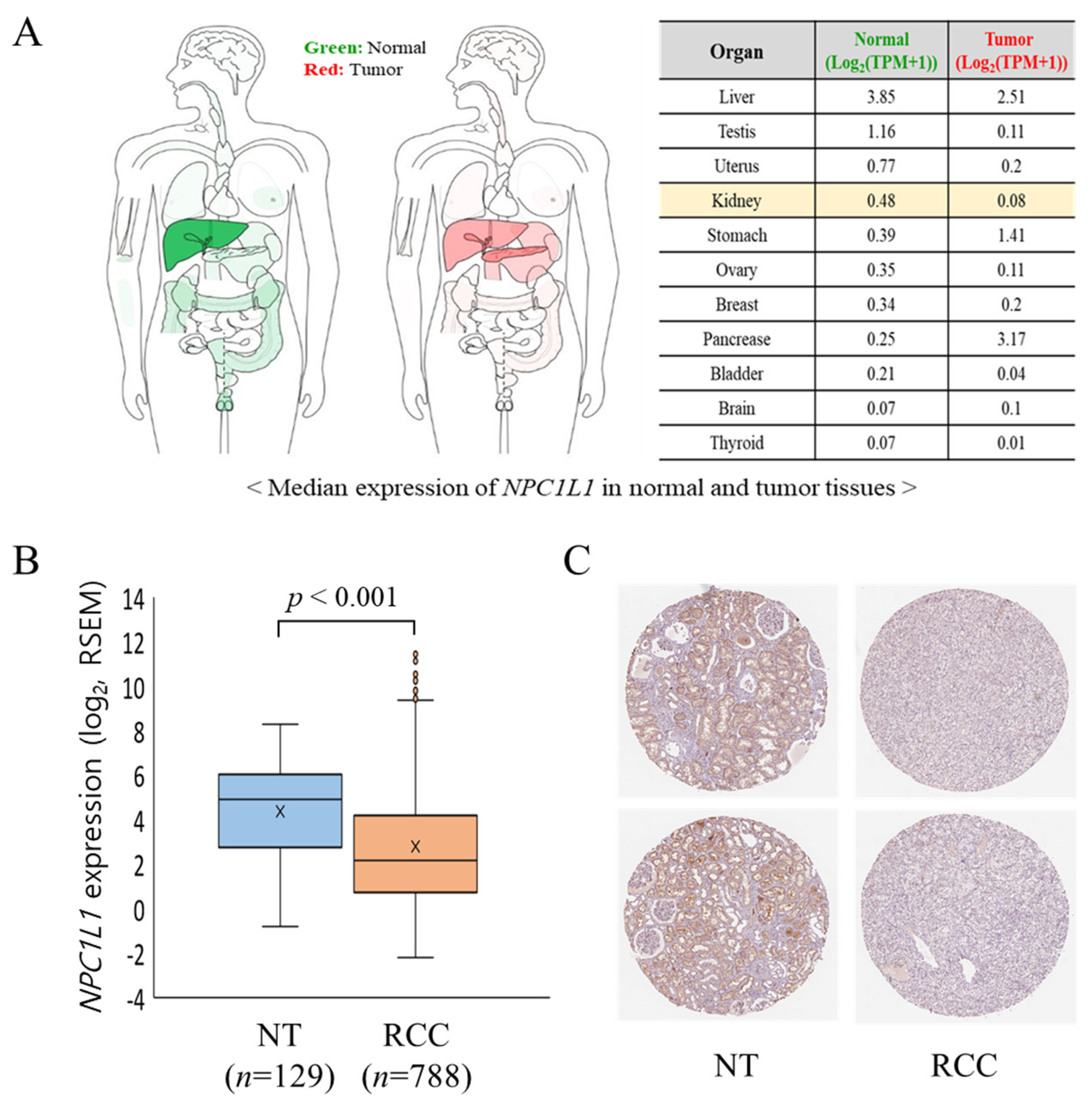
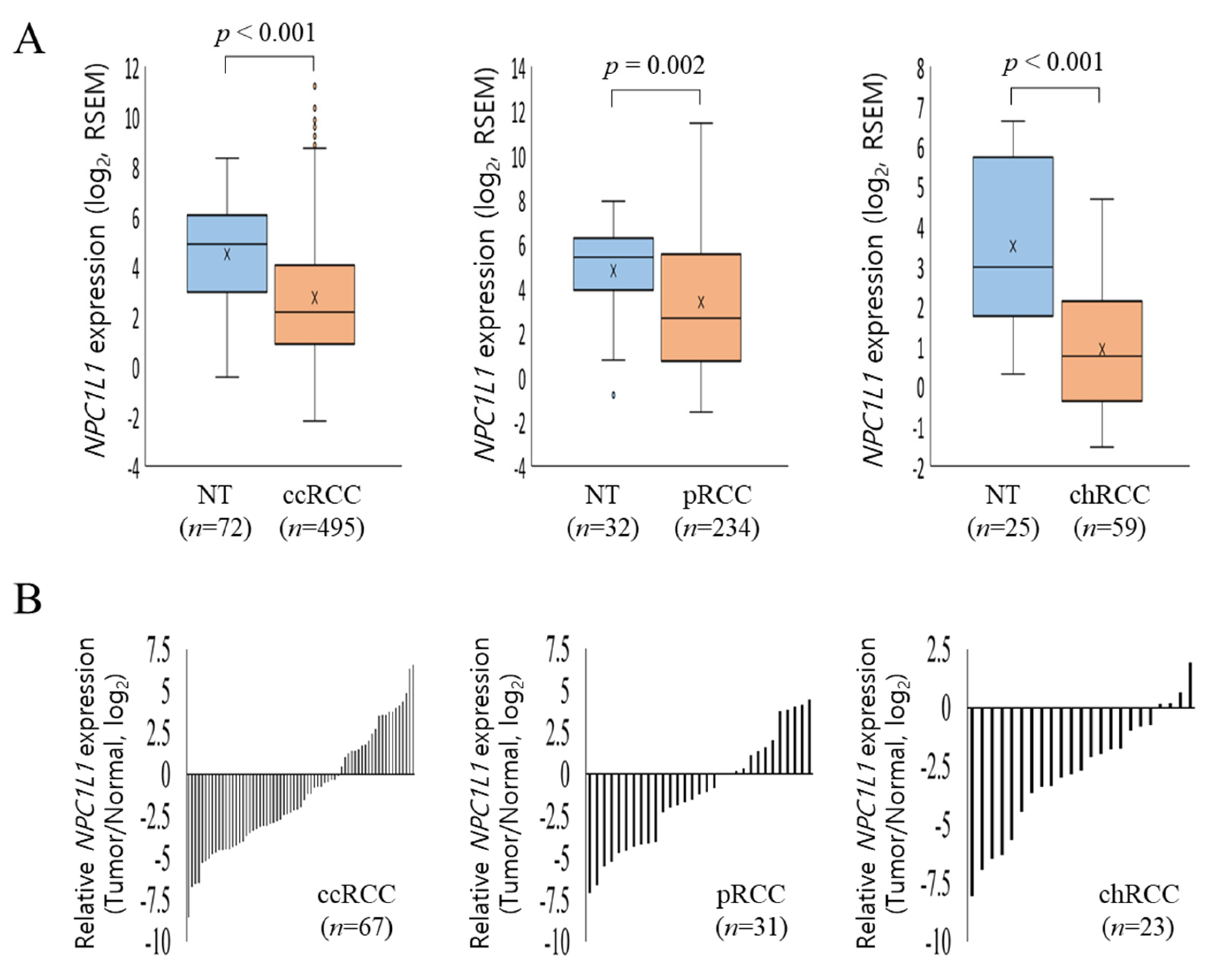
3.2. NPC1L1 Expression in Normal and RCC Tissues
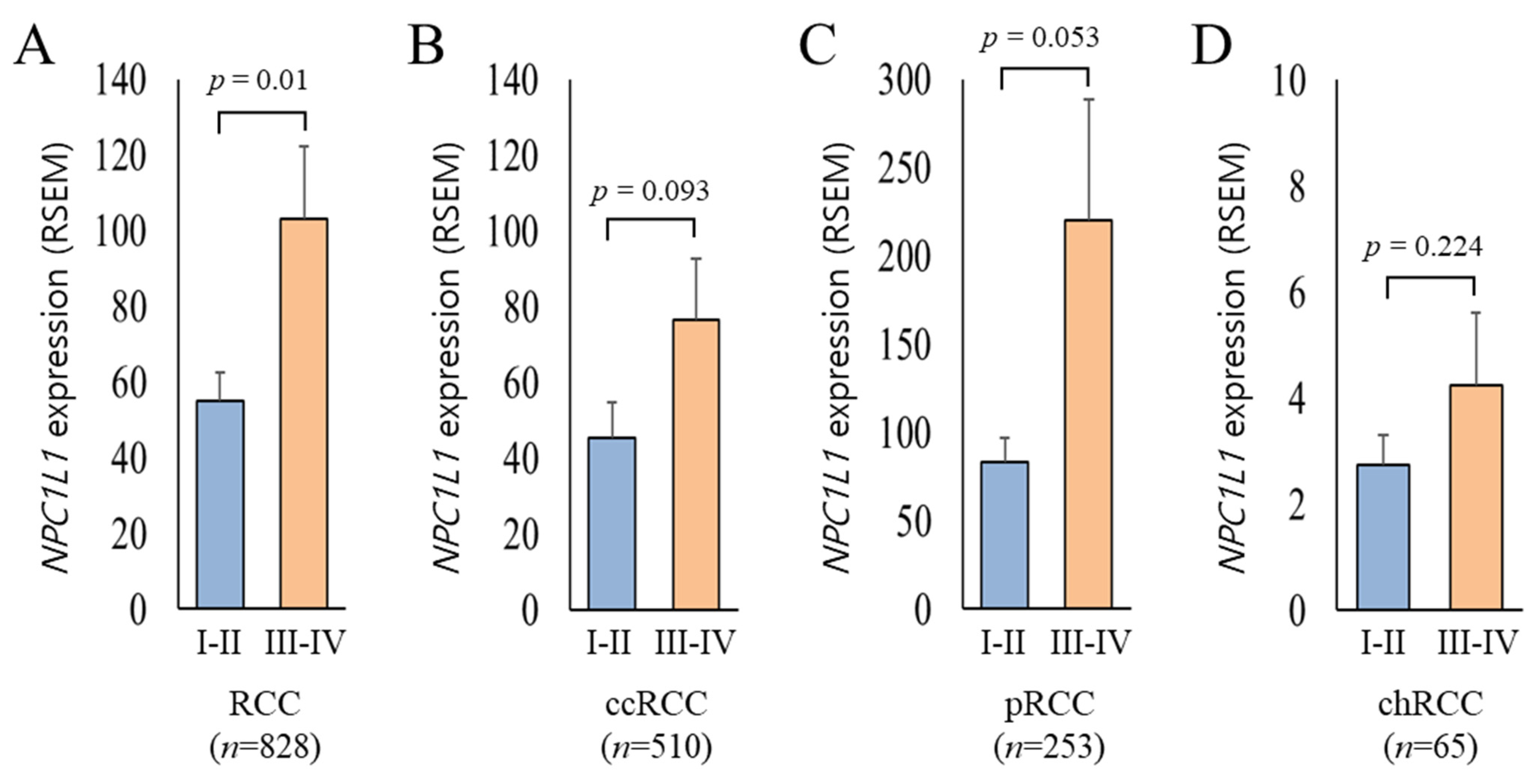
3.3. NPC1L1 Expression Based on RCC Stages
3.4. Relationship Between NPC1L1 Expression and Clinical Features
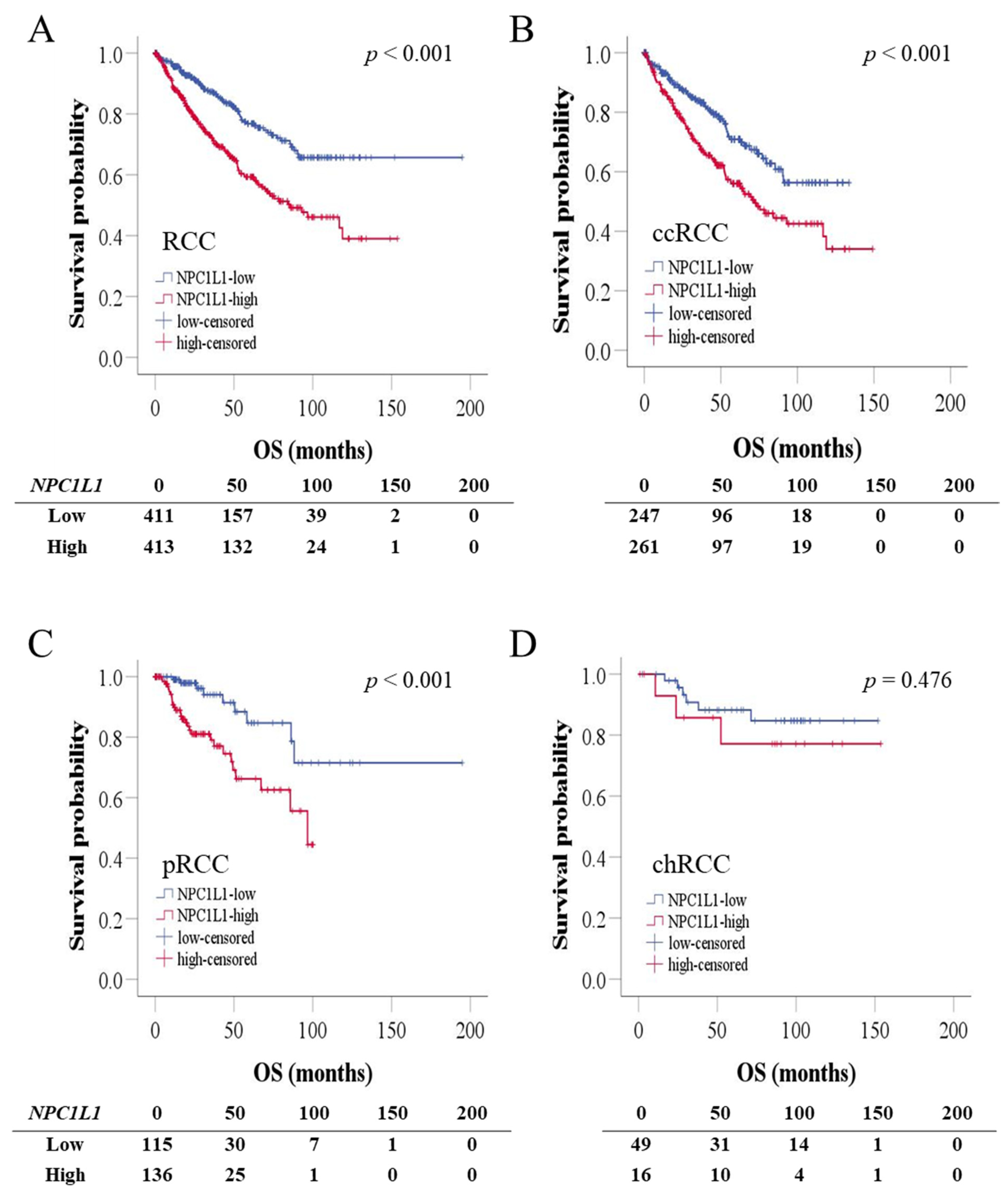
3.5. OS and PFS Based on NPC1L1 Expression in RCC
3.6. Prognostic Significance of NPC1L1 Expression in RCC Patients
3.7. OS According to Combined NPC1L1 Expression and Stage in RCC
3.8. Gene Effect Scores for NPC1L1 in Renal Cancer Cell Lines
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| HCC | Hepatocellular Carcinoma |
| HPA | The Human Protein Atlas |
| ccRCC | Clear Cell Renal Cell Carcinoma |
| chRCC | Chromophobe Renal Cell Carcinoma |
| NPC1L1 | Niemann-Pick C1-Like 1 |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| pRCC | Papillary Renal Cell Carcinoma |
| RCC | Renal Cell Carcinoma |
| SR-B1 | Scavenger Receptor Class B Member 1 |
| TCGA | The Cancer Genome Atlas |
| VLDL-R | Very-Low-Density Lipoprotein Receptor |
References
- Kwon, R.J.; Kim, Y.H.; Jeong, D.C.; Han, M.E.; Kim, J.Y.; Liu, L.; Jung, J.S.; Oh, S.O. Expression and prognostic significance of zinc fingers and homeoboxes family members in renal cell carcinoma. PLoS ONE 2017, 12, e0171036. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Physician 2019, 99, 179–184. [Google Scholar] [PubMed]
- Zhan, X.; Chen, T.; Liu, Y.; Wan, H.; Liu, X.; Deng, X.; Fu, B.; Xiong, J. Trends in cause of death among patients with renal cell carcinoma in the United States: A SEER-based study. BMC Public Health 2023, 23, 770. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansour, M.M.; Aga, S.S.; Alharbi, H.A.; Alsulami, M.N.; Fallatah, H.A.; Albedaiwi, T.B.; Anbari, L.K.; Surrati, T.R.; Algethami, A.A.; Althubaiti, A.; et al. Real-World Survival Outcomes of First-Line Therapies in Patients with Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Analysis from Two Centres in Saudi Arabia. Cancers 2024, 16, 3234. [Google Scholar] [CrossRef]
- Eichelberg, C.; Junker, K.; Ljungberg, B.; Moch, H. Diagnostic and prognostic molecular markers for renal cell carcinoma: A critical appraisal of the current state of research and clinical applicability. Eur. Urol. 2009, 55, 851–863. [Google Scholar] [CrossRef]
- Schiavoni, V.; Campagna, R.; Pozzi, V.; Cecati, M.; Milanese, G.; Sartini, D.; Salvolini, E.; Galosi, A.B.; Emanuelli, M. Recent Advances in the Management of Clear Cell Renal Cell Carcinoma: Novel Biomarkers and Targeted Therapies. Cancers 2023, 15, 3207. [Google Scholar] [CrossRef]
- Muglia, V.F.; Prando, A. Renal cell carcinoma: Histological classification and correlation with imaging findings. Radiol. Bras. 2015, 48, 166–174. [Google Scholar] [CrossRef]
- Coffey, N.J.; Simon, M.C. Metabolic alterations in hereditary and sporadic renal cell carcinoma. Nat. Rev. Nephrol. 2024, 20, 233–250. [Google Scholar] [CrossRef]
- Tanturovska, B.S.; Manaila, R.; Fabbro, D.; Huwiler, A. Lipids as Targets for Renal Cell Carcinoma Therapy. Int. J. Mol. Sci. 2023, 24, 3272. [Google Scholar] [CrossRef]
- Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Li, J.; Xu, H.; Zhao, Y.; Yu, X.; Shi, S. Functional significance of cholesterol metabolism in cancer: From threat to treatment. Exp. Mol. Med. 2023, 55, 1982–1995. [Google Scholar] [CrossRef]
- Liu, W.; Chakraborty, B.; Safi, R.; Kazmin, D.; Chang, C.Y.; McDonnell, D.P. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat. Commun. 2021, 12, 5103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Liu, S.; Cao, Q. Cholesterol promotes the migration and invasion of renal carcinoma cells by regulating the KLF5/miR-27a/FBXW7 pathway. Biochem. Biophys. Res. Commun. 2018, 502, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Calvo, M.; Lisnock, J.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R., Jr.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed]
- Kwon, R.J.; Cho, Y.H.; Park, E.J.; Lee, Y.; Lee, S.R.; Choi, J.I.; Lee, S.Y.; Son, S.M. Effect of Combination Therapy with Ezetimibe and Statins versus Statin Monotherapy on Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2023, 59, 1980. [Google Scholar] [CrossRef]
- Kwon, R.J.; Park, E.J.; Lee, S.Y.; Lee, Y.; Hwang, C.; Kim, C.; Cho, Y.H. Expression and prognostic significance of Niemann-Pick C1-Like 1 in colorectal cancer: A retrospective cohort study. Lipids Health Dis. 2021, 20, 104. [Google Scholar] [CrossRef]
- Nicolle, R.; Blum, Y.; Marisa, L.; Loncle, C.; Gayet, O.; Moutardier, V.; Turrini, O.; Giovannini, M.; Bian, B.; Bigonnet, M.; et al. Pancreatic Adenocarcinoma Therapeutic Targets Revealed by Tumor-Stroma Cross-Talk Analyses in Patient-Derived Xenografts. Cell Rep. 2017, 21, 2458–2470. [Google Scholar] [CrossRef]
- Chen, K.J.; Jin, R.M.; Shi, C.C.; Ge, R.L.; Hu, L.; Zou, Q.F.; Cai, Q.Y.; Jin, G.Z.; Wang, K. The prognostic value of Niemann-Pick C1-like protein 1 and Niemann-Pick disease type C2 in hepatocellular carcinoma. J. Cancer 2018, 9, 556–563. [Google Scholar] [CrossRef]
- Jaworska, M.; Szczudło, J.; Pietrzyk, A.; Shah, J.; Trojan, S.E.; Ostrowska, B.; Kocemba-Pilarczyk, K.A. The Warburg effect: A score for many instruments in the concert of cancer and cancer niche cells. Pharmacol. Rep. 2023, 75, 876–890. [Google Scholar] [CrossRef]
- Heravi, G.; Yazdanpanah, O.; Podgorski, I.; Matherly, L.H.; Liu, W. Lipid metabolism reprogramming in renal cell carcinoma. Cancer Metastasis Rev. 2022, 41, 17–31. [Google Scholar] [CrossRef]
- Xu, G.H.; Lou, N.; Shi, H.C.; Xu, Y.C.; Ruan, H.L.; Xiao, W.; Liu, L.; Li, X.; Xiao, H.B.; Qiu, B.; et al. Up-regulation of SR-BI promotes progression and serves as a prognostic biomarker in clear cell renal cell carcinoma. BMC Cancer 2018, 18, 88. [Google Scholar] [CrossRef]
- Xu, H.; Xu, W.H.; Ren, F.; Wang, J.; Wang, H.K.; Cao, D.L.; Shi, G.H.; Qu, Y.Y.; Zhang, H.L.; Ye, D.W. Prognostic value of epithelial-mesenchymal transition markers in clear cell renal cell carcinoma. Aging 2020, 12, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.H.; Visapaa, H.; Seligson, D.; Kim, H.; Han, K.R.; Huang, Y.; Horvath, S.; Stanbridge, E.J.; Palotie, A.; Figlin, R.A.; et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J. Urol. 2004, 171, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Minardi, D.; Lucarini, G.; Santoni, M.; Mazzucchelli, R.; Burattini, L.; Conti, A.; Principi, E.; Bianconi, M.; Scartozzi, M.; Milanese, G.; et al. Survival in patients with clear cell renal cell carcinoma is predicted by HIF-1α expression. Anticancer Res. 2015, 35, 433–438. [Google Scholar] [PubMed]
- Jiang, Z.; Chu, P.G.; Woda, B.A.; Rock, K.L.; Liu, Q.; Hsieh, C.C.; Li, C.; Chen, W.; Duan, H.O.; McDougal, S.; et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: A retrospective study. Lancet Oncol. 2006, 7, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, X.; Li, Y.; Liu, Q.; Wu, F.; Qu, H.; Gao, H.; Ge, J.; Xu, Y.; Wang, H.; et al. Expression and prognostic significance of fatty acid synthase in clear cell renal cell carcinoma. Pathol. Res. Pract. 2020, 216, 153227. [Google Scholar] [CrossRef]


| Histological Type (n = 828) | Clear Cell | Papillary | Chromophobe | Total (%) | |
|---|---|---|---|---|---|
| 510 (61.6) | 253 (30.6) | 65 (7.8) | |||
| Overall survival months (mean ± SD) | 44.25 ± 32.54 | 33.38 ± 29.74 | 70.27 ± 40.05 | 42.97 ± 33.66 | |
| Age (mean ± SD) | 60.49 ± 12.05 | 61.64 ± 12.13 | 51.15 ± 14.10 | 60.11 ± 12.52 | |
| Sex | Male | 325 (63.7) | 190 (75.1) | 38 (58.5) | 553 (66.8) |
| Female | 185 (36.3) | 63 (24.9) | 27 (41.5) | 275 (33.2) | |
| T stage | T1–T2 | 320 (62.7) | 195 (77.1) | 45 (69.2) | 560 (67.6) |
| T3–T4 | 190 (37.3) | 56 (22.1) | 20 (30.8) | 266 (32.1) | |
| Unknown | 0 (0.0) | 2 (0.8) | 0 (0.0) | 2 (0.3) | |
| M stage | M0 | 401 (78.6) | 83 (32.8) | 34 (52.3) | 518 (62.6) |
| M1 | 78 (15.3) | 9 (3.6) | 2 (3.1) | 89 (10.7) | |
| Unknown | 31 (6.1) | 161 (63.6) | 29 (44.6) | 221 (26.7) | |
| N stage | N0 | 228 (44.7) | 46 (18.2) | 39 (60.0) | 313 (37.8) |
| N1–N2 | 16 (3.1) | 27 (10.7) | 5 (7.7) | 48 (5.8) | |
| Unknown | 266 (52.2) | 180 (71.1) | 21 (32.3) | 467 (56.4) | |
| AJCC stage | Stage I | 249 (48.8) | 168 (66.4) | 20 (30.8) | 437 (52.8) |
| Stage II | 54 (10.6) | 21 (8.3) | 25 (38.5) | 100 (12.0) | |
| Stage III | 124 (24.3) | 49 (19.4) | 14 (21.5) | 187 (22.6) | |
| Stage IV | 83 (16.3) | 15 (5.9) | 6 (9.2) | 104 (12.6) | |
| Characteristics | n | NPC1L1 (Low) | NPC1L1 (High) | p-Value |
|---|---|---|---|---|
| Age (years) | 828 | p = 0.187 | ||
| <60 | 219 | 200 | ||
| ≥60 | 195 | 214 | ||
| Sex | 828 | p = 0.712 | ||
| Male | 279 | 274 | ||
| Female | 135 | 140 | ||
| T stage | 826 | p < 0.001 | ||
| T1-T2 | 308 | 252 | ||
| T3-T4 | 105 | 161 | ||
| M stage | 607 | p < 0.001 | ||
| M0 | 266 | 252 | ||
| M1 | 25 | 64 | ||
| N stage | 361 | p < 0.001 | ||
| N0 | 160 | 153 | ||
| N1-N2 | 13 | 35 | ||
| AJCC stage | 828 | p < 0.001 | ||
| Stage I–II | 301 | 236 | ||
| Stage III–IV | 113 | 178 |
| RCC (n = 828) | Univariable | Multivariable | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value * | Hazard Ratio (95% CI) | p-Value * | |
| Age < 60 (vs. ≥ 60) | 1.774 (1.348–2.335) | p < 0.001 | 1.519 (1.151–2.004) | p = 0.003 |
| Sex Male (vs. Female) | 1.091 (0.825–1.443) | p = 0.541 | 1.145 (0.864–1.516) | p = 0.347 |
| Stage I + II (vs. III + IV) | 4.853 (3.640–6.470) | p < 0.001 | 4.385 (3.278–5.867) | p < 0.001 |
| NPC1L1 Low (vs. High) | 2.115 (1.598–2.801) | p < 0.001 | 1.664 (1.252–2.211) | p < 0.001 |
| ccRCC (n = 510) | Univariable | Multivariable | ||
| Hazard Ratio (95% CI) | p-value * | Hazard Ratio (95% CI) | p-value * | |
| Age < 60 (vs. ≥ 60) | 1.833 (1.341–2.505) | p < 0.001 | 1.573 (1.144–2.164) | p = 0.005 |
| Sex Male (vs. Female) | 1.043 (0.763–1.426) | p = 0.792 | 1.059 (0.770–1.455) | p = 0.725 |
| Stage I + II (vs. III + IV) | 4.010 (3.640–6.470) | p < 0.001 | 3.634 (2.598–5.083) | p < 0.001 |
| NPC1L1 Low (vs. High) | 1.771 (2.890–5.564) | p < 0.001 | 1.315 (0.951–1.810) | p = 0.098 |
| pRCC (n = 253) | Univariable | Multivariable | ||
| Hazard Ratio (95% CI) | p-value * | Hazard Ratio (95% CI) | p-value * | |
| Age < 60 (vs. ≥ 60) | 1.087 (0.566–2.085) | p = 0.803 | 1.035 (0.534–2.005) | p = 0.919 |
| Sex Male (vs. Female) | 1.594 (0.765–3.321) | p = 0.213 | 1.239 (0.592–2.596) | p = 0.569 |
| Stage I + II (vs. III + IV) | 6.268 (3.240–12.128) | p < 0.001 | 6.309 (3.258–12.217) | p < 0.001 |
| NPC1L1 Low (vs. High) | 3.446 (1.626–7.288) | p = 0.001 | 3.446 (1.606–7.397) | p = 0.001 |
| chRCC (n = 65) | Univariable | Multivariable | ||
| Hazard Ratio (95% CI) | p-value * | Hazard Ratio (95% CI) | p-value * | |
| Age < 60 (vs. ≥ 60) | 2.284 (0.612–8.526) | p = 0.219 | 3.194 (0.635–16.066) | p = 0.159 |
| Sex Male (vs. Female) | 0.643 (0.160–2.575) | p = 0.532 | 2.392 (0.395–14.476) | p = 0.342 |
| Stage I + II (vs. III + IV) | 10.223 (2.120–49.293) | p =0.004 | 14.727 (2.241–96.773) | p = 0.006 |
| NPC1L1 Low (vs. High) | 1.647 (0.412–6.593) | p = 0.481 | 3.058 (0.654–18.810) | p = 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, R.J.; Kim, H.J.; Lee, Y.-S.; Lee, H.S.; Lee, S.Y.; Park, E.-J.; Lee, Y.; Lee, S.R.; Choi, J.-I.; Son, S.M.; et al. Niemann–Pick C1-like 1 as a Prognostic Marker in Renal Cell Carcinoma: A Retrospective Cohort Study. Life 2024, 14, 1444. https://doi.org/10.3390/life14111444
Kwon RJ, Kim HJ, Lee Y-S, Lee HS, Lee SY, Park E-J, Lee Y, Lee SR, Choi J-I, Son SM, et al. Niemann–Pick C1-like 1 as a Prognostic Marker in Renal Cell Carcinoma: A Retrospective Cohort Study. Life. 2024; 14(11):1444. https://doi.org/10.3390/life14111444
Chicago/Turabian StyleKwon, Ryuk Jun, Ho Jun Kim, Young-Shin Lee, Hye Sun Lee, Sang Yeoup Lee, Eun-Ju Park, Youngin Lee, Sae Rom Lee, Jung-In Choi, Soo Min Son, and et al. 2024. "Niemann–Pick C1-like 1 as a Prognostic Marker in Renal Cell Carcinoma: A Retrospective Cohort Study" Life 14, no. 11: 1444. https://doi.org/10.3390/life14111444
APA StyleKwon, R. J., Kim, H. J., Lee, Y.-S., Lee, H. S., Lee, S. Y., Park, E.-J., Lee, Y., Lee, S. R., Choi, J.-I., Son, S. M., Lee, J. G., Yi, Y. H., Tak, Y. J., Lee, S.-H., Kim, G. L., Ra, Y. J., & Cho, Y. H. (2024). Niemann–Pick C1-like 1 as a Prognostic Marker in Renal Cell Carcinoma: A Retrospective Cohort Study. Life, 14(11), 1444. https://doi.org/10.3390/life14111444









