Neurosurgical Outcomes for Intracerebral Hemorrhage in Patients Undergoing Dialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Features and Outcomes
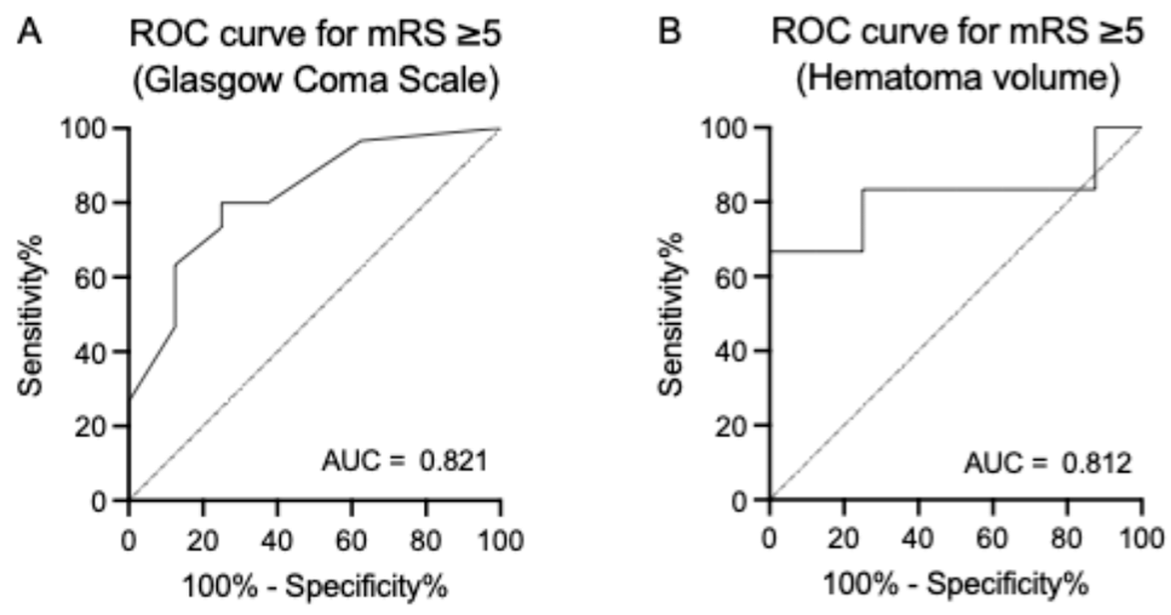
3.3. Predictors of Clinical Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of global kidney health care status. JAMA 2017, 317, 1864–1881. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of haemodialysis outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Power, A. Stroke in dialysis and chronic kidney disease. Blood Purif. 2013, 36, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Gillen, D.L.; Longstreth, W.T., Jr.; Kestenbaum, B.; Stehman-Breen, C.O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003, 64, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Fujii, K.; Fujimi, S.; Kumai, Y.; Tsuchimochi, H.; Ibayashi, S.; Iida, M. Stroke in patients on maintenance hemodialysis: A 22-year single-center study. Am. J. Kidney Dis. 2005, 45, 1058–1066. [Google Scholar] [CrossRef]
- Sakamoto, N.; Ishikawa, E.; Aoki, K.; Uemae, Y.; Komatsu, Y.; Matsumura, A. Clinical outcomes of intracerebral hemorrhage in hemodialysis patients. World Neurosurg. 2014, 81, 538–542. [Google Scholar] [CrossRef][Green Version]
- Murakami, M.; Hamasaki, T.; Kimura, S.; Maruyama, D.; Kakita, K. Clinical features and management of intracranial hemorrhage in patients undergoing maintenance dialysis therapy. Neurol. Med. Chir. 2004, 44, 225–232. [Google Scholar] [CrossRef]
- Suzuki, S.; Hashimoto, N.; Kimura, G.-J. Neurosurgical procedures in hemodialytic patients-analysis of 26 cases of intracranial hemorrhagic diseases and review of literature on cerebral aneurysms with renal failure. Surg. Cereb. Stroke 1996, 24, 193–198. [Google Scholar] [CrossRef]
- Gondo, G.; Fujitsu, K.; Kuwabara, T.; Mochimatsu, Y.; Ishiwata, Y.; Oda, H.; Takagi, N.; Yamashita, T.; Fujino, H.; Kim, I.; et al. Comparison of Five Modes of Dialysis in Neurosurgical Patients with Renal Failure. Neurol. Med. Chir. 1989, 29, 1125–1131. [Google Scholar] [CrossRef][Green Version]
- Saver, J.L.; Chaisinanunkul, N.; Campbell, B.C.V.; Grotta, J.C.; Hill, M.D.; Khatri, P.; Landen, J.; Lansberg, M.G.; Venkatasubramanian, C.; Albers, G.W.; et al. Standardized Nomenclature for Modified Rankin Scale Global Disability Outcomes: Consensus Recommendations From Stroke Therapy Academic Industry Roundtable XI. Stroke 2021, 52, 3054–3062. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ogasawara, K.; Kuroda, S.; Itabashi, R.; Toyoda, K.; Itoh, Y.; Iguchi, Y.; Shiokawa, Y.; Takagi, Y.; Ohtsuki, T.; et al. Japan Stroke Society Guideline 2021 for the Treatment of Stroke. Int. J. Stroke 2022, 17, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Suzuki, K.; Inoue, T.; Kurita, H.; Okada, H. Functional prognosis following spontaneous intracerebral hemorrhage in patients on hemodialysis: A retrospective study of 100 consecutive cases. Ren. Replace. Ther. 2024, 10, 12. [Google Scholar] [CrossRef]
- Hanafusa, N.; Abe, M.; Joki, N.; Hoshino, J.; Wada, A.; Kikuchi, K.; Goto, S. 2021 Annual Dialysis Data Report, JSDT Renal Data Registry. Jpn. Soc. Dial. Ther. 2022, 55, 665–723. [Google Scholar] [CrossRef]
- Naganuma, T.; Takemoto, Y. New aspects of cerebrovascular diseases in dialysis patients. Contrib. Nephrol. 2015, 185, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Gilbertson, D.T.; Murray, T.; Collins, A.J. Long interdialytic interval and mortality among patients receiving hemodialysis. N. Engl. J. Med. 2011, 365, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Power, A.; Chan, K.; Singh, S.K.; Taube, D.; Duncan, N. Appraising stroke risk in maintenance hemodialysis patients: A large single-center cohort study. Am. J. Kidney Dis. 2012, 59, 249–257. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, K.B.; Jang, I.M.; Roh, H.; Ahn, M.Y.; Woo, H.Y.; Hwang, H.W. Low glomerular filtration rate increases hemorrhagic transformation in acute ischemic stroke. Cerebrovasc. Dis. 2013, 35, 53–59. [Google Scholar] [CrossRef]
- Molshatzki, N.; Orion, D.; Tsabari, R.; Schwammenthal, Y.; Merzeliak, O.; Toashi, M.; Tanne, D. Chronic kidney disease in patients with acute intracerebral hemorrhage: Association with large hematoma volume and poor outcome. Cerebrovasc. Dis. 2011, 31, 271–277. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Sanossian, N.; Liebeskind, D.S.; Kim, D.; Ali, L.K.; Pineda, S.; Saver, J.L. Indices of kidney dysfunction and discharge outcomes in hospitalized stroke patients without known renal disease. Cerebrovasc. Dis. 2009, 28, 582–588. [Google Scholar] [CrossRef]
- Hirakata, H.; Nitta, K.; Inaba, M.; Shoji, T.; Fujii, H.; Kobayashi, S.; Tabei, K.; Joki, N.; Hase, H.; Nishimura, M.; et al. Japanese Society for Dialysis Therapy guidelines for management of cardiovascular diseases in patients on chronic hemodialysis. Ther. Apher. Dial. 2012, 16, 387–435. [Google Scholar] [CrossRef]
- Chen, A.; Stecker, E.; Bruce, A.W. Direct oral anticoagulant use: A practical guide to common clinical challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Ageno, W.; Agnelli, G.; Chan, N.C.; Diener, H.C.; Hylek, E.; Raskob, G.E.; Siegal, D.M.; Verheugt, F.W.A.; Lip, G.Y.H.; et al. The non-vitamin K antagonist oral anticoagulants in heart disease: Section V-Special Situations. Thromb. Haemost. 2019, 119, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Ayman, K. Advances in Hemodialysis Techniques. In Hemodialysis; Hiromichi, S., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 20. [Google Scholar] [CrossRef]
- Suga, S.; Koike, K.; Inoue, S.; Katayama, M. The current state of non-traumatic intracranial hemorrhage treatment in Japan using the diagnosis procedure combination data from 2014. Surg. Cereb. Stroke 2018, 46, 348–353. [Google Scholar] [CrossRef][Green Version]
- Holden, R.M.; Harman, G.J.; Wang, M.; Holland, D.; Day, A.G. Major bleeding in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Gejyo, F.; Narita, I. Current clinical and pathogenetic understanding of beta2-m amyloidosis in long-term haemodialysis patients. Nephrology 2003, 8, S45–S49. [Google Scholar] [CrossRef]
- Fushihara, G.; Kamide, T.; Kimura, T.; Takeda, R.; Ikeda, T.; Kikkawa, Y.; Araki, R.; Kurita, H. Factors associated with early seizures after surgery of unruptured intracranial aneurysms. Clin. Neurol. Neurosurg. 2019, 178, 93–96. [Google Scholar] [CrossRef]
- Jabbari, B.; Vaziri, N.D. The nature, consequences, and management of neurological disorders in chronic kidney disease. Hemodial. Int. 2018, 22, 150–160. [Google Scholar] [CrossRef]
- Asconapé, J.J. Use of antiepileptic drugs in hepatic and renal disease. Handb. Clin. Neurol. 2014, 119, 417–432. [Google Scholar] [CrossRef]
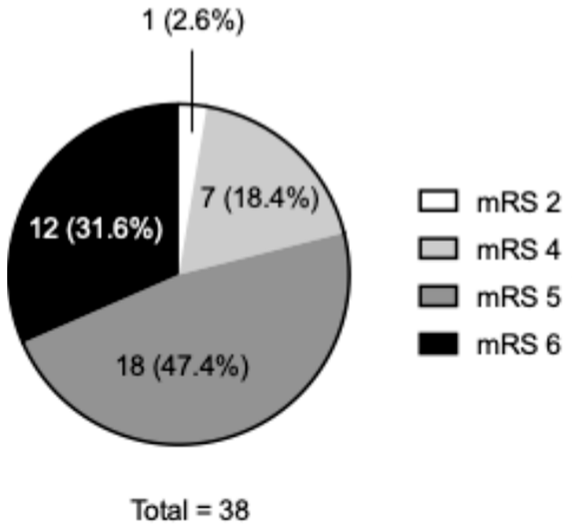
| All | Poor or Better | Very Poor | p Value | |
|---|---|---|---|---|
| No. of patients | 38 (100) | 8 (21.1) | 30 (78.9) | |
| Age (yrs), median [IQR] | 63 [56–71] | 60 [53–64] | 64 [56–71] | 0.390 |
| Female sex | 11 (28.9) | 3 (37.5) | 8 (26.7) | 0.667 |
| Past medical history | ||||
| Hypertension | 34 (89.5) | 8 (100) | 26 (86.7) | 0.560 |
| Diabetes | 15 (39.5) | 3 (37.5) | 12 (40.0) | 1.000 |
| Hyperlipidemia | 3 (7.9) | 1 (12.5) | 2 (6.7) | 0.519 |
| Any stroke | 9 (23.7) | 1 (12.5) | 8 (26.7) | 0.650 |
| Oral antithrombotic medication | 13 (34.2) | 1 (12.5) | 12 (40.0) | 0.222 |
| Antiplatelets | 11 (28.9) | 1 (12.5) | 10 (33.3) | 0.395 |
| Anticoagulants | 3 (7.9) | 1 (12.5) | 2 (6.7) | 0.519 |
| Basic disease for dialysis | ||||
| Chronic glomerulonephritis | 7 (18.4) | 0 (0) | 7 (23.3) | 0.307 |
| Chronic interstitial nephritis | 1 (2.6) | 1 (12.5) | 0 (0) | 0.211 |
| Diabetic nephropathy | 15 (39.5) | 3 (37.5) | 12 (40.0) | 1.000 |
| IgA nephropathy | 3 (7.9) | 1 (12.5) | 2 (6.7) | 0.519 |
| Nephrosclerosis | 1 (2.6) | 0 (0) | 1 (3.3) | 1.000 |
| Polycystic kidney disease | 2 (5.3) | 0 (0) | 2 (6.7) | 1.000 |
| Others | 9 (23.7) | 3 (37.5) | 6 (20.0) | 0.363 |
| Duration of dialysis (yrs), median [IQR] | 9 [4–15] | 8 [6–16] | 9.0 [4–14] | 1.000 |
| All | Poor or Better | Very Poor | p Value | |
|---|---|---|---|---|
| Glasgow Coma Scale, median [IQR] | 6 [4–13] | 13 [10–14] | 6 [3–8] | 0.006 |
| Hematoma volume (mL), median [IQR] | 99 [55–167] | 53 [50–65] | 114 [79–170] | 0.008 |
| Hematoma enlargement | 6 (15.8) | 4 (50.0) | 2 (6.7) | 0.012 |
| Location | ||||
| Putamen | 22 (57.9) | 2 (25.0) | 20 (66.7) | 0.049 |
| Thalamus | 3 (7.9) | 0 (0) | 3 (10.0) | 1.000 |
| Lobar | 12 (31.6) | 6 (75.0) | 6 (20.0) | 0.007 |
| Cerebellum | 2 (5.3) | 0 (0) | 2 (6.7) | 1.000 |
| Venticular perforation | 29 (76.3) | 5 (62.5) | 24 (80.0) | 0.363 |
| Operative time (min), median [IQR] | 200 [163–259] | 145 [98–189] | 214 [176–269] | 0.012 |
| Intraoperative blood loss (mL), median [IQR] | 320 [164–444] | 245 [98–420] | 321 [166–442] | 0.485 |
| Intraoperative blood transfusion | 17 (44.7) | 2 (25.0) | 15 (50.0) | 0.258 |
| Perioperative complication | 18 (47.4) | 2 (25.0) | 16 (53.3) | 0.238 |
| Rebleeding * | 4 (10.5) | 0 (0) | 4 (13.3) | 0.560 |
| Seizure | 3 (7.9) | 1 (12.5) | 2 (6.7) | 0.519 |
| Infection | 3 (7.9) | 1 (12.5) | 2 (6.7) | 0.519 |
| Severe cerebral edema | 3 (7.9) | 0 (0) | 3 (10.0) | 1.000 |
| Hospital stay (day), median [IQR] | 28 [12–48] | 24 [23–28] | 35 [9–65] | 0.531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, T.; Miyata, M.; Naito, N.; Onodera, K.; Take, Y.; Shibata, A.; Suzuki, K.; Ooigawa, H.; Kurita, H. Neurosurgical Outcomes for Intracerebral Hemorrhage in Patients Undergoing Dialysis. Life 2024, 14, 1366. https://doi.org/10.3390/life14111366
Maeda T, Miyata M, Naito N, Onodera K, Take Y, Shibata A, Suzuki K, Ooigawa H, Kurita H. Neurosurgical Outcomes for Intracerebral Hemorrhage in Patients Undergoing Dialysis. Life. 2024; 14(11):1366. https://doi.org/10.3390/life14111366
Chicago/Turabian StyleMaeda, Takuma, Mayuko Miyata, Nobuaki Naito, Koki Onodera, Yushiro Take, Aoto Shibata, Kaima Suzuki, Hidetoshi Ooigawa, and Hiroki Kurita. 2024. "Neurosurgical Outcomes for Intracerebral Hemorrhage in Patients Undergoing Dialysis" Life 14, no. 11: 1366. https://doi.org/10.3390/life14111366
APA StyleMaeda, T., Miyata, M., Naito, N., Onodera, K., Take, Y., Shibata, A., Suzuki, K., Ooigawa, H., & Kurita, H. (2024). Neurosurgical Outcomes for Intracerebral Hemorrhage in Patients Undergoing Dialysis. Life, 14(11), 1366. https://doi.org/10.3390/life14111366






