Possibilities for Methanogenic and Acetogenic Life in Molecular Clouds
Abstract
1. Introduction
2. Possibilities for Methanogenic Life in Molecular Clouds
2.1. The Calculation of Gibbs Free Energy
| Molecule | H (kJ mol−1) | S (J/mol K) | Abundance | |||
|---|---|---|---|---|---|---|
| 0 | 130.68 [19] | [20] | ||||
| −74.6 [21] | 186.3 [21] | [22] | ||||
| 227.400 [21] | 200.927 [21] | [23] | ||||
| −241.83 [24] | 188.84 [24] | (for ortho-) [25] | ||||
| −110.53 [24] | 197.66 [24] | |||||
| −427.4 | 51.07 [26] | - | ||||
| −433 | 282.84 [27] | [28] |
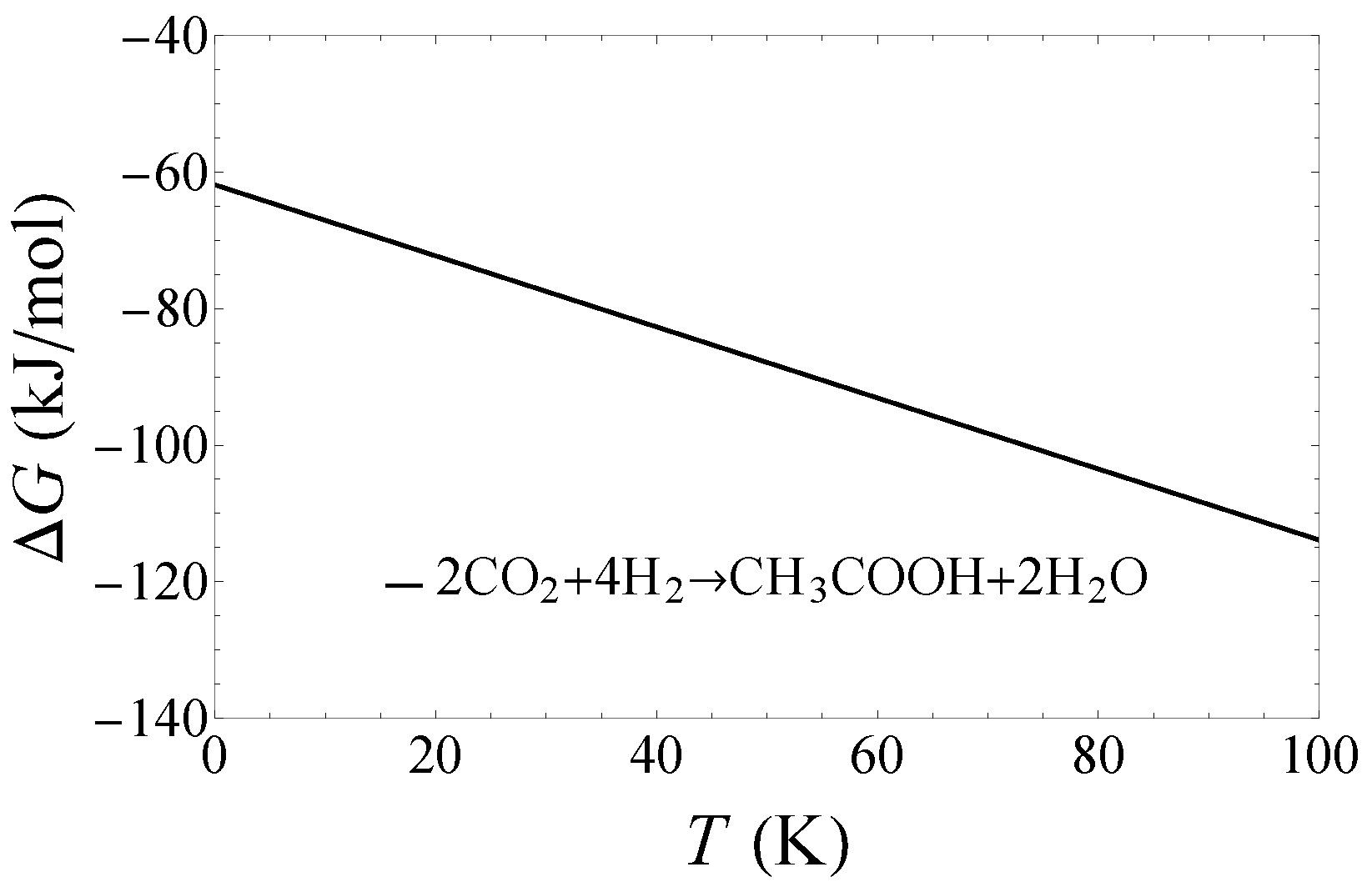
2.2. The Results
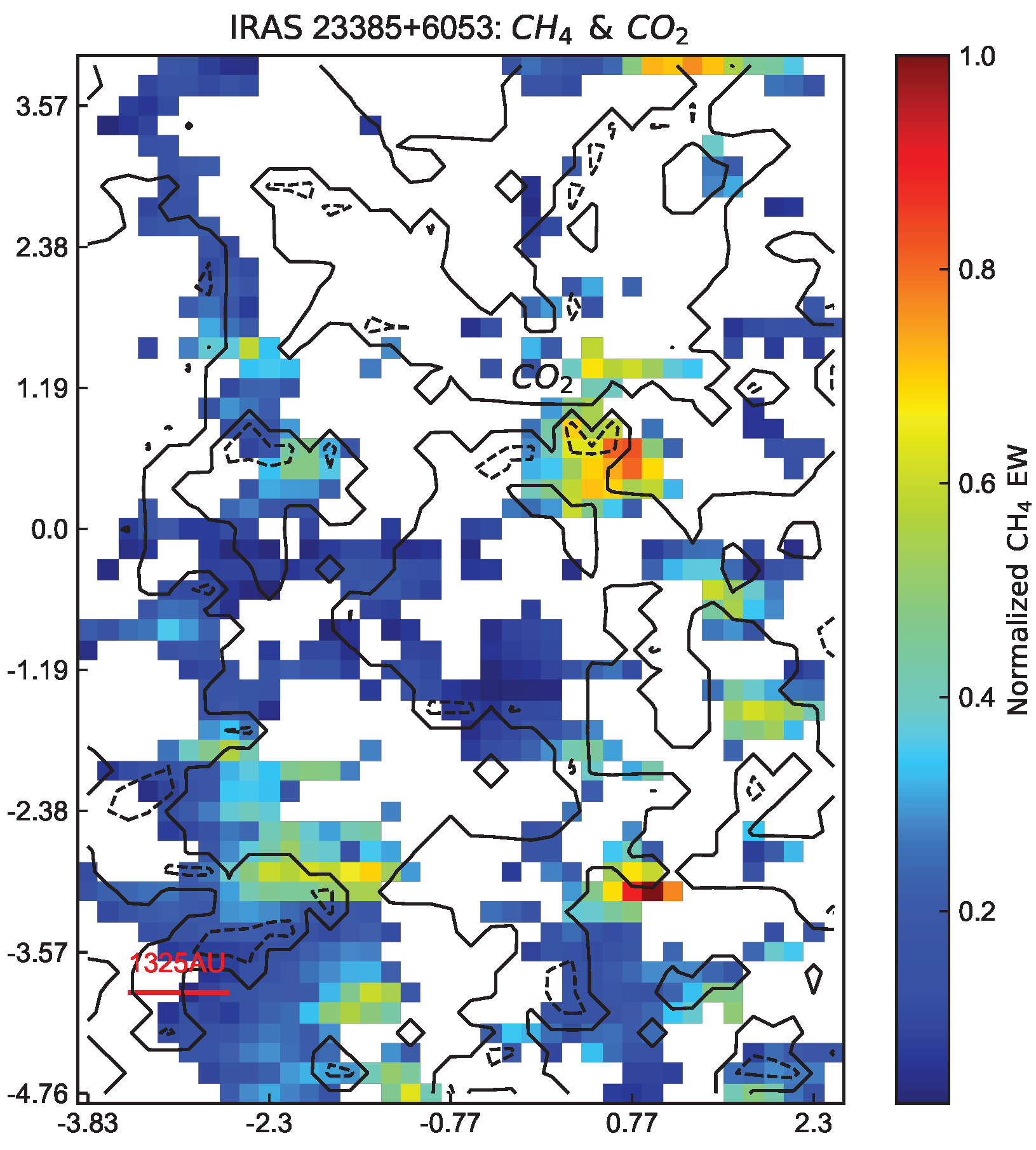
2.3. A Possible Distinguishing Signal
3. The Relationship with LUCA
4. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moody, E.R.R.; Álvarez-Carretero, S.; Mahendrarajah, T.A.; Clark, J.W.; Betts, H.C.; Dombrowski, N.; Szánthó, L.L.; Boyle, R.A.; Daines, S.; Chen, X.; et al. The nature of the last universal common ancestor and its impact on the early Earth system. Nat. Ecol. Evol. 2024, 8, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.E. Panspermia: Unlikely, unsupported, but just possible. Acta Astron. 1988, 17, 129–135. [Google Scholar] [CrossRef]
- Feng, L. Nebula-relay hypothesis: Primitive life in nebula and origin of life on Earth. Acta Astron. Sin. 2021, 62, 28. [Google Scholar]
- Feng, L. Nebula-relay hypothesis: The chirality of biological molecules in molecular clouds. Front. Astron. Space Sci. 2022, 9, 794067. [Google Scholar] [CrossRef]
- Paschek, K.; Semenov, D.A.; Pearce, B.K.D.; Lange, K.; Henning, T.K.; Pudritz, R.E. Meteorites and the RNA World: Synthesis of Nucleobases in Carbonaceous Planetesimals and the Role of Initial Volatile Content. Astrophys. J. 2023, 942, 50. [Google Scholar] [CrossRef]
- Krasnokutski, S.A. Did life originate from low-temperature areas of the Universe? Low Temp. Phys. 2021, 47, 199. [Google Scholar] [CrossRef]
- Wolman, Y.; Haverland, W.J.; Miller, S.L. Nonprotein Amino Acids from Spark Discharges and Their Comparison with the Murchison Meteorite Amino Acids. Proc. Natl. Acad. Sci. USA 1972, 69, 809–811. [Google Scholar] [CrossRef]
- Engel, M.H.; Nagy, B. Distribution and enantiomeric composition of amino acids in the Murchison meteorite. Nature 1982, 296, 837–840. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [Google Scholar] [CrossRef]
- Feng, L. Cosmic ray-driven bioenergetics for life in molecular clouds. arXiv 2024, arXiv:2206.12816. [Google Scholar]
- McKay, C.P.; Smith, H.D. Possibilities for methanogenic life in liquid methane on the surface of Titan. Icarus 2005, 178, 274–276. [Google Scholar] [CrossRef]
- Minh, Y.C.; Irvine, W.M.; Ziurys, L.M. Observations of interstellar HOCO+: Abundance enhancements toward the galactic center. Astrophys. J. 1988, 334, 175–181. [Google Scholar] [CrossRef]
- Gerakines, P.A.; Whittet, D.C.B.; Ehrenfreund, P.; Boogert, A.C.A.; Tielens, A.G.G.M.; Schutte, W.A.; Chiar, J.E.; van Dishoeck, E.F.; Prusti, T.; Helmich, F.P.; et al. Observations of solid carbon dioxide in molecular clouds with the Infrared Space Observatory. Astrophys. J. 1999, 522, 357. [Google Scholar] [CrossRef]
- Whittet, D.C.B.; Gerakines, P.A.; Tielens, A.G.G.M.; Adamson, A.J.; Boogert, A.C.A.; Chiar, J.E.; de Graauw, T.; Ehrenfreund, P.; Prusti, T.; Schutte, W.A.; et al. Detection of abundant CO2 ice in the quiescent dark cloud medium toward elias 16. Astrophys. J. Lett. 1998, 498, L159–L163. [Google Scholar] [CrossRef]
- Stull, D.R.; Westrum, E.F.; Sinke, G.C. The Chemical Thermodynamics of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1969. [Google Scholar]
- Miller, S.L.; SmithMagowan, D.J. The thermodynamics of the Krebs cycle and related compounds. Phys. Chem. Ref. Data 1990, 19, 1049–1073. [Google Scholar] [CrossRef]
- Kral, T.A.; Brink, K.M.; Miller, S.L.; McKay, C.P. Hydrogen consumption by methanogens on the early Earth. Orig. Life Evol. B 1998, 28, 311–319. [Google Scholar] [CrossRef]
- Chase, M. NIST-JANAF Thermochemical Tables, 4th ed.; American Institute of Physics: College Park, MD, USA, 1998. [Google Scholar]
- Frerking, M.A.; Langer, W.D.; Wilson, R.W. The relationship between carbon monoxide abundance and visual extinction in interstellar clouds. Astrophys. J. 1982, 262, 590–605. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498754293. [Google Scholar]
- Lacy, J.H.; Carr, J.S.; Evans, N.J., II; Baas, F.; Achtermann, J.M.; Arens, J.F. Discovery of interstellar methane: Observations of gaseous and solid CH4 absorption toward young stars in molecular clouds. Astrophys. J. 1991, 376, 556. [Google Scholar] [CrossRef]
- Lacy, J.H.; Evans, N.J., II; Achtermann, J.M.; Bruce, D.E.; Arens, J.F.; Carr, J.S. Discovery of interstellar acetylene. Astrophys. J. Lett. 1989, 342, L43. [Google Scholar] [CrossRef]
- Peter, J.L.; William, G.M. (Eds.) NIST Chemistry Webbook; SRD 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. Available online: http://webbook.nist.gov (accessed on 23 October 2024).
- Snell, R.L.; Howe, J.E.; Erickson, N.R.; Ashby, M.L.N.; Bergin, E.A.; Kleiner, S.C.; Melnick, G.J.; Patten, B.M.; Plume, R.; Stauffer, J.R.; et al. Water in molecular clouds. Bull. Am. Astron. Soc. 1999, 31, 1465. [Google Scholar]
- Giauque, W.F.; Egan, C.J. Carbon dioxide. The heat capacity and vapor pressure of the solid. The heat of sublimation. Thermodynamic and spectroscopic values of the entropy. J. Chem. Phys. 1937, 5, 45–54. [Google Scholar] [CrossRef]
- Weltner, W., Jr. The vibrational spectrum, associative and thermodynamic properties of acetic acid vapor. J. Am. Chem. Soc. 1955, 77, 3941–3950. [Google Scholar] [CrossRef]
- Mehringer, D.M.; Snyder, L.E.; Miao, Y.; Lovas, F.J. Detection and confirmation of interstellar acetic acid. Astrophys. J. Lett. 1997, 480, L71. [Google Scholar] [CrossRef]
- Wilson, C.; Walker, C.; Thornley, M. The density and temperature of molecular clouds in M33. Astrophys. J. 1997, 483, 210–219. [Google Scholar] [CrossRef]
- Formisano, V.; Atreya, S.; Encrenaz, T.; Ignatiev, N.; Giuranna, M. Detection of Methane in the Atmosphere of Mars. Science 2004, 306, 1758–1761. [Google Scholar] [CrossRef]
- Krasnopolsky, V.A.; Maillard, J.P.; Owen, T.C. Detection of methane in the martian atmosphere: Evidence for life? Icarus 2004, 172, 537–547. [Google Scholar] [CrossRef]
- Mumma, M.J.; Villanueva, G.L.; Novak, R.E.; Hewagama, T.; Bonev, B.P.; DiSanti, M.A.; Mandell, A.M.; Smith, M.D. Strong release of methane on Mars in northern summer 2003. Science 2009, 323, 1041–1045. [Google Scholar] [CrossRef]
- Atreya, S.K.; Mahaffy, P.R.; Wong, A.S. Methane and related trace species on Mars: Origin, loss, implications for life, and habitability. Planet. Space Sci. 2007, 55, 358–369. [Google Scholar] [CrossRef]
- Korablev, O.; Vandaele, A.C.; Montmessin, F.; Fedorova, A.A.; Trokhimovskiy, A.; Forget, F.; Lefèvre, F.; Daerden, F.; Thomas, I.R.; Trompet, L.; et al. No detection of methane on Mars from early ExoMars Trace Gas Orbiter observations. Nature 2019, 568, 517–520. [Google Scholar] [CrossRef]
- Narang, M.; Manoj, P.; Tyagi, H.; Watson, D.M.; Megeath, S.T.; Federman, S.; Rubinstein, A.E.; Gutermuth, R.; Garatti, A.C.; Beuther, H.; et al. Discovery of a collimated jet from the low luminosity protostar IRAS 16253-2429 in a quiescent accretion phase with the JWST. arXiv 2023, arXiv:2310.14061. [Google Scholar] [CrossRef]
- Beuther, H.; van Dishoeck, E.F.; Tychoniec, L.; Gieser, C.; Kavanagh, P.J.; Perotti, G.; Gelder, M.L.v.; Klaassen, P.; Garatti, A.C.; Francis, L.; et al. JWST Observations of Young protoStars (JOYS): Outflows and accretion in the high-mass star-forming region IRAS 23385+6053. Astron. Astrophys. 2023, 673, A121. [Google Scholar] [CrossRef]
- Lei, L.; Feng, L.; Fan, Y. The Spatial Distribution of CH4 and CO2 Ice around Protostars IRAS 16253-2429 and IRAS 23385+6053. arXiv 2024, arXiv:2409.04217. [Google Scholar]
- Herbst, E.; Klemperer, W. The formation and depletion of molecules in dense interstellar clouds. Astrophys. J. 1973, 185, 505–534. [Google Scholar] [CrossRef]
- Caselli, P.; Ceccarelli, C. Our astrochemical heritage. Astron. Astrophys. Rev. 2012, 20, 56. [Google Scholar] [CrossRef]
- Molinari, S.; Testi, L.; Br, J.; Cesaroni, R.; Palla, F. IRAS 23385+ 6053: A prototype massive class 0 object. Astrophys. J. Lett. 1998, 505, L39. [Google Scholar] [CrossRef][Green Version]
- Fontani, F.; Cesaroni, R.; Testi, L.; Walmsley, C.M.; Molinari, S.; Neri, R.; Shepherd, D.; Br, J.; Palla, F.; Zhang, Q. IRAS 23385+ 6053: A candidate protostellar massive object. Astron. Astrophys. 2004, 414, 299–315. [Google Scholar] [CrossRef]
- Wolf-Chase, G.; Smutko, M.; Sherman, R.; Harper, D.A.; Medford, M. Near-infrared and Millimeter-wavelength Observations of Mol 160: A Massive Young Protostellar Core. Astrophys. J. 2012, 745, 116. [Google Scholar] [CrossRef]
- Francis, L.; van Gelder, M.L.; van Dishoeck, E.F.; Gieser, C.; Beuther, H.; Tychoniec, L.; Perotti, G.; o Garatti, A.C.; Kavanagh, P.J.; Ray, T.; et al. JOYS: MIRI/MRS spectroscopy of gas-phase molecules from the high-mass star-forming region IRAS 23385+ 6053. Astron. Astrophys. 2024, 683, A249. [Google Scholar] [CrossRef]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef]
- Kasting, J.F. Bolide impacts and the oxidation state of carbon in the Earth’s early atmosphere. Origins Life 1990, 20, 199–231. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F. Earth’s Early Atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef]
- Holland, H.D. The Chemical Evolution of the Atmosphere and Oceans; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Miller, S.L.; Orgel, L.E. The Origins of Life on the Earth; Prentice-Hall: Englewood Cliffs, NJ, USA, 1974. [Google Scholar]
- DiSanti, M.A.; Mumma, M.J.; Russo, N.D.; Magee-Sauer, K.; Novak, R.; Rettig, T.W. Identification of two sources of carbon monoxide in Comet Hale–Bopp. Nature 1999, 399, 662–665. [Google Scholar] [CrossRef]
- Pierce, E.; Xie, G.; Barabote, R.D.; Saunders, E.; Han, C.S.; Detter, J.C.; Richardson, P.; Brettin, T.S.; Das, A.; Ljungdahl, L.G.; et al. The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ. Microbiol. 2008, 10, 2550–2573. [Google Scholar] [CrossRef]
- Rother, M.; Metcalf, W.W. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: An unusual way of life for a methanogenic archaeon. Proc. Natl. Acad. Sci. USA 2004, 101, 16929–16934. [Google Scholar] [CrossRef]
- Mat, W.; Xue, H.; Wong, J.T. The genomics of LUCA. Front. Biosci. J. Virtual Libr. 2008, 13, 5605–5613. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef]
- Berkemer, S.J.; McGlynn, S.E. A new analysis of archaea—Cbacteria domain separation: Variable phylogenetic distance and the tempo of early evolution. Mol. Biol. Evol. 2020, 37, 2332–2340. [Google Scholar] [CrossRef]
- Preiner, M.; Igarashi, K.; Muchowska, K.B.; Yu, M.; Varma, S.J.; Kleinermanns, K.; Nobu, M.K.; Kamagata, Y.; Tüysüz, H.; Moran, J.; et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 2020, 4, 534–542. [Google Scholar] [CrossRef]
- Xavier, J.C.; Gerhards, R.E.; Wimmer, J.L.E.; Brueckner, J.; Tria, F.D.K.; Martin, W.F. The metabolic network of the last bacterial common ancestor. Commun. Biol. 2021, 4, 413. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L. Possibilities for Methanogenic and Acetogenic Life in Molecular Clouds. Life 2024, 14, 1364. https://doi.org/10.3390/life14111364
Feng L. Possibilities for Methanogenic and Acetogenic Life in Molecular Clouds. Life. 2024; 14(11):1364. https://doi.org/10.3390/life14111364
Chicago/Turabian StyleFeng, Lei. 2024. "Possibilities for Methanogenic and Acetogenic Life in Molecular Clouds" Life 14, no. 11: 1364. https://doi.org/10.3390/life14111364
APA StyleFeng, L. (2024). Possibilities for Methanogenic and Acetogenic Life in Molecular Clouds. Life, 14(11), 1364. https://doi.org/10.3390/life14111364






