Accounting for Red Cell Distribution Width Improves Risk Stratification by Commonly Used Mortality/Deterioration Risk Scores in Adult Patients Hospitalized Due to COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Outline
2.2. Statistical Analysis
3. Results
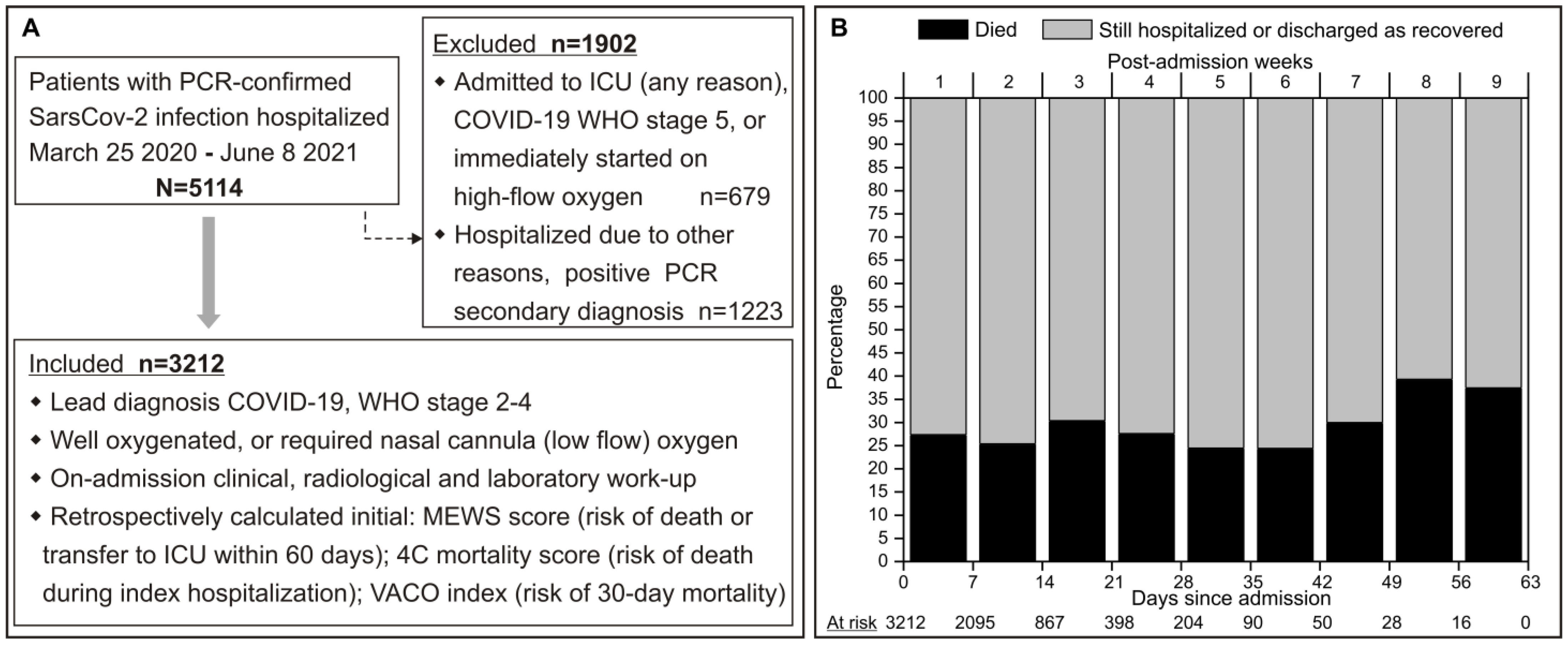
3.1. Patient Eligibility and Characteristics
3.2. Accounting for RDW Improves MEWS Scoring System-Based Risk Stratification
3.3. Accounting for RDW Improves 4C Score-Based Risk Stratification
3.4. Accounting for RDW Improves VACO 30-Day Mortality Scoring System-Based Risk Stratification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tong, Z.; Guan, X.; Du, B.; Qiu, H.; Slutsky, A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020, 46, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Telenti, A.; Arvin, A.; Corey, L.; Corti, D.; Diamond, M.S.; García-Sastre, A.; Garry, R.F.; Holmes, E.C.; Pang, P.S.; Virgin, H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin. Chem. Lab. Med. 2014, 52, 1247–1249. [Google Scholar] [CrossRef]
- Abrahan, L.L., IV; Ramos, J.D.A.; Cunanan, E.L.; Tiongson, M.D.A.; Punzalan, F.E.R. Red Cell Distribution Width and Mortality in Patients with Acute Coronary Syndrome: A Meta-Analysis on Prognosis. Cardiol. Res. 2018, 9, 144–152. [Google Scholar] [CrossRef]
- Milas, G.P.; Karageorgiou, V.; Cholongitas, E. Red cell distribution width to platelet ratio for liver fibrosis: A systematic review and meta-analysis of diagnostic accuracy. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 877–891. [Google Scholar] [CrossRef]
- Goyal, H.; Awad, H.; Hu, Z.D. Prognostic value of admission red blood cell distribution width in acute pancreatitis: A systematic review. Ann. Transl. Med. 2017, 5, 342. [Google Scholar] [CrossRef]
- Song, S.Y.; Hua, C.; Dornbors, D., III; Kang, R.J.; Zhao, X.X.; Du, X.; He, W.; Ding, Y.C.; Meng, R. Baseline Red Blood Cell Distribution Width as a Predictor of Stroke Occurrence and Outcome: A Comprehensive Meta-Analysis of 31 Studies. Front. Neurol. 2019, 10, 1237. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Pérez-Cejas, A.; González-Rivero, A.F.; Ramos-Gómez, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; et al. Early Mortality of Brain Infarction Patients and Red Blood Cell Distribution Width. Brain Sci. 2020, 10, 196. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Tang, Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. Dis. Markers 2017, 2017, 7089493. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Montazerin, S.M.; Jamil, A.; Jamil, U.; Marszalek, J.; Chuang, M.L.; Chi, G. Association between red blood cell distribution width and mortality and severity among patients with COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Kannan, S.; Khanna, P.; Singh, A.K. Role of red blood cell distribution width, as a prognostic indicator in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2022, 32, e2264. [Google Scholar] [CrossRef]
- Barnett, W.R.; Radhakrishnan, M.; Macko, J.; Hinch, B.T.; Altorok, N.; Assaly, R. Initial MEWS score to predict ICU admission or transfer of hospitalized patients with COVID-19: A retrospective study. J. Infect. 2021, 82, 282–327. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 371, m4334. [Google Scholar] [CrossRef] [PubMed]
- King, J.T., Jr.; Yoon, J.S.; Rentsch, C.T.; Tate, J.P.; Park, L.S.; Kidwai-Khan, F.; Skanderson, M.; Hauser, R.G.; Jacobson, D.A.; Erdos, J.; et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: The Veterans Health Administration COVID-19 (VACO) Index. PLoS ONE 2020, 15, e0241825. [Google Scholar] [CrossRef]
- Ministarstvo Zdravstva Republike Hrvatske. Smjernice za Liječenje Oboljelih od COVID-19, Verzija 1 od 8. Rujna 2020. 2020. Available online: https://zdravlje.gov.hr/UserDocsImages//2020%20CORONAVIRUS//Smjernice%20za%20liječenje%20oboljelih%20od%20COVID-19,%20verzija%201%20od%2008.09.2020..pdf (accessed on 2 May 2024).
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, Geneva: World Health Organization (2020). Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 2 May 2024).
- Adeli, K.; Raizman, J.E.; Chen, Y.; Higgins, V.; Nieuwesteeg, M.; Abdelhaleem, M.; Wong, S.L.; Blais, D. Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: Establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin. Chem. 2015, 61, 1075–1086. [Google Scholar] [CrossRef]
- Hornick, A.; Tashtish, N.; Osnard, M.; Shah, B.; Bradigan, A.; Albar, Z.; Tomalka, J.; Dalton, J.; Sharma, A.; Sekaly, R.P.; et al. Anisocytosis is Associated with Short-Term Mortality in COVID-19 and May Reflect Proinflammatory Signature in Uninfected Ambulatory Adults. Pathog. Immun. 2020, 5, 312–326. [Google Scholar] [CrossRef]
- Guaní-Guerra, E.; Torres-Murillo, B.; Muñoz-Corona, C.; Rodríguez-Jiménez, J.C.; Macías, A.E.; Scavo-Montes, D.A.; Alvarez, J.A. Diagnostic Accuracy of the RDW for Predicting Death in COVID-19. Medicina 2022, 58, 613. [Google Scholar] [CrossRef]
- Lucijanic, M.; Soric, E.; Sedicnic Lacko, M.; Sabljic, A.; Krecak, I.; Bistrovic, P.; Jordan, A.; Manola, S.; Jaksic, O.; Lucijanic, T.; et al. Gradual increase in red cell distribution width is similarly prognostic for in-hospital mortality in both anemic and non-anemic COVID-19 patients. J. Med. Virol. 2022, 94, 3509–3511. [Google Scholar] [CrossRef]
- Gama, S. RDW shows prognostic potential in hospitalized patients with COVID-19. J. Med. Virol. 2022, 94, 3498–3500. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Benoit, J.L.; Benoit, S.; Pulvino, C.; Berger, B.A.; Olivera, M.H.S.; Crutchfield, C.A.; Lippi, G. Red Blood Cell Distribution Width (RDW) Predicts COVID-19 Severity: A Prospective, Observational Study from the Cincinnati SARS-CoV-2 Emergency Department Cohort. Diagnostics 2020, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Gajendran, M.; Perisetti, A.; Elkholy, K.O.; Chakraborti, A.; Lippi, G.; Goyal, H. Red Blood Cell Distribution Width in Hospitalized COVID-19 Patients. Front. Med. 2021, 8, 582403. [Google Scholar] [CrossRef] [PubMed]
- Said, A.S.; Spinella, P.C.; Hartman, M.E.; Steffen, K.M.; Jackups, R.; Holubkov, R.; Wallendorf, M.; Doctor, A. RBC Distribution Width: Biomarker for Red Cell Dysfunction and Critical Illness Outcome? Pediatr. Crit. Care Med. 2017, 18, 134–142. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef]
- Tziakas, D.; Chalikias, G.; Grapsa, A.; Gioka, T.; Tentes, I.; Konstantinides, S. Red blood cell distribution width: A strong prognostic marker in cardiovascular disease: Is associated with cholesterol content of erythrocyte membrane. Clin. Hemorheol. Microcirc. 2012, 51, 243–254. [Google Scholar] [CrossRef]
- Qurtom, H.A.; al-Saleh, Q.A.; Lubani, M.M.; Hassanein, A.; Kaddoorah, N.; Qurtom, M.A.; al-Sheikh, T. The value of red cell distribution width in the diagnosis of anaemia in children. Eur. J. Pediatr. 1989, 148, 745–748. [Google Scholar] [CrossRef]
- Sultana, G.S.; Haque, S.A.; Sultana, T.; Rahman, Q.; Ahmed, A.N. Role of red cell distribution width (RDW) in the detection of iron deficiency anaemia in pregnancy within the first 20 weeks of gestation. Bangladesh Med. Res. Counc. Bull. 2011, 37, 102–105. [Google Scholar] [CrossRef]
- Emans, M.E.; Gaillard, C.A.; Pfister, R.; Tanck, M.W.; Boekholdt, S.M.; Wareham, N.J.; Khaw, K.T. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int. J. Cardiol. 2013, 168, 3550–3555. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Danese, E.; Montagnana, M. Association of red blood cell distribution width with plasma lipids in a general population of unselected outpatients. Kardiol. Pol. 2013, 71, 931–936. [Google Scholar] [CrossRef]
- Lucijanic, M.; Krecak, I.; Soric, E.; Sabljic, A.; Galusic, D.; Holik, H.; Perisa, V.; Peric, M.M.; Zekanovic, I.; Budimir, J.; et al. Evaluation of Absolute Neutrophil, Lymphocyte and Platelet Count and Their Ratios as Predictors of Thrombotic Risk in Patients with Prefibrotic and Overt Myelofibrosis. Life 2024, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- García-Escobar, A.; Vera-Vera, S.; Tébar-Márquez, D.; Rivero-Santana, B.; Jurado-Román, A.; Jiménez-Valero, S.; Galeote, G.; Cabrera, J.; Moreno, R. Neutrophil-to-lymphocyte ratio an inflammatory biomarker, and prognostic marker in heart failure, cardiovascular disease and chronic inflammatory diseases: New insights for a potential predictor of anti-cytokine therapy responsiveness. Microvasc. Res. 2023, 150, 104598. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Krečak, I.; Heidel, F.H.; De Stefano, V.; Bryan, K.; Zuurman, M.W.; Zaiac, M.; Morelli, M.; Smyth, A.; Redondo, S.; et al. Identifying Patients with Polycythemia Vera at Risk of Thrombosis after Hydroxyurea Initiation: The Polycythemia Vera—Advanced Integrated Models (PV-AIM) Project. Biomedicines 2023, 11, 1925. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, J.; Liu, X.; Deng, J.; Sun, C.; Tang, J.; Chen, H.; Cao, W.; Wang, W.; Duan, X.; et al. Interpretable machine learning for 28-day all-cause in-hospital mortality prediction in critically ill patients with heart failure combined with hypertension: A retrospective cohort study based on medical information mart for intensive care database-IV and eICU databases. Front. Cardiovasc. Med. 2022, 9, 994359. [Google Scholar] [CrossRef]
- Prozan, L.; Shusterman, E.; Ablin, J.; Mitelpunkt, A.; Weiss-Meilik, A.; Adler, A.; Choshen, G.; Kehat, O. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 compared with Influenza and respiratory syncytial virus infection. Sci. Rep. 2021, 11, 21519. [Google Scholar] [CrossRef]
- Park, J.; Dean, L.S.; Jiyarom, B.; Gangcuangco, L.M.; Shah, P.; Awamura, T.; Ching, L.L.; Nerurkar, V.R.; Chow, D.C.; Igno, F.; et al. Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals. Front. Immunol. 2023, 14, 1151780. [Google Scholar] [CrossRef]
- Lucijanic, M.; Krecak, I.; Soric, E.; Sedinic, M.; Sabljic, A.; Derek, L.; Jaksic, O.; Kusec, R. Thrombocytosis in COVID-19 patients without myeloproliferative neoplasms is associated with better prognosis but higher rate of venous thromboembolism. Blood Cancer J. 2021, 11, 189. [Google Scholar] [CrossRef]
- Lucijanic, M.; Krecak, I.; Soric, E.; Sabljic, A.; Vasilj, T.; Cicic, D.; Vuk, A.V.; Kremer, Z.; Dilber, I.; Glasnovic, A.; et al. Secondary polycythemia in acutely ill COVID-19 patients is associated with higher mortality but not markedly higher thrombotic risk. Scand. J. Clin. Lab. Investig. 2024, 84, 84–90. [Google Scholar] [CrossRef]


| All Patients | RDW Normal Range | RDW > Normal Range | p Value | |
|---|---|---|---|---|
| N | 3212 | 2584 | 628 | - |
| Age | 72 (63–82) | 71 (62–81) | 77 (68–84) | <0.001 |
| Men | 1804 (56.2) | 1347 (52.1) | 457 (72.8) | <0.001 |
| X-ray pneumonia on admission | 3101 (96.5) | 2501 (96.8) | 600 (95.5) | 0.768 |
| Started oxygen upon admission | 2962 (92.2) | 2377 (92.0) | 585 (93.2) | 0.308 |
| Remdesivir before progression | 566 (17.6) | 494 (19.1) | 72 (11.5) | <0.001 |
| MEWS | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.109 |
| MEWS 0–2 | 1765 (54.9) | 1391 (53.8) | 374 (59.5) | 0.012 |
| MEWS 3–4 | 1447 (45.1) | 1193 (46.2) | 254 (40.5) | 0.012 |
| 4C score | 11 (8–13) | 10 (7–13) | 13 (10–15) | <0.001 |
| 4C score 0–3 (low, 1.2–1.7%) | 156 (4.9) | 145 (5.6) | 11 (1.7) | <0.001 |
| 4C score 4–8 (medium, 9.1–9.9%) | 814 (25.3) | 740 (28.6) | 74 (11.8) | <0.001 |
| 4C score 9–14 (high, 31.4–34.9%) | 1734 (54.0) | 1393 (53.9) | 341 (54.3) | 0.819 |
| 4C score ≥15 (very high, 61.5–66.2%) | 508(15.8) | 306 (11.8) | 202 (32.2) | <0.001 |
| VACO index | 16.4 (8.8–24.2) | 14.7 (8.8–23.6) | 21.3 (14.5–31.9) | <0.001 |
| Low (0–8.7%) | 707 (22.0) | 630 (24.4) | 77 (12.3) | <0.001 |
| Medium (8.8–16.0%) | 777 (24.2) | 684 (26.5) | 93 (14.8) | <0.001 |
| High (16.1–21.2%) | 671 (20.9) | 540 (20.9) | 131 (20.9) | 0.976 |
| Extreme (≥21.3%) | 1057 (32.9) | 730 (28.3) | 328 (52.1) | <0.001 |
| Need transfer to ICU | 405 (12.6) | 295 (11.4) | 110 (17.5) | <0.001 |
| Need mechanical ventilation | 346 (10.8) | 258 (10.0) | 88 (14.0) | 0.003 |
| Died during hospitalization | 879 (27.4) | 591 (22.9) | 288 (45.9) | <0.001 |
| Died within 30 days | 841 (26.2) | 561 (21.7) | 280 (44.6) | <0.001 |
| Death or ICU within 60 days | 939 (29.2) | 633 (24.5) | 306 (48.7) | <0.001 |
| Charlson comorbidity index (CCI) | 4 (2–6) | 4 (2–5) | 5.5 (4–7) | <0.001 |
| CCI 0 | 181 (5.6) | 170 (6.6) | 11 (1.7) | <0.001 |
| CCI 1–2 | 639 (19.9) | 600 (23.2) | 39 (6.2) | <0.001 |
| CCI 3–4 | 1047 (32.6) | 899 (34.8) | 148 (23.6) | <0.001 |
| CCI ≥5 | 1345 (41.9) | 915 (35.4) | 430 (68.5) | <0.001 |
| Diabetes | 1007 (31.4) | 768 (29.7) | 239 (38.1) | <0.001 |
| Obesity | 1004 (31.3) | 819 (31.7) | 185 (29.5) | 0.273 |
| Chronic heart failure | 429 (13.4) | 259 (10.0) | 170 (27.1) | <0.001 |
| Chronic renal failure | 354 (11.0) | 210 (8.1) | 144 (22.9) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordan, A.; Trkulja, V.; Jurin, I.; Marević, S.; Đerek, L.; Lukšić, I.; Manola, Š.; Lucijanić, M. Accounting for Red Cell Distribution Width Improves Risk Stratification by Commonly Used Mortality/Deterioration Risk Scores in Adult Patients Hospitalized Due to COVID-19. Life 2024, 14, 1267. https://doi.org/10.3390/life14101267
Jordan A, Trkulja V, Jurin I, Marević S, Đerek L, Lukšić I, Manola Š, Lucijanić M. Accounting for Red Cell Distribution Width Improves Risk Stratification by Commonly Used Mortality/Deterioration Risk Scores in Adult Patients Hospitalized Due to COVID-19. Life. 2024; 14(10):1267. https://doi.org/10.3390/life14101267
Chicago/Turabian StyleJordan, Ana, Vladimir Trkulja, Ivana Jurin, Sanja Marević, Lovorka Đerek, Ivica Lukšić, Šime Manola, and Marko Lucijanić. 2024. "Accounting for Red Cell Distribution Width Improves Risk Stratification by Commonly Used Mortality/Deterioration Risk Scores in Adult Patients Hospitalized Due to COVID-19" Life 14, no. 10: 1267. https://doi.org/10.3390/life14101267
APA StyleJordan, A., Trkulja, V., Jurin, I., Marević, S., Đerek, L., Lukšić, I., Manola, Š., & Lucijanić, M. (2024). Accounting for Red Cell Distribution Width Improves Risk Stratification by Commonly Used Mortality/Deterioration Risk Scores in Adult Patients Hospitalized Due to COVID-19. Life, 14(10), 1267. https://doi.org/10.3390/life14101267









